Abstract
Introduction: Breast cancer is the most prevalent cancer among women in Egypt. Trastuzumab is administered with chemotherapy for patients with HER2-positive advanced breast cancer (HER2 + ve ABC) in the metastatic and adjuvant settings resulting in improved treatment outcomes, and long-term follow-up. Some studies have evaluated whether equivalent outcomes can be achieved with reduced treatment duration. This study evaluates the cost-effectiveness of 6-month versus 1-year trastuzumab treatments from payer perspective over a 10 year time horizon.
Methods: A half-cycle corrected Markov model was developed with five mutually exclusive health states; patient with HER2 +ve ABC, disease-free survival (DFS), local or regional relapse, metastatic relapse, and death. A cycle length of 6 months was applied, direct medical costs including cost of treatments, day-care, surgery, health states and follow-up visits were collected, and indirect costs such as lost productivity were not estimated. The transition probabilities and utilities were extracted from published literature, and deterministic sensitivity analyses were conducted.
Results: Among the HER2 +ve ABC patient population in Egypt, the total QALYs of the 6-month trastuzumab were estimated to be 2.99 compared with 2.93 for the 1-year trastuzumab which resulted in a difference of 0.06 QALYs. The total costs were EGP 271,647 ($106,947) and EGP 381,248 ($150,097), respectively. These costs yielded an ICER of –109,600 EGP/QALY (–43,149 $/QALY) for the 6-month trastuzumab. The 6-month trastuzumab is a dominant strategy when compared to 1-year trastuzumab, resulting in improved effectiveness at a reduced cost. All analyses results confirmed the dominance of 6-month trastuzumab and our model robustness.
Conclusions: This study concluded that 6-month trastuzumab is a cost-effective option when compared to 1-year trastuzumab in patients with HER2 +ve ABC in Egypt. Our findings provide health care decision makers with additional insights to best allocate available resources concurrently with the improvement of the Egyptian patient’s outcomes.
Introduction
Breast cancer is the most prevalent cancer among women in most developed and developing countries. In Egypt, it is the most common cancer among females and constitutes 29% of National Cancer Institute casesCitation1. Breast cancer has three molecular subtypes: luminal estrogen receptor (ER) positive, human epidermal growth factor receptor 2 (HER2) enriched and basal-likeCitation2. HER2 forms 20–25% of all breast cancer cases that tend to grow faster than luminal breast cancers and can have a worse prognosis if not treatedCitation3,Citation4. A retrospective study conducted at the Oncology Center – Mansoura University (September 2006 to August 2015) among all breast cancer patients reported that 8.19% were 21–35 years of age, and among these 4.7% had metastatic disease and 75.7% showed positive lymph nodes. Among this age group, 37.8% were HER2 positive and presented with a highly aggressive diseaseCitation5.
Trastuzumab is a monoclonal antibody that is used in the treatment of breast cancer. It is administered with chemotherapy for breast cancer patients who overexpress HER2 in the metastatic and adjuvant settings resulting in improved treatment outcomes confirmed by long-term follow-up studiesCitation6–10. Randomized controlled trials have also shown a significant survival improvement of trastuzumab and conventional chemotherapy, for 1-year, with a reduction in the rate of relapse for early breast cancerCitation8,Citation11. Treatment with trastuzumab for 1 year is therefore the recommended duration of treatment.
Studies have evaluated whether equivalent outcomes can be achieved with reduced treatment durationCitation12–14. The studies PHARE, PERSEPHONE, and HORG compared 6-month with 1-year trastuzumab treatmentsCitation12–14. In PHARE and HORG, the results were inconclusive for the non-inferiority hypothesis but PERSEPHONE trial showed that 6-month trastuzumab was noninferior to 12 month treatment in patients with in HER2 +ve early BC. Short-HER and SOLD compared treatment for 1 year with treatment for 9 weeks, and E2198 compared treatment for 1 year with 12-week treatmentsCitation15–17. Five of these six de-escalation trials were supported by governmental payers and aimed to balance efficacy, safety, and total costs for the health services utilized.
In Egypt, the limited healthcare budget and the higher acquisition costs of trastuzumab requires that economic considerations complement the assessment of clinical benefits to ensure equitable and sustainable access to costly treatments. Given the uncertainty in the cost-effectiveness of 1-year trastuzumab (Herceptin©) in Egypt, this study set-out to evaluate the cost-effectiveness of 6-month versus 1-year trastuzumab treatment in HER2 +ve ABC patients from the perspective of army hospital over a 10 year time horizon.
