Abstract
Aims: The efficacy and safety of oral semaglutide, the first glucagon-like peptide-1 (GLP-1) receptor agonist developed for oral administration for the treatment of type 2 diabetes, was evaluated in the PIONEER clinical trial program, and a recently published network meta-analysis allowed comparison with further injectable GLP-1 receptor agonists. The present study aimed to assess the short-term cost- effectiveness of oral semaglutide 14 mg versus subcutaneous once-weekly dulaglutide 1.5 mg, once-weekly exenatide 2 mg, twice-daily exenatide 10 µg, once-daily liraglutide 1.8 mg, once-daily lixisenatide 20 µg, and once-weekly semaglutide 1 mg, in terms of the cost per patient achieving glycated hemoglobin (HbA1c) targets (cost of control).
Materials and methods: Cost of control was calculated by dividing the annual treatment costs associated with an intervention by the proportion of patients achieving the treatment target with an intervention, with outcomes calculated for targets of HbA1c ≤6.5% and HbA1c <7.0% for all included GLP-1 receptor agonists. Annual treatment costs were accounted in 2019 United States dollars (USD), based on 2019 wholesale acquisition cost.
Results: For the treatment target of HbA1c ≤6.5%, once-weekly semaglutide 1 mg and oral semaglutide 14 mg were associated with the lowest costs of control, at USD 15,430 and USD 17,383 per patient achieving target, respectively. Similarly, the cost of control was lowest with once-weekly semaglutide 1 mg at USD 12,627 per patient achieving target, followed by oral semaglutide 14 mg at USD 13,493 per patient achieving target for the target of HbA1c <7.0%. All other interventions were associated with higher cost of control values for both targets.
Conclusions: Oral semaglutide 14 mg is likely to be cost-effective versus dulaglutide, exenatide (once weekly and twice daily), liraglutide, and lixisenatide in terms of bringing people with type 2 diabetes to glycemic control targets of HbA1c ≤6.5% and HbA1c <7.0% in the US.
Introduction
Clinicians have a range of treatment options available to treat people with type 2 diabetes not achieving glycemic control on metformin monotherapy, including modern interventions such as glucagon-like peptide-1 (GLP-1) receptor agonists, sodium-glucose cotransporter-2 (SGLT-2) inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, and older interventions such as thiazolidinediones and sulfonylureasCitation1. GLP-1 receptor agonists are recommended when there is a need to minimize the risk of hypoglycemia, and when there is a need to minimize weight gain or promote weight lossCitation1. Furthermore, GLP-1 receptor agonists with a proven positive impact on the risk of cardiovascular disease are preferred for treatment of patients at high risk of cardiovascular diseaseCitation1.
Within the GLP-1 receptor agonists class, options include twice daily (BID), once daily (QD), and once weekly (QW) interventions. Exenatide BID was the first GLP-1 receptor agonist to receive Food and Drug Administration (FDA) approval in 2005. Since then, liraglutide (approved in 2010) and lixisenatide (approved in 2016) have become available as once-daily treatment options, and a long-acting formulation of exenatide (approved in 2012), dulaglutide (approved in 2014), and semaglutide (approved in 2017) have become available as once-weekly treatment options (albiglutide once weekly was also approved in 2014, but has since been withdrawn by the manufacturer). In September 2019, oral semaglutide became the first orally administered GLP-1 receptor agonist to be approved by the FDACitation2. Oral semaglutide uses an absorption enhancer, sodium N-(8-[2-hydroxybenzoyl] amino) caprylate, to facilitate absorption across the gastric mucosa, and aims to provide the benefits of existing GLP-1 receptor agonists without the requirement for daily or weekly injectionCitation3. Patients are required to take oral semaglutide at least 30 min before the first food, beverage, or other oral medications of the day with no more than 4 ounces of water only. Waiting less than the full 30 min reduces the effect of oral semaglutide. The dosing conditions of oral semaglutide have been described in more detail in previous publications and the prescribing informationCitation4–12.
