Abstract
Background and aims: Patients with acute intermittent porphyria (AIP) may suffer from acute non-specific attacks that often result in hospitalizations or emergency room (ER) visits. Prior to the recent approval of givosiran (November 2019), hemin was the only FDA-approved therapy for AIP attacks in the US. Our aim was to estimate the annual healthcare utilization and expenditures for AIP patients treated with hemin using real-world data.
Methods: Patients with ≥1 hemin claim and confirmed AIP diagnosis – 1 inpatient claim or 2 outpatient claims ≥30 d apart for AIP (2015–2017) or acute porphyria (prior to 2015) – were identified in MarketScan administrative claims dataset between 2007 and 2017. Continuous enrolment for ≥6 months from confirmed diagnosis was required. A secondary analysis (“active disease population”) limited the sample to adult patients with ≥3 attacks or 10 months of prophylactic use of hemin within a 12-month pre-index period. AIP-related care was defined by hemin use during an attack (daily glucose and/or hemin use) or prophylaxis (non-attack hemin use). Outcomes were annualized and expenditures were inflated to 2017.
Results: Across 10 years, patients with a confirmed AIP diagnosis (N = 8,877) and ≥1 hemin claim (N = 164) were restricted by ≥6 months continuous follow-up (N = 139). AIP patients were mostly female (N = 112; 81%), had median age of 40 and 3 years average follow-up. Annualized average total expenditures for AIP-related care were $113,477. Annualized average all-cause (any diagnosis) hospitalizations were statistically significantly lower for patients treated with hemin prophylaxis vs. acute treatment (1.0 vs. 2.1; p < .001). In the secondary analysis (N = 27), annualized average total expenditures for AIP-related care were higher ($187,480).
Conclusions: For AIP patients treated with hemin, patients treated for acute attacks may use a greater number of resources compared to patients treated prophylactically.
Introduction
Acute intermittent porphyria (AIP) is a rare metabolic disorder characterized by deficiency of the enzyme hydroxymethylbilane synthase (HMBS; also known as porphobilinogen deaminase, PBGD)Citation1. As the most common acute porphyria, AIP has an expected prevalence of 1 in 1,700 populationCitation2–4. However, only 30–50% of individuals with the HMBS deficiency will experience clinical symptoms of AIP during their lifetimeCitation1. The prevalence of symptomatic disease is approximately 1–2/100,000 populationCitation5. Women with AIP are more likely to have symptomatic disease than menCitation5.
Symptomatic AIP patients suffer from intermittent, mild attacks, or acute, moderate to severe non-specific attacksCitation6. Acute AIP attacks often result in a high amount of healthcare resource utilization including emergency room (ER) visits and inpatient hospitalizationsCitation7,Citation8. However, due to vague and non-specific symptomatology, an AIP attack is often misdiagnosed, therefore, confusing the process of disease identification and delaying treatmentCitation9.
Symptoms of acute AIP attacks, which are characterized as neurovisceral crises, when severe can be life-threatening. The most commonly reported clinical features include severe neuropathic abdominal pain, constipation, nausea/vomiting, back pain, hypertension, muscle weakness, a rapid heartbeat (tachycardia), behavioural changes, and seizuresCitation10–13. Paresis is usually symmetrical and begins proximally in the upper extremities, but it may be focal and may involve cranial nerves. Weakness may progress to respiratory and bulbar paralysis and death, especially with delayed diagnosisCitation13. Sudden death, presumably from cardiac arrhythmia, may also occur during an acute attackCitation13. Although only 3–5% of AIP patients suffer from recurrent, severe attacksCitation1, the symptoms of attacks are similar between patients with and without recurrent diseaseCitation7.
Symptomatic AIP is associated with long-term complications including peripheral neuropathy, systemic arterial hypertension, chronic kidney disease, chronic liver damage, hepatocellular carcinoma, psychiatric conditions, palpitations, and chronic fatigueCitation11,Citation13. The prevalence of chronic medical conditions increases with severity of AIP defined by the number of annual attacksCitation7. Some patients experience chronic neuropathic pain, which may account for an increased risk for depression and suicideCitation13.