Methods
Decision model
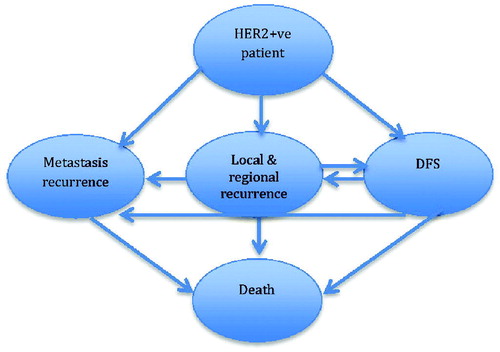
We simulated a hypothetical cohort of patients with HER2-positive advanced breast cancer (HER2 + ve ABC), aged 18 years or older, same age of PERSEPHONE study target population. To evaluate the cost-effectiveness of 6-month versus 1-year trastuzumab treatments, a half-cycle corrected Markov cohort model was built in MS ExcelCitation18. The adjuvant chemotherapy regimen used in our model was anthracycline (AC) based regimens plus taxanes (AC for four cycles plus either four cycles docetaxel or 12 weeks of paclitaxel). The model was developed with five mutually exclusive health states; patient with HER2 + ve ABC, disease-free survival (DFS), local or regional relapse, metastatic relapse, and death (). In this model, HER2 + ve ABC newly diagnosed patients (target population) received adjuvant therapy and remained in the DFS state until Death (a transition based on mortality rates) or they experienced either a loco-regional or metastatic relapse. Patients who survive with local or regional relapse can move to the DFS state or the Metastatic Relapse state. Patients remain in metastatic relapse state until death, that is mortality from breast cancer or from other reasons. The model structure represents the natural history of breast cancer, current clinical practice in Egypt, and published literature in this areaCitation12–14. This approach to decision modeling is preferred when analyzing clinical problems resulting in risks that change continuously over timeCitation18.
The model structure was validated by clinical experts in Egypt to reflect current management approaches for HER2 + ve ABC relapse in the health care settings. The model simulates patients aged 18 years or older with a histological diagnosis of invasive early breast cancer with overexpression of HER2 receptor, defined according to the American Society of Clinical Oncology and College of American Pathologists guidelinesCitation19. The cycle length of this model is 6 months to represent the natural progression of breast cancer, and a 10 year time horizon to capture the long-term consequences of the decision in a plausible survival period in HER2 breast cancer based on real practice situation in Egypt. The perspective is that of army hospital concerned with maximizing health gains for patients while making the most efficient use of finite resources. All costs and effects were discounted at 3.5% annually, as recommended by Egyptian guidelines for reporting economic evaluationsCitation20.
A comprehensive search of PubMed and Medline was conducted between January 1990 and July of 2019 for English-language articles on the treatment of patients with HER2 positive breast cancer, health states probabilities, cardiovascular risks, quality of life in the health states and cost effectiveness of trastuzumab using the following terms “trastuzumab”, “6 months “1 year”, “cost-effectiveness”, “HER 2 positive”, “quality of life”, “early breast cancer”, “randomized”, “controlled trial”, and “meta-analysis”. Randomized controlled trials were prioritized because they provide the robust and less biased evidence regarding patient outcomes. Published trials exclusively focused on patient populations other than HER2 +ve ABC were excluded.
Clinical parameters
All clinical input variables (including costs and utilities – discussed below), their ranges, and sources are noted in . The transition probabilities of DFS, local or regional relapse, metastatic relapse, and death were derived from the PERSEPHONE study; an open-label, randomized phase 3 non-inferiority trial, included a large sample size of total 4,088 patients with HER2-positive early breast cancer recruited from 152 centers in the UKCitation24. We used the median follow up of PERSEPHONE study to derive the probabilities of cardio toxicity per cycle. Transition probabilities of local or regional relapse and metastatic relapse from DFS, and metastatic relapse and DFS from local or regional relapse were derived from long-term follow-up results on DFS and overall survival (OS) of trastuzumab plus adjuvant chemotherapy for operable HER 2-positive breast cancer, a joint analysis of randomized data from NCCTG N9831 and NSABP B-31 studiesCitation22. The mortality rates included in the model were obtained from PERSEPHONE study and a risk-adjustment for metastatic breast cancer for patients was applied. The increased risk of metastasis after local or regional relapse from year 1 to 5 and year 5 to 10 were extracted from pooled data of four randomized phase III clinical trials included 7,751 early stage breast cancer patients conducted by the European Organisation for Research and Treatment of Cancer (EORTC) Breast Cancer Group and the EORTC Radiotherapy GroupCitation21.