The efficacy and safety of oral semaglutide was assessed in the PIONEER clinical trial program, which was comprised of a series of global phase 3 studies, comparing oral semaglutide (3, 7, or 14 mg) with placebo, empagliflozin 25 mg, sitagliptin 100 mg, and liraglutide 1.8 mg, with a further study to establish cardiovascular safetyCitation4–11,Citation13. An additional two phase 3 studies comparing oral semaglutide with liraglutide 0.9 mg and dulaglutide 0.75 mg were conducted in JapanCitation14,Citation15. To allow comparison of the efficacy of oral semaglutide with other GLP-1 receptor agonists not included in the PIONEER trial program, a network meta-analysis (NMA) has been conductedCitation16. This found that oral semaglutide 14 mg was associated with significantly greater reductions in glycated hemoglobin (HbA1c) than dulaglutide 0.75 mg QW, exenatide 2 mg QW, exenatide 10 µg BID, liraglutide 1.2 mg QD, and lixisenatide 20 µg QD. Oral semaglutide 14 mg was associated with numerically greater (but not statistically significant) reductions in HbA1c than dulaglutide 1.5 mg QW, liraglutide 1.8 mg QD, and semaglutide 0.5 mg QW. Only semaglutide 1 mg QW was associated with a numerically greater reduction in HbA1c than oral semaglutide 14 mg, with this difference found not to be statistically significant. When change from baseline in bodyweight was analyzed, oral semaglutide was associated with significantly greater reductions than all included GLP-1 receptor agonists, with the exception of semaglutide 0.5 mg QW and semaglutide 1 mg QW, where no significant difference was observed.
Choosing treatment regimens that are both effective and cost-effective is becoming increasingly important, as the prevalence of type 2 diabetes continues to grow, particularly in the United States (US)Citation17. Improving glycemic control in people with type 2 diabetes has been shown to reduce the incidence of micro- and macrovascular diabetes-related complications, and therefore the American Diabetes Association (ADA) and the American Association of Clinical Endocrinologists (AACE) have proposed HbA1c targets that should be aimed for as part of routine care. There is increasing acknowledgement that the glycemic control target should be individualized depending on the risk of adverse effects of treatment (such as hypoglycemia), disease duration, life expectancy, comorbidities, available support, and patient preferences including consideration of tolerability and dosing conditions, but the ADA recommend an HbA1c target of <7.0% for the majority of people with type 2 diabetes, with a more stringent target of HbA1c <6.5% if this can be achieved without significant hypoglycemia or other adverse effects of treatmentCitation1,Citation18. The AACE suggest an HbA1c target of ≤6.5% for most adults with type 2 diabetes, if this can be achieved safelyCitation19. Data from the National Health and Nutrition Examination Survey (NHANES) in the US show that only 50.9% of people with diabetes had HbA1c levels lower than 7.0% in the 2011–2014 cohort (N = 1,325)Citation20. Failure to intensify treatment despite not achieving glycemic control targets may be due to therapeutic inertia, increasing the time patients spend in sub-optimal glycemic control, and therefore increasing the risk of diabetes-related complicationsCitation21. Therefore, there is a significant opportunity to improve care for people with type 2 diabetes, and thereby reduce the clinical and economic burden of the disease. Achieving improvements in glycemic control in a cost-effective manner is crucial to the future management of type 2 diabetes.
The aim of the present analysis was to compare the short-term cost-effectiveness of oral semaglutide 14 mg versus injectable GLP-1 receptor agonists, in terms of the cost per patient achieving HbA1c targets from a healthcare payer perspective in the US.
Methods
Evaluation of the cost of control
Cost of control evaluates the short-term cost-effectiveness of interventions in a simple and transparent manner, allowing the value for money of each intervention to be assessed. In the present analysis, cost of control was calculated by dividing the annual treatment costs associated with an intervention by the proportion of patients achieving the treatment target with an intervention. The methodology used was consistent with a previously published cost of control analysis of oral semaglutide, as well as a number of other cost of control analyses published in the peer-reviewed literatureCitation22–26. The cost of control calculation was carried out in a cost of control model, programmed in Microsoft Excel (Microsoft Corporation, Redmond, WA, US). Outcomes were not projected beyond a 1-year time horizon, and therefore discounting of cost and clinical outcomes was not required. Cost of control was evaluated for two treatment targets: HbA1c ≤6.5% and HbA1c <7.0%. A total of seven GLP-1 receptor agonists were included in the evaluation: oral semaglutide 14 mg QD, dulaglutide 1.5 mg QW, exenatide 2 mg QW, exenatide 10 µg BID, liraglutide 1.8 mg QD, lixisenatide 20 µg QD, and semaglutide 1 mg QW. Cost of control analyses require data on the clinical efficacy of interventions in terms of the proportion of patients who achieve treatment targets, and data on the costs of interventions, the sources of which are outlined below.