Although investigations have been led into gene therapyCitation14, liver transplant is currently the only curative treatment available for AIP but remains uncommon due to high rates of morbidity and mortalityCitation15,Citation16. In the case of moderate or severe AIP attacks, Panhematin (Recordati Rare Disease; Lebanon, NJ) has been FDA-approved for treatment of AIP in the US since 1983Citation17. Panhematin, a hemin infusion of 3–4 mg/kg/d for 4 d, is indicated for the amelioration of recurrent attacks of AIP temporally related to the menstrual cycle in susceptible women after initial carbohydrate therapy is known or suspected to be inadequateCitation17. More recently, Givlaari (givosiran; Alnylam Pharmaceuticals; Cambridge, MA) was approved by the FDA with an indication for the treatment of acute hepatic porphyria (AHP) and a recommended monthly dosing scheduleCitation18.
In practice, patients may also receive Panhematin prophylactically (i.e. weekly, bi-weekly, or monthly) as an off-label but common treatment to prevent recurrent AIP attacksCitation19. Moderate to severe attacks are often healthcare resource intensive and may result in an ER visit or hospitalization. Therefore, prophylactic use of hemin that leads to lower attack incidence may also lower healthcare costs and contribute to better patient quality of lifeCitation8. A case study of two patients showed that average direct hospital costs decreased by 25% after starting prophylaxis treatment with hemin expenses accounting for 63% of average direct costsCitation8.
Current estimates of the annual cost of AIP-related expenditures are limited. A study by Gouya et al. estimates AIP-related costs with a simple model based on utilization inputs that are not AIP- or US-specific and average cost estimates from US sourcesCitation20. A Dutch study of the medical and financial burden of AIP does not report annualized expendituresCitation7.
It is important to have an estimate of annual, real-world expenditures for AIP patients to inform decision-makers at every level. The aim of this study was to estimate the annual healthcare utilization and expenditures for AIP patients treated in the US with hemin using real-world data.
Patients and methods
Data
The IBM MarketScan Commercial and Medicare pharmacy and medical claims database 2007–2017 was analyzed in this retrospective, observational study. The MarketScan database captures total cost of care (inpatient, outpatient, drug, laboratory, etc.) for over 240 million de-identified patients in the US with Commercial and Medicare Supplemental insurance. The MarketScan database is commonly used for health economic studies of healthcare resource utilization and expenditures in insured populations including rare disease population such as patients with acute porphyriaCitation9,Citation21,Citation22.
Information contained in each record includes demographic and eligibility related data as well as detailed records for inpatient services (i.e. primary diagnosis, up to ten additional diagnoses, diagnosis-related grouping, principal procedure, procedure code and up to ten procedure modifiers, revenue code, admit date, discharge date, dates of first and last service, discharge status, and length of hospital stay), outpatient services (i.e. primary diagnosis, up to three additional diagnoses, principal procedure, procedure code, and up to four procedure modifiers, admit date, discharge date, provider identifier, provider, and provider type), and prescription claims (i.e. outpatient drug name, therapeutic class, national drug code (NDC), days of supply, and quantity of drug prescribed). Expenditure information is available on all records, inpatient, outpatient, and prescription, from the carrier (total and net payments) and beneficiary (coinsurance, copayment, and deductible) perspectives.
Study design
AIP was only added as a specific diagnosis code starting with the introduction of the 10th edition of the International Classification of Diseases (ICD-10) in October 2015. Prior to this, AIP patients would have been classified as having acute porphyria in the 9th edition of the ICD (ICD-9). Therefore, the study population, AIP patients treated with hemin, was defined both by inpatient and outpatient diagnosis codes (ICD-10 = E80.21 AIP or ICD-9 = 277.1 Acute porphyria) and by any medical or pharmaceutical claim for hemin (J-code = J1640 or NDC = 55292-0701-54, 55292-0701-55, 55292-0702-54, 55292-0702-55, 00074-2000-43, 67386-0701-54). After limiting the MarketScan database to patients with at least one diagnosis of AIP or acute porphyria and at least one claim for hemin, the sample was further limited to patients with a confirmed diagnosis of AIP or acute porphyria (≥1 inpatient claim or ≥2 outpatient claims ≥30 d apart) and with 6+ months of continuous coverage (“follow-up period”) from the date of confirmed diagnosis (“index date”). The primary analysis of AIP patients treated with hemin examines the all-cause (any diagnosis) and AIP-related healthcare resource utilization and expenditures during the follow-up period.