Table 1. Model input variables.
The incidence of side effects associated with the adjuvant therapy was also considered, particularly the elevated risk of cardiotoxicityCitation24. In the model, trastuzumab patients experience cardiotoxicity only within the first year of treatment so they stop treatment for one month per each cardiotoxicity. Therefore, the risk of cardiotoxicity was applied in “patient with HER2 +ve ABC” health state. Cardiac death related to trastuzumab was studied in PERSEPHONE study but did not show any evidence of it so it was not included in our studyCitation24. To simplify the model, the population was assumed to be free of any comorbidity.
Outcomes
The outcome of the two strategies was compared with quality-adjusted life years (QALYs). Utilities in the DFS, HER 2 +ve early stage and local or regional relapse health states were retrieved from a study that evaluated health-related quality of life (HRQoL) in different breast cancer disease states using preference-based measures (EQ5D and Time-Trade-Off)Citation25. The utility of symptomatic cardiotoxicity was obtained from an evaluation using the EQ-5D and Minnesota Living with Heart Failure QuestionnaireCitation27. The utility of metastatic relapse was elicited from the largest preference study in breast cancer designed to survey a representative general public sample using Standard Gamble MethodCitation26. These inputs were varied over a wide range in a sensitivity analyses because the validity and reliability of reported QALYs are uncertain.
Costs
The study perspective is that of army hospital (the health care payer perspective). Direct medical care costs including cost of treatments, day-care, surgery, health states and follow-up visits were obtained from Maadi Oncology and Hematology Military Hospital (Cairo Egypt), and supplemented with the local resource utilization validated by the expert panel. The total cost of each health state included the cost of all medications administered, hospitalization, surgery and the follow-up. There was a difference in the resource use between the two arms. A macro-costing analysis was conducted in 2019 Egyptian pounds () based on billable amounts that would patient consume, in the future (rounding applied). Indirect costs such as lost productivity, were not estimated. After consultation with experts at the army hospital, this secondary research method was the best reliable evidence from the perspective of the local health care payer.
Sensitivity analyses
Deterministic, one-way sensitivity analyses were conducted to examine the robustness of study results due to the inherent uncertainty in applying the clinical, cost and utility inputs (), consistent with recommendations by Consolidated Health Economic Evaluation Reporting Standards (CHEERS): ISPOR Task force reportCitation28. Clinical Inputs were varied along the 95% confidence intervals reported in published literature. The utility and cost data were varied along the standard above and below percentage. All sensitivity analyses were performed using Microsoft Excel 2010.
Results
The total costs of each strategy (6-month versus 1-year trastuzumab treatments) and the corresponding QALYs were estimated (). The incidences of recurrent metastasis over the 10 year-time horizon were 64% and 67% in 6-month and 1-year trastuzumab groups, respectively. The incidences of local or regional relapse were 4.6% in 6-month trastuzumab group and 4.5% in 1-year trastuzumab group. Among the HER2 +ve ABC patient population in Egypt, the total QALYs of the 6-month trastuzumab were estimated to be 2.99 compared with 2.93 for the 1-year trastuzumab which resulted in a difference of 0.06 QALYs. The total costs were EGP 271,647 and EGP 381,248, respectively. These costs yielded an ICER of –109,600 EGP/QALY. The 6-month trastuzumab option is a dominant strategy when compared to 1-year trastuzumab resulting in improved effectiveness at reduced cost.
Table 2. Decision analytic model results.
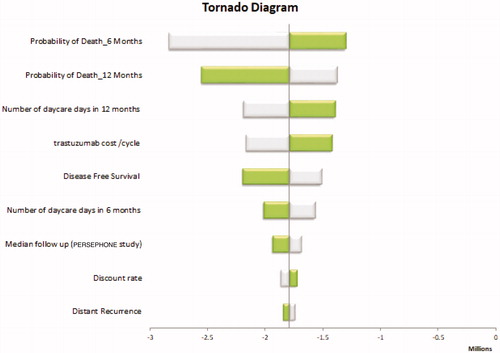
Among all inputs included in the deterministic, one-way sensitivity analyses, probability of metastatic relapse for 6-month had the greatest impact (). It was found that the probability of death for 6-month and 12-month had the greatest effects on ICER but without changing the conclusion. None of the inputs when varied across their defined ranges modify the result of the cost-effectiveness of the 6-month trastuzumab option. The model was also analyzed with 15 and 20 year time horizons but results did not alter the conclusion. Similarly, a scenario analysis for a subgroup of high-risk patients based on hormone receptor status was performed, that is women with hormone receptor negative tumors at increased risk of relapse and death. This subgroup analysis also did not change the study conclusion. All sensitivity analyses result confirmed the dominance of 6-month trastuzumab versus 1-year trastuzumab in patients with HER 2 positive early breast cancer and our model robustness.