Proportions of patients achieving targets
Data on the proportions of patients achieving the two treatment targets of HbA1c ≤6.5% and HbA1c <7.0% were taken from an NMA ()Citation16. The NMA was based on a systematic literature review, which identified 108 publications reporting on 71 trials in people with type 2 diabetes. A total of 25 studies were taken forward into the network used to assess the proportion of patients achieving a target of HbA1c ≤6.5%, and 26 studies were used in the network to evaluate the proportion of patients achieving a target of HbA1c <7.0%. Both outcomes were assessed based on 26-week trial data (±4 weeks). The NMA used WinBUGS software (MRC Biostatistics Unit, Cambridge, UK) and employed a Bayesian framework with the use of uninformative prior distributions. Three Markov Monte Carlo chains were used, starting from different initial values of selected unknown parameters with a burn-in of 50,000 iterations. Convergence for all models was assessed by analyzing history and density plots, and Brooks–Gelman–Rubin diagnostic plots. In addition, autocorrelation plots were assessed to detect the presence of autocorrelation in the chains. Following this, model convergence inferences were made from data obtained by sampling for a further 10,000 iterations on the three chains. Dichotomous outcomes were evaluated using a binomial likelihood, logit link model, with a random-effects model usedCitation16.
Table 1. Estimated proportion of patients achieving treatment targets.
Costs of interventions
The costs of treatment with oral semaglutide 14 mg, dulaglutide 1.5 mg, exenatide 2 mg, exenatide 10 µg, liraglutide 1.8 mg, lixisenatide 20 µg, and semaglutide 1 mg were accounted over a 1-year time horizon, based on wholesale acquisition cost (WAC) ()Citation27. Needles are not required for administration of oral semaglutide, and are included in the packs of the once-weekly GLP-1 receptor agonists (dulaglutide 1.5 mg, exenatide 2 mg, and semaglutide 1 mg). However, there is a requirement to purchase needles separately for injection of once daily (liraglutide 1.8 mg and lixisenatide 20 µg) and twice daily (exenatide 10 µg) GLP-1 receptor agonists. Costs relating to self-monitoring of blood glucose were not included in the analysis, as GLP-1 receptor agonists are associated with a low risk of hypoglycemia and resource use would be unlikely to differ between interventions.
Table 2. Wholesale acquisition cost applied in the analysis.
Probabilistic sensitivity analysis
To evaluate the uncertainty around the cost of control outcomes, a probabilistic sensitivity analysis (PSA) was performed. The proportion of patients achieving the target endpoints of HbA1c ≤6.5% and HbA1c <7.0% with each intervention was sampled based on the 95% credible intervals identified in the NMA (). The cost of control with each intervention was calculated based on the sampled values, with this process repeated 1,000 times (as results were stable at this number of model iterations). The mean and standard deviation cost of control across the 1,000 sampled values was then calculated for each intervention.
Results
Annual cost of treatment
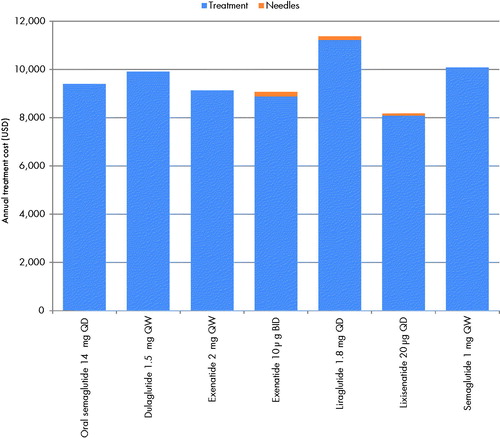
Annual pharmacy costs ranged from United States dollars (USD) 8,090 with lixisenatide 20 µg, to USD 11,223 with liraglutide 1.8 g, a difference of USD 3,133 between the most and least costly interventions (). Exenatide 10 µg, liraglutide 1.8 mg, and lixisenatide 20 µg were also associated with needle costs, as these are not included with these interventions. However, these costs were relatively low, ranging from USD 90 to USD 180 per year depending on the frequency of injection. In total, annual costs of treatment (the sum of pharmacy and any needles costs) associated with each treatment ranged from USD 8,180 with lixisenatide 20 µg, to USD 11,367 with liraglutide 1.8 mg, with oral semaglutide associated with an annual cost of USD 9,404 ().