In a sub-group analysis, the primary analysis sample was divided into two groups based on each patient’s pattern of hemin use. The acute treatment sub-group was defined by a pattern of intermittent, daily hemin use associated with attacks. The prophylaxis sub-group was defined by monthly or other scheduled doses of hemin (i.e. weekly and bi-weekly) for at least 10 months in a 12-month period. Patients in the prophylaxis sub-group were also allowed to use hemin for the treatment of acute attacks. Attacks were defined by daily treatment with glucose and/or hemin in either inpatient or outpatient settings. The end of the attack was defined as the end of the inpatient stay or when daily infusion of glucose or hemin was stopped. All other observed doses of hemin were categorized as prophylaxis doses. Patients were classified into the sub-groups once based on the full patient history observed in the data.
As a secondary analysis (“active disease population”), the population of AIP patients treated with hemin was limited to patients with ≥3 attacks or at least 10 months of hemin prophylaxis within 12 months (“pre-index period”). In the secondary analysis, the index date was set when the patient met the pre-index period criteria. The active disease population was further limited to patients with continuous coverage during the pre-index period and 12+ months of follow-up, longer than in the primary analysis, and with age ≥18 years at the index date. Sub-group analysis of the active disease population by acute treatment and prophylaxis was not possible due to small sample size (sub-group size ≤10).
Study outcomes
Patient demographic characteristics were reported including mean and median patient age at index date, gender, geographic region, and insurance plan type. In addition to demographic characteristics, the Charlson comorbidity index (CCI) score, a method of categorizing comorbidities (i.e. diabetes, congestive heart failure, and renal disease) based on ICD-9 and ICD-10 diagnosis codes, was calculated for each patientCitation23,Citation24. A CCI score of zero indicates that no comorbidities are present. The CCI score was summarized as both a continuous variable and as a categorical variable.
Annualized healthcare resource utilization and expenditures were calculated per person per year for hospitalizations, ER visits, primary care (PCP) visits, specialist visits, home services, and prescription medications during the follow-up period. The length of attack in days and expenditures associated with attacks were calculated by days of continuous glucose or hemin administration or hospital length of stay (LOS). Outcomes are reported for all-cause (any diagnosis) and AIP-related health care resource utilization and expenditures. AIP-related claims were flagged by hemin administration during hospitalization or by outpatient visits on the same day as hemin administration. Expenditures were inflated to 2017 using the Medical Consumer Price Index (CPI)Citation25 to match the last year of data analyzed.
Incremental health care resource utilization and expenditures were calculated for attacks, medication use during attacks (including hemin, glucose, and opioids), prophylaxis, and hemin administration.
Statistical analysis
Categorical variables were presented as frequencies and percentages. Continuous variables were summarized using mean and standard deviation (SD). In the sub-group comparison of the primary analysis, differences in outcome variables between acute treatment and prophylaxis sub-groups were tested for statistical significance using a chi-square test for categorical and binary variables, a t-test (two-tailed) for means, and a Kruskal–Wallis test for medians. A chi-squared test is used for the incidence of hospitalization, ER visits, PCP visits, specialist visits, other outpatient services, and prescriptions. Significance is defined as p < .05. Reported results are unweighted. No multivariate analysis was conducted due to small sample size and similar patient characteristics between subgroups. Python 3.6 (Python Software Foundation, https://www.python.org/) was used to process the data and generate results including statistical analysis. This study uses de-identified data and is, therefore, exempt from Institutional Review Board review.
Results
Patient sample
Between 2015 (introduction of ICD-10) and 2017, 214 patients had a confirmed ICD-10 diagnosis of AIP (N = 214) (). Among patients with a confirmed ICD-10 diagnosis of AIP, 16.4% (N = 35) received ≥1 hemin claim. 97.1% (N = 34) of those patients had 6+ months of continuous enrolment after the index date.
Table 1. Study sample selection.
In order to expand the sample size and because the analysis population is specific to AIP patients treated with hemin, which is only indicated for patients with AIP, patients from 2007 to 2017 with an ICD-9 diagnosis of acute porphyria were also considered. A total of 8,663 patients had a confirmed ICD-9 diagnosis of acute porphyria. Among patients with a confirmed ICD-9 diagnosis of acute porphyria, 1.5% (N = 129) received ≥1 hemin claim, and 81.4% (N = 105) had 6+ months of continuous enrolment after the index date.