Discussion
This study evaluated the cost-effectiveness of 6-month versus 1-year trastuzumab among HER2 +ve early breast cancer patients. The 6-month trastuzumab treatment strategy is more effective and less costly than 1-year trastuzumab and may be the preferred option among patients with HER2 positive early breast cancer (clinical impact is still in question). Although 6-month trastuzumab exhibited a small gain in QALYs compared with the 1-year trastuzumab, the costs associated with 6-month trastuzumab were much lower compared with 1-year trastuzumab. The main drivers of the dominance of 6-month trastuzumab in this model were the probability of death for 6-month and 12-month.
Studies in IranCitation29, ColumbiaCitation23, ChinaCitation30 and BelgiumCitation31 have also calculated the cost-effectiveness of adjuvant trastuzumab and reported similar results (that is, the cost-effectiveness of the 6-month trastuzumab treatment strategy), while other economic evaluations in the USACitation32,Citation33 AustraliaCitation34, and the UKCitation35 estimated higher ICERs (that is, favored the 1-year trastuzumab treatment strategy) and were therefore different compared to this study. This study is different in results to other studies due to the variable cost of trastuzumab and the country threshold for the willingness to pay. However, given the nature of economic evaluations, study inputs varied widely such as costs of trastuzumab and healthcare services.
The strength of our model is the use of effectiveness data from RCTs for decision modeling in Egypt. This is the first economic evaluation in Egypt focused on trastuzumab treatment among HER2 +ve early breast cancer patients. Our results are more dynamic because we used the expensive branded trastuzumab in our model but introducing biosimilars to the market means a decrease in the cost of acquisition of highly effective monoclonal antibodies and enhancement in the possibility of more cost effective alternatives. It is also the first to examine the impact of age heterogeneity of patients on the cost-effectiveness of two trastuzumab strategies. Another strength includes the testing of the model over a 20 year time horizon to determine the long-term consequences of each treatment strategy for decision making purposes. The study insights were strengthened by evaluating the internal validity and applying a standard economic evaluation checklistCitation36.
The study explicitly evaluated model input uncertainty by assigning confidence intervals and plausible ranges based on published sources to the clinical, cost, and utility inputs in the model. The costs in the base-case analysis were derived from a local hospital and therefore the results are limited to patients treated in these settings. The sensitivity analysis used a range of different values with no qualitatively different conclusion, suggesting that clinical, cost, and utility values could vary widely in practice with no need to modify the treatment recommendation. The results of our sensitivity analyses were similar to the results of sensitivity analyses conducted across defined ranges in the above cost-effectiveness studies.
This economic evaluation is limited by the adoption of a narrow health care payer perspective rather than a broader, societal perspective. Consequently, the study results are not generalizable to other public or private health care institutions in Egypt, particularly given the cost inputs collected from an army hospital and therefore applicable to this setting. Another limitation includes the use of quality of life values from international studies because no data are available for HER2 +ve early breast cancer patients in Egypt. Nevertheless, one-way sensitivity analyses for utility values used in the model showed no impact on the study conclusion.
Conclusions
A 6-month trastuzumab treatment strategy is a cost-effective and robust alternative to 1-year trastuzumab among patients with HER2 +ve early breast cancer over a 10 year time horizon in Egypt. However, further economic evaluations are needed with real-world effectiveness data derived from a patient population in Egypt and quality of life values specific to Egypt. Our findings provide health care decision makers with additional insights to best allocate available resources concurrently with the improvement of the Egyptian patient’s outcomes.
Transparency
Declaration of funding
There are no funders to report for this submission.
Declaration of financial/other relationships
All authors have nothing to disclose. A peer reviewer on this manuscript has disclosed that they are an academic competitor in the field of cost-effectiveness analysis of treatments for early breast cancer and that the lead author is a former student of theirs. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
None reported.
References
- Ibrahim AS, Khaled HM, Mikhail NN, et al. Cancer incidence in Egypt: results of the national population-based cancer registry program. J. Cancer Epidemiol. 2014;2014. Article ID 437971.