Number needed to treat
Once-weekly semaglutide 1 mg (subcutaneous) was associated with the lowest number needed to treat to bring one patient to a target of HbA1c ≤6.5%, with 1.53 patients requiring treatment to bring one patient to target (). Oral semaglutide 14 mg was the second most effective intervention, with 1.85 patients requiring treatment to bring one patient to target. Lixisenatide 20 µg was the least effective treatment, with 4.26 patients requiring treatment to bring one patient to target.
Figure 2. Number needed to treat to bring one patient to target. Abbreviations. BID, twice daily; HbA1c, glycated hemoglobin; QD, once daily; QW, once weekly.
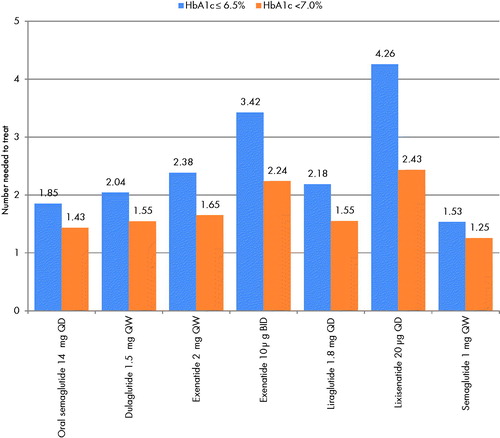
For the target of HbA1c <7.0%, a similar pattern was observed (), with once-weekly semaglutide 1 mg found to be the most effective treatment (1.25 patients requiring treatment to bring one patient to target), followed by oral semaglutide 14 mg (1.43 patients requiring treatment to bring one patient to target). Lixisenatide 20 µg remained the least effective treatment (2.43 patients requiring treatment to bring one patient to target).
Cost of control
For the treatment target of HbA1c ≤6.5%, once-weekly semaglutide 1 mg was associated with the lowest cost of control, at USD 15,430 per patient achieving target, followed by oral semaglutide 14 mg at USD 17,383 per patient achieving target (). The cost of control was the highest with lixisenatide 20 µg, with a cost of USD 34,809 per patient achieving a target of HbA1c ≤6.5%. Cost of control values for dulaglutide 1.5 mg, exenatide 2 mg, liraglutide 1.8 mg, and exenatide 10 µg ranged from USD 20,217 to USD 21,039 per patient achieving target.
Figure 3. Cost of control. Abbreviations. BID, twice daily; HbA1c, glycated hemoglobin; QD, once daily; QW, once weekly; USD, 2019 United States dollars.
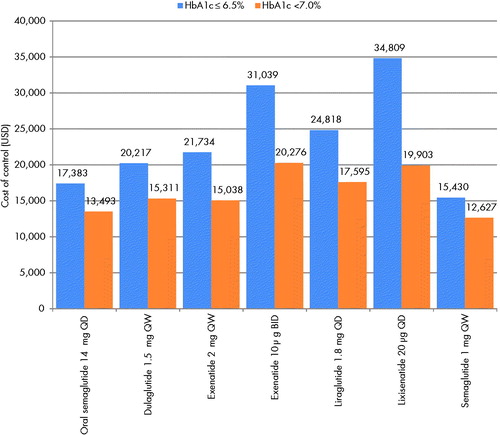
Cost of control values for the treatment target of HbA1c <7.0% were lower for all interventions than when the treatment target of HbA1c ≤6.5% was considered, driven by the greater proportion of patients achieving the less strict glycemic control target. The cost of control was lowest with once-weekly semaglutide 1 mg at USD 12,627 per patient achieving target, and oral semaglutide 14 mg was associated with the second lowest cost of control, at USD 13,493 per patient achieving target (). The intervention with the highest cost of control was found to be exenatide 10 µg, with a cost of USD 20,276 per patient achieving target.
Probabilistic sensitivity analysis
Results produced using a probabilistic approach were consistent with the deterministic approach used in the base case analysis. In the PSA, for the analysis based on a treatment target of HbA1c ≤6.5%, once-weekly semaglutide 1 mg was associated with the lowest cost of control, with a mean (standard deviation) value of USD 15,651 (USD 1,726) per patient achieving target, followed by oral semaglutide 14 mg at USD 17,791 (USD 2,820) per patient achieving target (). All other interventions were associated with a higher cost of control, with lixisenatide 20 µg associated with the highest cost of control at USD 36,552 (USD 8,293) per patient achieving target.