The total sample size for the primary analysis of AIP patients treated with hemin was 139. The primary analysis was divided into two sub-groups of patients with primary acute treatment (N = 54; 38.8%) or prophylaxis (N = 85; 61.2%) use of hemin.
The selection of the active disease population started with 164 patients with a confirmed diagnosis and hemin use. 31.1% of patients met the inclusion criteria for the pre-index period (N = 51). Twenty-eight patients also had continuous enrolment during the 12+ months of follow-up. Applying the age exclusion criteria reduced the sample for the secondary analysis of the active disease population to 27.
Patient characteristics
AIP patients treated with hemin were mostly female (N = 112; 80.6%), had a median age of 40 years old (mean = 39.0), and had on average 36.1 months follow-up (). Most patients (N = 103; 74.1%) had comorbid disease (CCI score ≥1). No demographic characteristics were statistically significantly different between the two sub-groups of interest (acute treatment and prophylaxis).
Table 2. Patient characteristics.
All-cause (any diagnosis) annualized healthcare resource utilization
In the sample of AIP patients treated with hemin, the annualized average hospitalizations were 1.4 with an average LOS of 5.5 d (). Annualized average outpatient visits included ER visits (3.2), PCP visits (10.3), specialist visits (18.1), home services (7.2), and other outpatient services (23.1). Annualized average number of prescriptions was 38.1.
Table 3. Annualized healthcare resource utilization (per person per year).
As expected, patients identified as part of the prophylaxis subgroup compared to the acute treatment subgroup had statistically significantly lower annualized mean all-cause (any diagnosis) healthcare resource utilization including hospitalizations (1.0 vs. 2.1; p < .001), ER visits (2.8 vs. 3.8; p < .001), PCP visits (10.3 vs. 10.3; p < .001), home services (6.9 vs. 7.6; p < .001), and other outpatient services (22.0 vs. 24.8; p < 0.001) ().
AIP-related annualized healthcare resource utilization
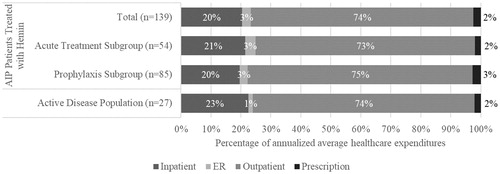
Healthcare resource utilization and expenditures associated with doses of hemin were summarized as AIP-related healthcare resource utilization and expenditures. AIP-related healthcare resource utilization was lower than all-cause (any diagnosis) (hospitalizations: 0.0; ER visits: 0.4; PCP visits: 1.5; specialist visits: 3.5; home services: 0.5; other outpatient services: 8.6) but with higher average LOS for hospitalizations (7.5) (). When limited to AIP-related utilization, patients identified as part of the prophylaxis subgroup compared to the acute treatment subgroup had statistically significantly lower annualized mean hospitalizations (0.0 vs. 0.1; p < .001) and other outpatient services (7.9 vs. 9.6; p < .001), similar ER visits (0.3 vs. 0.4; p = .942), and home services (0.5 vs. 0.5; p = .938), and statistically significantly higher PCP visits (1.6 vs. 1.3; p < .001) and specialist visits (4.1 vs. 2.6; p < .001).
The annualized number of attacks treated with hemin among AIP patients was 1.2 with a length of 3.3 d (). Glucose was used in only a fifth of those attacks on average (average days of glucose per attack = 0.2). AIP patients treated with hemin received on average 2.9 d of hemin per attack. The annualized average number of prophylaxis hemin doses was 9.
Table 4. Characteristics of hemin use.
Annualized healthcare expenditures
Annualized average total all-cause (any diagnosis) expenditures for AIP patients treated with hemin were $188,752 (). Annualized mean all-cause (any diagnosis) total expenditures were not statistically significantly lower in the prophylaxis subgroup compared to the acute treatment subgroup ($186,788 vs. $191,844; p = .907). AIP-related annualized average expenses were $113,477. Annualized mean AIP-related total expenditures were lower, but not statistically significant, in the prophylaxis subgroup compared to the acute treatment subgroup ($109,582 vs. $119,607; p = .781). The greatest expenditures were for outpatient expenses across all groups ().