- Schnitt SJ. Classification and prognosis of invasive breast cancer: from morphology to molecular taxonomy. Mod Pathol. 2010;23:S60–S64.
- Maximov PY, Lee TM, Jordan VC. The discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol. 2013;8:135–155.
- Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368.
- Farouk O, Ebrahim MA, Senbel A, et al. Breast cancer characteristics in very young Egyptian women ≤35 years. Breast Cancer Targets Ther. 2016;8:53–58.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684.
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283.
- Cameron D, Piccart-Gebhart MJ, Gelber RD, et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. 2017;389:1195–1205.
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752.
- Gianni L, Dafni U, Gelber RD, et al. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 2011;12:236–244.
- Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. 2013;14:741–748.
- Earl HM, Cameron DA, Miles D, et al. PERSEPHONE: duration of trastuzumab with chemotherapy in women with HER2-positive early breast cancer – six versus twelve months. J Clin Oncol. 2014;32:TPS667.
- Mavroudis D, Saloustros E, Malamos N, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol. 2015;26:1333–1340.
- Conte P, Frassoldati A, Bisagni G, et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER study double dagger. Ann Oncol. 2018;29:2328–2333.
- Joensuu H, Fraser J, Wildiers H, et al. Effect of adjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol. 2018;4:1199–1206.
- Schneider BP, O'Neill A, Shen F, et al. Pilot trial of paclitaxel-trastuzumab adjuvant therapy for early stage breast cancer: a trial of the ECOG-ACRIN cancer research group (E2198). Br J Cancer. 2015;113:1651–1657.
- Weinstein MC, Brien BO, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health. 2003;6:9–17.
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013.
- Elsisi GH, Kaló Z, Eldessouki R, et al. Recommendations for reporting pharmacoeconomic evaluations in Egypt. Value Health RI. 2013;2:319–327.
- Tanis E, van de Velde CJH, Bartelink H, et al. Locoregional relapse after breast-conserving therapy remains an independent prognostic factor even after an event free interval of 10 years in early stage breast cancer. Eur J Cancer. 2012;48:1751–1756.
- Pérez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373.
- Buendía JA, Vallejos C, Pichón-Rivière A. An economic evaluation of trastuzumab as adjuvant treatment of early HER2-positive breast cancer patients in Colombia. Biomedica. 2013;33:411–417.
- Earl HM, Hiller L, Vallier AL, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet. 2019;393:2599–2612.
- Lidgren M, Wilking N, Jonsson B, et al. Health-related quality of life in different states of breast cancer. Qual Life Res. 2007;16:1073–1081.
- Lloyd A, Nafees B, Narewska J, et al. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95:683–690.
- Calvert MJ, Freemantle N, Cleland J. The impact of chronic heart failure on health-related quality of life data acquired in the baseline phase of the CARE-HF study. Eur J Heart Fail. 2005;7:243–251.
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: are part of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–250.
- Ansaripour A, Uyl-de Groot CA, Redekop WK. Adjuvant trastuzumab therapy for early HER2-positive breast cancer in Iran: a cost-effectiveness and scenario analysis for an optimal treatment strategy. Pharmacoeconomics. 2017;36:91–103.
- Chen W, Jiang Z, Shao Z, et al. An economic evaluation of adjuvant trastuzumab therapy in HER2-positive early breast cancer. Value Health. 2009;12:S82–S84.
- Van Vlaenderen I, Canon JL, Cocquyt V, et al. Trastuzumab treatment of early stage breast cancer is cost effective from the perspective of the Belgian health care authorities. Acta Clin Belg. 2009;64:100–112.
- Kurian AW, Thompson RN, Gaw AF, et al. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu – positive breast cancer. J Clin Oncol. 2007;25:634–641.
- Garrison LP, Lubeck D, Lalla D, et al. Cost-effectiveness analysis of trastuzumab in the adjuvant setting for treatment of HER2-positive breast cancer. Cancer. 2007;110:489–498.
- Millar JA, Millward MJ. Cost effectiveness of trastuzumab in the adjuvant treatment of early breast cancer: a lifetime model. Pharmacoeconomics. 2007;25:429–442.
- Hall P, Hulme C, McCabe C, et al. Updated cost-effectiveness analysis of trastuzumab for early breast cancer. Pharmacoeconomics. 2011;29:415–432.
- Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the economic evaluation of health care programmes. 4th ed. New York: Oxford University Press; 2015.