Table 3. Results of the probabilistic sensitivity analysis.
Once-weekly semaglutide 1 mg and oral semaglutide 14 mg were also associated with the lowest cost of control values for the endpoint of HbA1c <7.0%, at USD 12,653 (USD 707) and USD 13,587 (USD 1,333) per patient achieving target, respectively. As in the base case analysis, exenatide 10 µg was found to have the highest cost of control, at USD 20,695 (USD 3,590) per patient achieving target.
Discussion
The present study compared the short-term cost-effectiveness of a number of GLP-1 receptor agonists based on a published NMA, and found that once-weekly semaglutide 1 mg and oral semaglutide 14 mg were associated with the lowest cost per patient achieving treatment targets of HbA1c ≤6.5% and HbA1c <7.0%, and therefore these interventions are likely to be the most cost-effective GLP-1 receptor agonists in the US in terms of bringing patients to glycemic control targets. A key strength of this cost of control study is that these glycemic control targets are recommended in guidelines for the treatment of type 2 diabetes released by the ADA and the AACE, and therefore the analysis is highly applicable to clinical practice in the USCitation1,Citation18,Citation19.
A further strength of the study is the transparency of the cost-effectiveness analysis, which can be easily replicated by other research groups based on the information provided in and Citation2. Furthermore, the analysis can be easily updated should new clinical data become available or if costs change. Conventional, long-term modeling of the cost-effectiveness of interventions for type 2 diabetes involves projection of short-term trial data over patient lifetimes based on published risk equations, and there is always uncertainty around how well risk equations represent the cohort of interestCitation28. The present analysis avoids this uncertainty by evaluating cost-effectiveness over a 1-year period in natural units (i.e. cost per patient achieving target). However, a key strength of long-term modeling is that projections of quality-adjusted life expectancy (measured in quality-adjusted life years [QALYs]) and costs are made. Interventions can be compared, with calculation of an incremental cost-effectiveness ratio (ICER) expressed in terms of the cost per QALY gained with the new intervention compared with the existing intervention. ICERs can then be compared against willingness to pay thresholds to evaluate whether an intervention represents good value for money, and can be compared across therapeutic areas with the aim of maximizing healthcare gains across a population within a constrained healthcare budget. The use of natural units in the present analysis limits the comparability of the findings, as no defined willingness to pay threshold per patient achieving glycemic control target have been published and, as such, the present analysis is intended to provide additional, complementary information to conventional, long-term analyses, rather than replace them.
Cost-effectiveness analyses are only as robust as the clinical data used to inform them, and therefore the strengths and weaknesses of the previously published NMA must be consideredCitation16. The NMA was conducted using robust methodology, in line with guidance released by the National Institute for Health and Care Excellence, the International Society of Pharmacoeconomics and Outcomes Research, and the Cochrane CollaborationCitation29–33. The networks for each endpoint included a large number of trials, across which the risk of bias was considered to be low. However, pooling of data across multiple studies is always associated with some limitations, including heterogeneity in patient populations enrolled in the randomized controlled trials, heterogeneity in study design across the randomized controlled trials, and that publication bias may result in some studies of GLP-1 receptor agonists not being published (and, therefore, these unpublished studies cannot be captured in the systematic literature review). Furthermore, the authors of the NMA did not identify potential effect modifiers for the endpoints used in the present analysis (HbA1c <7% and HbA1c ≤6.5%), and this represents a potential weakness of the analysis. A patient-level meta-analysis would provide a robust and useful data source for future cost of control analyses evaluating oral semaglutide, with similar analyses conducted to assess the impact of liraglutide and insulin glargine on glycemic control and safety outcomesCitation34–36. However, the patient-level data required for such an analysis are not currently publicly available, and therefore this is not possible at the present time. While the NMA used in the present analysis has a number of limitations, it is the best available data source to inform the present analysis, expanding the number of comparisons that can be made beyond head-to-head trials.