Figure 1. Annualized average all-cause (any diagnosis) and AIP-related total healthcare expenditures (per person per year).
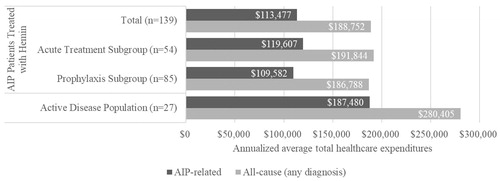
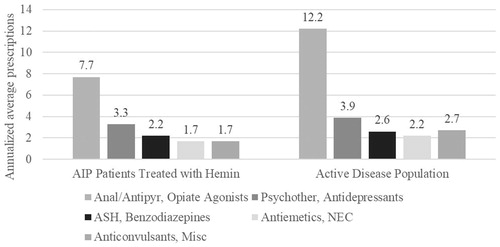
Figure 2. Annualized average all-cause (any diagnosis) healthcare expenditures by service type (per person per year).
Annualized average hemin expenditures for attacks and prophylaxis were $44,223 and $88,964, respectively (). The average payment for a dose of hemin was $7,745 in the outpatient setting and $6,225 in the inpatient setting (not shown).
Active disease population (secondary analysis)
Patient characteristics in the secondary analysis of AIP patients treated with hemin with active disease, the active disease population, were similar compared to the primary analysis sample (). AIP patients with active disease were mostly female (N = 24; 88.9%), had a median age of 40 years old (mean = 40.8), and had on average 38.4 months follow-up. 81.5% (N = 22) of active disease patients had comorbid disease.
Among the active disease population, the annualized average hospitalizations were 1.5 with an average LOS of 5.3 d (). Annualized average outpatient visits included ER visits (2.1), PCP visits (11.0), specialist visits (13.1), home services (4.5), and other outpatient services (27.3). Annualized average number of prescriptions was 47.4.
AIP-related healthcare resource utilization, visits or inpatient stays with hemin infusion, was lower compared to all-cause (any diagnosis) utilization (hospitalizations: 0.1; ER visits: 0.3; PCP visits: 2.3; specialist visits: 2.8; home services: 0.4; other outpatient services: 14.4) but with higher average LOS for hospitalizations (9.0) ().
The annualized number of attacks treated with hemin among AIP patients with active disease was 1.9 with a length of 3.2 d (). Glucose was used in less than one-fifth of those attacks on average (average days of glucose per attack = 0.1). On average, 2.8 d of hemin were used per attack. The annualized average number of prophylaxis hemin doses was 15. Annualized average hemin expenditures for attacks and prophylaxis were $66,374 and $127,297, respectively ().
Annualized average total all-cause (any diagnosis) expenditures for AIP patients treated with hemin with active disease were $280,405 (). AIP-related care annualized average expenses were $187,480 (). The greatest expenditures were for outpatient expenses (annualized average healthcare expenditures=$207,652) ().
Discussion
Healthcare expenditures among AIP patients treated with hemin lower than previous estimates
Because AIP is a rare disease and in order to construct the largest sample of AIP patients treated with hemin from the MarketScan data, the primary analysis did not apply any criteria for a pre-index period and applied minimal criteria for the follow-up period (pre-index: none; follow-up: 6+ months; N = 139). As expected, the percentage of the population based on the ICD-10 code for AIP treated with hemin (16.4%) was greater than the percentage of the population based on the ICD-9 code for acute porphyria treated with hemin (1.5%) likely due to the fact that hemin is a therapy for AIP, not more broadly for other acute porphyria. Based on these assumptions, the annualized average healthcare expenditures for AIP patients treating with hemin were $188,752 and $113,477 for all-cause (any diagnosis) and AIP-related healthcare utilization, respectively.
Prior to the annual spending estimates in this study, the authors were only aware of one study, presented at an academic conference by Gouya et al. that reported estimates of the annual AIP-related healthcare expenses for a large sample of AIP patientsCitation20. However, the ability to compare the expenditures estimated by Gouya et al. to this study, which are more relevant from a US payer perspective, is limited because the study by Gouya et al. is not US- or AIP-specific and relies on a simple model that does not accurately represent real-world AIP patient behaviorCitation20. The international survey-based utilization study applied unit costs to average patient-reported AHP-related healthcare resource utilization to estimate the annual cost of an AHP patient treated with hemin and with active disease ($398,463 based on hospital costs and $655,418 based on hospital charges per year)Citation20.
The intention of our more restricted active disease population was to compare directly to the inclusion/exclusion criteria used by Gouya et al.Citation20. The secondary analysis of the active disease population with more restrictive pre-index and follow-up criteria (pre-index: ≥3 attacks or at least 10 months of hemin prophylaxis within 12 months; follow-up: 12+ months; N = 27) provided much lower estimates (all-cause (any diagnosis): $280,405; AIP-related: $187,480) than by Gouya et al.Citation20
Givlaari was approved by the FDA in November 2019 for the monthly treatment of AHP in adults. The average annual cost of Givlaari prophylaxis will be $575,000 per year with an expected discounted average annual price of $442,000Citation26. Compared to the annual cost of an AIP patient treated with Panhematin, even the discounted Givlaari price, is more than twice the expected AIP-related costs of Panhematin prophylaxis in the active disease population calculated by this study (all-cause (any diagnosis): $280,405; AIP-related: $187,480). Additional research could be conducted to more directly compare the cost of treating a patient with Panhematin vs. Givlaari.
AIP patients treated with hemin use a wide range of healthcare resources
As expected, the analysis of MarketScan commercial and Medicare claims showed that AIP patients use a wide range of healthcare resources. AIP patients treated with hemin on average had less than two hospitalizations a year (annualized average hospitalizations: all-cause (any diagnosis) = 1.4, AIP-related: 0.0), visited the ER approximately three times (annualized average ER visits: all-cause (any diagnosis) = 3.2, AIP-related: 0.4), used a large number of outpatient services, and filled more than three prescriptions per month (annualized average prescriptions = 38.1). The active disease population on average had a similar number of hospitalizations per year (annualized average hospitalizations: all-cause (any diagnosis) = 1.5, AIP-related: 0.1) and ER visits (annualized average ER visits: all-cause (any diagnosis) = 2.1, AIP-related: 0.3) but used a larger number of outpatient services and filled almost four prescriptions per month (annualized average prescriptions = 47.4).
Hemin is almost exclusively billed as a medical service and is not filled as a prescription in the claims database. This is consistent with the clinical use of hemin during attacks or as prophylaxis. Counter to expectations only 1 in 5 attacks on average included at least a day of glucose use, which may indicate a desire by physicians to start hemin infusions more quickly.
Healthcare resource utilization lower for AIP patients treated with hemin prophylaxis
Over half of the sample of AIP patients treated with hemin (61%; 85/139) received prophylaxis (10+ months of hemin prophylaxis within 12 months) within the MarketScan claims data. Among the prophylaxis subgroup, all-cause (any diagnosis) healthcare resource utilization was statistically significantly lower than for patients treated with hemin for acute attacks (hospitalizations: 1.0 vs. 2.1, p < .001; ER visits: 2.8 vs. 3.8, p < .001; PCP visits: 10.3 vs. 10.3, p < .001; home services: 6.9 vs. 7.6, p < .001; other outpatient services: 22.0 vs. 24.8, p < .001). Although not statistically significant, both all-cause (any diagnosis) and AIP-related annualized average healthcare expenditures were lower for patients categorized with prophylaxis vs. acute treatment use of hemin (all-cause (any diagnosis): $186,788 vs. $191,844, p = .907; AIP-related: $109,582 vs. $119,607, p = .781). These positive economic results are consistent with the recent results reported for two AIP patients treated with hemin prophylaxisCitation8 and warrants further study into the effectiveness and cost-effectiveness of hemin prophylaxis.
Prescriptions for pain relief common among AIP patients treated with hemin
Consistent with the literature which reports long-term pain and neurological symptoms that increase with disease severity among AIP patientsCitation7 as well as the common use of opioids among US AIP patients (50%)Citation11, the most common prescription based on annualized average prescriptions and expenditures in the analysis of MarketScan data was opiate agonists (AIP patients treated with hemin: 7.7) (). In the secondary analysis of AIP patients with active disease, patients were on average filling approximately one prescription for opioids per month (annualized average prescriptions = 12.2). Patient advocates have argued for the continued access to pain treatment options, but more research may be warranted to potentially identify a reduction in opioid pain relief associated with arresting porphyria attacks in the group of AIP patients with recurrent, severe attacks.