The present analysis focused on glycemic control targets, but treatment guidelines recommend that treatment of type 2 diabetes should take a patient-centered approach, with the impact on other risk factors for diabetes-related complications taken into account when selecting treatments; particularly the risk of hypoglycemia and the impact of medications on bodyweightCitation1,Citation18,Citation19,Citation37. While all GLP-1 receptor agonists are associated with a low risk of hypoglycemic events, efficacy in terms of weight loss varies. Cost of control analyses with endpoints based on weight loss or composite endpoints capturing both a glycemic control target and a weight loss target would be of further interest, but these were not possible in the present analysis, as these endpoints could not be evaluated in the published NMACitation16. The most common side effects with GLP-1 receptor agonists, including oral semaglutide, are gastrointestinal adverse events. The NMA found no statistically significant differences in the odds of experiencing a gastrointestinal adverse event across the GLP-1 receptor agonists. Achieving composite endpoints of glycemic control targets without experiencing a gastrointestinal adverse event could not be calculated in the NMA due to limitations in the published data, but these would be of interest in a future analysis, if such data become available.
A previously published cost of control analysis based on the PIONEER trial program included composite endpoints of ≥1.0%-point HbA1c reduction and weight loss ≥3.0%, and HbA1c <7.0% without hypoglycemia and without weight gain, as well as the glycemic control targets of HbA1c ≤6.5% and HbA1c <7.0% used in the present analysisCitation22. This study compared oral semaglutide 14 mg with empagliflozin 25 mg, sitagliptin 100 mg, and liraglutide 1.8 mg, and found that oral semaglutide 14 mg was associated with a lower cost of control for all endpoints versus all comparators. Therefore the previously published analysis and the present analysis concur that oral semaglutide 14 mg is associated with a lower cost of control for treatment targets of HbA1c ≤6.5% and HbA1c <7.0% than liraglutide 1.8 mg. Expansion of the present analysis to include further endpoints when additional clinical data comparing oral semaglutide with other GLP-1 receptor agonists are available would provide further information for clinicians and healthcare payers.
Previous studies have shown that there is a preference for avoidance of injections (both daily and weekly)Citation38,Citation39. Oral administration may remove a barrier to treatment intensification, with fear of injection often cited as a reason for therapeutic inertiaCitation40–44. For some patients, oral semaglutide may help overcome therapeutic inertia and therefore be associated with further benefits not captured in the present analysis in real-world clinical practice. While oral semaglutide avoids the requirement for daily or weekly injections, the required dosing conditions also need to be considered. Furthermore, some patients may have a preference for a weekly injectable medication over a daily oral medication. The optimal medication for each patient needs to be determined using a patient-centric, holistic approach as recommended by the ADA, through discussion with a physician, where a number of factors including preferences around dosing schedule and mode of administration (including dosing conditions) are consideredCitation1.
The present economic evaluation used clinical trial data (through an NMA) and unit costs of medications as inputs, and an inherent limitation of this approach is that it does not take into consideration the real-world use of the drugs or the impact of adherence of any of the drugs in this study. As with all medications, it will be important to assess adherence differences between the comparators, as the differences in modes of administration and dosing conditions may introduce variability in adherence and persistence in the real-world. To date, there are no real-world studies assessing adherence rates with oral semaglutide and the injectable GLP-1 receptor agonists, or the impact that nonadherence has on outcomes, and, therefore, it could not be included in the study. It will be important to assess adherence to oral semaglutide outside of a clinical trial setting, and comparing adherence with once-daily oral semaglutide, once-daily injectable GLP-1 receptor agonists and once-weekly GLP-1 receptor agonists represents a key aspect for future studies in order to assess the impact on both effectiveness and cost-effectiveness. Furthermore, capturing adherence within economic evaluations is difficult, as both costs and clinical outcomes are affected, but the extent of this is often unclear. Further real-world studies are required to elucidate adherence patterns and the impact of these on cost and clinical outcomes with GLP-1 receptor agonists, before these can be incorporated into cost-effectiveness analyses.
The use of WAC price for the drug costs may also represent a limitation of the analysis, as these prices may not reflect the costs borne by the healthcare payer due to privately-negotiated discounts, rebates, and patient co-pay. However, these factors differ from medication to medication and from payer to payer, and are confidential. Therefore these could not be included in the analysis, and use of WAC represents the best-available approach.
Conclusions
Oral semaglutide 14 mg is likely to be cost-effective versus dulaglutide, exenatide (once weekly and twice daily), liraglutide, and lixisenatide in terms of bringing people with type 2 diabetes to glycemic control targets of HbA1c ≤6.5% and HbA1c <7.0% in the US.
Transparency
Declaration of funding
This study was supported by funding from Novo Nordisk A/S.
Declaration of financial/other interests
Barnaby Hunt, Samuel Malkin, and William Valentine are employees of Ossian Health Economics and Communications, which received consulting fees from Novo Nordisk A/S to support preparation of the analysis. Brian Bekker Hansen and Solomon Nuhoho are employees of Novo Nordisk A/S. Sarah Naz Ali and Tam Dang-Tan are employees of Novo Nordisk Inc. Brian Bekker Hansen and Tam Dang-Tan are shareholders in Novo Nordisk.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
The study was conceived by all authors, and conducted by Barnaby Hunt. The paper was drafted by Barnaby Hunt and reviewed by Brian Bekker Hansen, Solomon Nuhoho, Sarah Naz Ali, Tam Dang-Tan T, William Valentine, and Samuel Malkin. All authors agree to be accountable for all aspects of the work, and have given their approval for this version to be published.
Previous presentations
The analysis presented in this manuscript has not been presented previously.
Data availability statement
All data used in the analysis are included in the manuscript.
Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in Type 2 diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Dia Care. 2018;41(12):2669–2701.
- US Food and Drug Administration. FDA approves first oral GLP-1 treatment for type 2 diabetes. [cited 2019 Sep 20]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-glp-1-treatment-type-2-diabetes.
- Buckley ST, Baekdal TA, Vegge A, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(467):pii:eaar7047.
- Bakdal TA, Borregaard J, Donsmark M, et al. Evaluation of the effects of water volume with dosing and post-dose fasting period on pharmacokinetics of oral semaglutide. Diabetes. 2017;66(suppl 1):A315.
- Aroda VR, Rosenstock J, Terauchi Y, PIONEER 1 Investigators, et al. PIONEER 1: randomized clinical trial of the efficacy and safety of oral semaglutide monotherapy in comparison with placebo in patients with type 2 diabetes. Dia Care. 2019;42(9):1724–1732.
- Rodbard HW, Rosenstock J, Canani LH, et al. Montanya E, for the PIONEER 2 investigators. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Dia Care. 2019;42(12):2272–2281.
- Rosenstock J, for the PIONEER 3 Investigators, Allison D, Birkenfeld AL, Blicher TM, Deenadayalan S, Jacobsen JB, Serusclat P, Violante R, Watada H, Davies M. PIONEER 3 investigators. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321(15):1466–1480.
- Pratley R, Amod A, Hoff ST, et al. PIONEER 4 investigators. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39–50.
- Mosenzon O, Blicher TM, Rosenlund S, et al. PIONEER 5 investigators. Efficacy and safety of oral semaglutide in patients with type 2 diabetes and moderate renal impairment (PIONEER 5): a placebo-controlled, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):515–527.
- Husain M, Birkenfeld AL, Donsmark M, et al. Bain SC; PIONEER 6 investigators. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381(9):841–851.
- Pieber TR, Bode B, Mertens A, et al. PIONEER 7 investigators. Efficacy and safety of oral semaglutide with flexible dose adjustment versus sitagliptin in type 2 diabetes (PIONEER 7): a multicentre, open-label, randomised, phase 3a trial. Lancet Diabetes Endocrinol. 2019;7(7):528–539.
- US Food and Drug Administration RYBELSUS (semaglutide) tablets, for oral use: Prescribing information. [cited 2020 Jan 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213182s000,213051s001lbl.pdf.
- Zinman B, for the Pioneer 8 investigators, Araki E; PIONEER 8 Investigators, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff ST, Pedersen KB, Tarp-Johansen MJ. 985-P: oral semaglutide as add-on to insulin in T2D: PIONEER 8. Diabetes. 2019;68(Supplement 1):985-P.
- Yamada Y, Katagiri H, Hamamoto Y, et al. Efficacy and safety of oral semaglutide monotherapy vs placebo or liraglutide in Japanese T2D patients: PIONEER 9 trial. J Diabetes Investig. 2019;10(S1):30.
- Yabe D, Nakamura J, Kaneto H, et al. Safety and efficacy of oral semaglutide vs dulaglutide in Japanese T2D patients: the PIONEER 10 trial. J Diabetes Investig. 2019;10(S1):30.
- Nuhoho S, Gupta J, Hansen BB, et al. Orally administered semaglutide versus GLP-1 RAs in patients with type 2 diabetes previously receiving 1-2 oral antidiabetics: systematic review and network meta-analysis. Diabetes Ther. 2019;10(6):2183–2199.