Limitations
As with any administrative data analysis, there are limitations to the data collected and reported by the third-party data provider. In the case of this analysis, biochemical test results are not available in the data to confirm a patient’s AIP status. Additionally, biochemical test results may be misinterpreted to positively identify AIP when it does not exist. The use of ICD codes assigned by the patient’s physician in combination with treatment with hemin as a proxy for correctly interpreted biochemical test results is not expected to substantially change the results of the analysis.
Without a direct indicator of disease severity in this analysis, we rely on attacks per year and months of prophylaxis use to identify patients with AIP-related healthcare resource utilization as a proxy for symptomatic disease. Acute treatment and prophylaxis use of hemin were identified based on the stated methods of examining hemin administration patterns in the data.
We expected to find fewer hospitalizations among AIP patients on hemin prophylaxisCitation8. However, in the current analysis, no AIP-related hospitalizations were observed in the data among patients treated with hemin prophylaxis. For this reason, we have reported both all-cause (any diagnosis) and AIP-related utilization and expenditures, which are both lower than estimates from the literature.
Although the AIP-specific ICD-10 code was not available until October 2015, the more general ICD-9 code was also used to select patients along with AIP-specific treatment Panhematin. The context of the sample selection based on ICD-9 codes can be put into context with prevalence data. All forms of porphyria affect approximately 200,000 patients in the US, and AIP is expected to affect one in 20,000 or 16,285 patients in the US or 8% of the total porphyria populationCitation5. The number of individuals with active disease would be expected to be 4,885 in the US based on an approximate US population of 325.7 million. Therefore, the expected proportion of patients with active disease would be approximately 30% (4,885/16,285). This is similar to our subset of patients with active disease prior to applying enrolment restrictions (31%; 51/164). Therefore, we are confident that our selection method identified eligible AIP patients treated with hemin in the full sample (2007–2017).
The identification of AIP patients not treated with hemin in the population of patients prior to the introduction of ICD-10 codes requires an algorithm to identify those patients. We did not conduct that analysis as part of this study because the primary goal of this study was to estimate the annual expenditures of AIP patients treated with hemin and not the comparison to AIP patients not treated with hemin.
In general, analysis of the IBM MarketScan database is not generalizable to the full US population. The results of this study are based on a sample of the full population of AIP patients treated with hemin and insured by commercial and Medicare supplemental health insurance plans and can only be generalized to that population.
Conclusions
AIP patients treated with hemin use a wide range of healthcare resources, and patients treated for acute attacks may use a greater number of resources compared to patients treated prophylactically (annualized all-cause (any diagnosis) average hospitalizations: 2.1 vs. 1.0; p < .001). Annualized average total healthcare expenditures for AIP patients treated with hemin were estimated from claims data to be $188,752 and $113,477 for all-cause (any diagnosis) and for AIP-related care, respectively. Among AIP patients treated with hemin with active disease, the annualized average total healthcare expenditures for all-cause (any diagnosis) and AIP-related care were higher as expected ($280,405 and $187,480, respectively). Real-world annualized total expenditures for all-cause (any diagnosis) and AIP-related care are much less than previous estimates of AIP treatment costs.
Transparency
Declaration of funding
This study was funded by Recordati Rare Diseases.
Declaration of financial/other interests
PS is a paid employee at Recordati Rare Diseases. BB and JE are employees at Stratevi, which was retained for this work.
A peer reviewer on this manuscript has disclosed that they have received honoraria for participation in disease training videos and advisory boards for Recordati. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
BB: study design, data analysis, drafting of the manuscript. JE: study design, data interpretation, and revision of the manuscript. PS: study design, data interpretation, review of the manuscript, and study supervision.
Previous presentations
A first look at results were shared in a poster presentation at the International Congress on Porphyrins and Porphyrias (Milan, Italy; September 8–11, 2019).
Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet. 2015;8:201–214.
- Sardh E, Harper P, Balwani M, et al. Phase 1 trial of an RNA interference therapy for acute intermittent porphyria. N Engl J Med. 2019;380(6):549–558.
- Chen B, Solis-Villa C, Hakenberg J, et al. Acute intermittent porphyria: predicted pathogenicity of HMBS variants indicates extremely low penetrance of the autosomal dominant disease. Hum Mutat. 2016;37(11):1215–1222.