- International Diabetes Federation. IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017.
- American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S61–S70.
- Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology – clinical practice guidelines for developing a diabetes mellitus comprehensive care plan – 2015. Endocr Pract. 2015;21 (Suppl 1):1–87.
- Carls G, Huynh J, Tuttle E, et al. Achievement of glycated hemoglobin goals in the US remains unchanged through 2014. Diabetes Ther. 2017;8(4):863–873.
- Okemah J, Peng J, Quiñones M. Addressing clinical inertia in type 2 diabetes mellitus: a review. Adv Ther. 2018;35(11):1735–1745.
- Hunt B, Hansen BB, Ericsson Å, et al. Evaluation of the cost per patient achieving treatment targets with oral semaglutide: a short-term cost-effectiveness analysis in the United States. Adv Ther. 2019;36(12):3483–3493.
- Wilkinson L, Hunt B, Johansen P, et al. Cost of achieving HbA1c treatment targets and weight loss responses with once-weekly semaglutide versus dulaglutide in the United States. Diabetes Ther. 2018;9(3):951–961.
- Johansen P, Hunt B, Iyer NN, et al. A relative cost of control analysis of once-weekly semaglutide versus exenatide extended-release and dulaglutide for bringing patients to HbA1c and weight loss treatment targets in the USA. Adv Ther. 2019;36(5):1190–1199.
- Hunt B, McConnachie CC, Gamble C, et al. Evaluating the short-term cost-effectiveness of liraglutide versus lixisenatide in patients with type 2 diabetes in the United States. J Med Econ. 2017;20(11):1117–1120.
- Langer J, Hunt B, Valentine WJ. Evaluating the short-term cost-effectiveness of liraglutide versus sitagliptin in patients with type 2 diabetes failing metformin monotherapy in the United States. JMCP. 2013;19(3):237–246.
- IBM Micromedex. RED BOOK. [cited 2019 Sep 30]. Available from: https://www.micromedexsolutions.com/home/dispatch.
- American Diabetes Association Consensus Panel. Guidelines for computer modeling of diabetes and its complications. Diabetes Care. 2004;27(9):2262–2265.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials; 2011 [updated April 2014; cited 2019 Aug 14]. Available from: http://www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015April2014.pdf.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta- regression, bias and bias-adjustment; 2011. [cited 2019 Aug 14]. Available from: http://www.nicedsu.org.uk.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials; 2011 [updated April 2014; cited 2019 Aug 14]. Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/TSD4-Inconsistency.final_.15April2014.pdf.
- Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 2. Value Health. 2011;14(4):429–437.
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2; 2009. [updated September 2009; cited 2019 Aug 14]. Available from: http://www.cochrane-handbook.org.
- Zinman B, Schmidt WE, Moses A, et al. Achieving a clinically relevant composite outcome of an HbA1c of <7% without weight gain or hypoglycaemia in type 2 diabetes: a meta-analysis of the liraglutide clinical trial programme. Diabetes Obes Metab. 2012;14(1):77–82.
- Ritzel R, Roussel R, Giaccari A, et al. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab. 2018;20(3):541–548.
- Ritzel R, Roussel R, Bolli GB, et al. Patient-level meta-analysis of the EDITION 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17(9):859–867.
- American Diabetes Association. 8. Obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes – 2019. Diabetes Care. 2019;42(Suppl 1):S81–S89.
- Boye KS, Matza LS, Walter KN, et al. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–230.
- Ridderstråle M, Evans LM, Jensen HH, et al. Estimating the impact of changes in HbA1c, body weight and insulin injection regimen on health related quality-of-life: a time trade off study. Health Qual Life Outcomes. 2016;14(1):13.
- Pantalone KM, Misra-Hebert AD, Hobbs TM, et al. Clinical inertia in type 2 diabetes management: evidence from a large, real-world data set. Diabetes Care. 2018;41(7):e113–e114.
- Fu AZ, Sheehan JJ. Treatment intensification for patients with type 2 diabetes and poor glycaemic control. Diabetes Obes Metab. 2016;18(9):892–898.
- Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11(1):3–12.
- Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20(2):427–437.
- Khunti K, Nikolajsen A, Thorsted BL, et al. Clinical inertia with regard to intensifying therapy in people with type 2 diabetes treated with basal insulin. Diabetes Obes Metab. 2016;18(4):401–409.