- Nordmann Y, Puy H, Da Silva V, et al. Acute intermittent porphyria: prevalence of mutations in the porphobilinogen deaminase gene in blood donors in France. J Intern Med. 1997;242(3):213–217.
- Ramanujam V-M, Anderson KE. Porphyria diagnostics-part 1: a brief overview of the porphyrias. Curr Protocol Hum Genet. 2015;86(1):17.20.1–17.20.26.
- Simon A, Pompilus F, Querbes W, et al. Patient perspective on acute intermittent porphyria with frequent attacks: a disease with intermittent and chronic manifestations. Patient. 2018;11(5):527–537.
- Neeleman RA, Wagenmakers MA, Koole Lesuis RH, et al. Medical and financial burden of acute intermittent porphyria. J Inherit Metab Dis. 2018;41(5):809–817.
- Yarra P, Faust D, Bennett M, et al. Benefits of prophylactic heme therapy in severe acute intermittent porphyria. Mol Genet Metab Rep. 2019;19:100450.
- Rudnick SR, Pedro H, Merkel M, et al. The patient odyssey to confirmed acute hepatic porphyria diagnosis: clinical characteristics and healthcare utilization of patients preceding diagnosis of acute hepatic porphyria. Program no. P1459. ACG 2018 Annual Scientific Meeting Abstracts; 2018; Philadelphia, PA.
- Kollipara VN, Su J, Smalligan RD, et al. Abdominal pain and quadriparesis: acute intermittent porphyria at its worst. Abstracts from the 36th annual meeting of the society of general internal medicine; 2013; Denver, CO.
- Bonkovsky HL, Maddukuri VC, Yazici C, et al. Acute porphyrias in the USA: features of 108 subjects from porphyrias consortium. Am J Med. 2014;127(12):1233–1241.
- Pischik E, Kauppinen R. Neurological manifestations of acute intermittent porphyria. Cell Mol Biol (Noisy-le-Grand)). 2009;55(1):72–83.
- Anderson KE, Bloomer JR, Bonkovsky HL, et al. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142(6):439–450.
- D’Avola D, Lopez-Franco E, Sangro B, et al. Phase i open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J Hepatol. 2016;65(4):776–783.
- Soonawalla ZF, Orug T, Badminton MN, et al. Liver transplantation as a cure for acute intermittent porphyria. Lancet. 2004;363(9410):705–706.
- Dowman JK, Gunson BK, Mirza DF, et al. Liver transplantation for acute intermittent porphyria is complicated by a high rate of hepatic artery thrombosis. Liver Transpl. 2012;18(2):195–200.
- Panhematin (R) (hemin for injection) [prescribing information]. Lebanon, NJ: Recordati Rare Diseases, Inc; 2017.
- Givlaari (R) (givosiran) [prescribing information]. Cambridge, MA: Alnylam Pharmaceuticals, Inc; 2019.
- Marsden JT, Guppy S, Stein P, et al. Audit of the use of regular haem arginate infusions in patients with acute porphyria to prevent recurrent symptoms. JIMD Rep. 2015;22:57–65.
- Gouya L, Bloomer JR, Balwani M, et al. An analysis of healthcare utilization and costs associated with patients with acute hepatic porphyrias (ahps) with recurrent attacks in explore: a prospective, multinational natural history study of patients with ahp. Value Health. 2018;21:S125.
- Nguyen MH, Burak Ozbay A, Liou I, et al. Healthcare resource utilization and costs by disease severity in an insured national sample of us patients with chronic hepatitis b. J Hepatol. 2019;70(1):24–32.
- Agarwal S, McManus A, Querbes W, et al. Developing an algorithm to identify patients with acute intermittent porphyria in an administrative claims database. Value Health. 2018;21:S213.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in icd-9-cm and icd-10 administrative data. Med Care. 2005;43(11):1130–1139.
- U.S. Bureau of Labor Statistics. Consumer price index for all urban consumers: medical care [cpimedsl]. FRED, Federal Reserve Bank of St Louis; 2019. Available from: https://fredstlouisfedorg/series/CPIMEDSL.
- Sheridan K. FDA approves Alnylam’s Givlaari, second-ever drug based on RNAi. Boston (MA): STAT; 2019.