Abstract
Aims: Modern pharmaceutical product development is a long and complex process associated with significant investments by pharmaceutical companies. The innovative pharmaceutical industry accounts for the vast majority of expenditures in clinical trials of potential new pharmaceuticals and therefore generates economic activity within a country. The aim was to assess the far-reaching economic impact of industry-sponsored clinical-trials (ISCTs) of pharmaceutical products for the healthcare system and the national economy.
Materials and methods: The study approach was based on three analytical steps. First, a survey among 15 pharmaceutical companies in Austria was conducted to evaluate the annual number of ISCTs subdivided according to trial phase, therapeutic areas and associated employees. Second, the monetary value of treatments performed in ISCTs was calculated based on a sample of clinical-trial protocols. Finally, the macroeconomic impact, measured in terms of value-added and jobs created by the conducted ISCTs, was calculated using Input–Output analysis by applying an extended Leontief-model.
Results: The study demonstrated that €116.22 million spent in ISCTs generated a total value added of €144 million, €74 million direct, in 2018. Each year a medical treatment value of €100 million was financed through 463 ISCTs, with an average value of medical treatment of €37,068 per recruited patient. This represents a significant 0.3% of annual current health-expenditures. In summary, each Euro invested by the pharmaceutical industry in ISCTs generates €1.95 for the Austrian economy. ISCTs also created and secured employment in the extent of 2,021 full-time-equivalents, thus resulting in an employment multiplier of 1.66.
Conclusions: In conclusion, conducting clinical-trials by pharmaceutical industry—beside its importance in its own domain—results in tangible benefits and a positive macroeconomic impact that contribute to the sustainability of the Austrian healthcare system by complementing its limited resources. Furthermore, it is a non-negligible factor in locational and industrial policy.
Introduction
Developing a new pharmaceutical product is a long and complex process with a high risk both of failure and of missing the target to successfully develop an effective and safe medication. Analysis across all therapeutic areas indicates that the development of a new pharmaceutical product, from target identification through to marketing authorization, takes over 12 years, and often significantly longerCitation1. The cost of developing a New Molecular Entity (NME; a small molecule compound) or New Biological Entity (NBE; an antibody, protein, gene therapy, or other biological medicine) has been estimated to be about $2.6 billionCitation2. Both the regulations required and an increasing need for safety and efficacy data are responsible for the rising costs in drug discovery and developmentCitation3. Additionally, clinical trials are systematically associated with multifactorial investigational insights about side-effects and health outcomes. Therefore, safety information, even about marketed pharmaceutical products and therapies, is captured in industry-sponsored clinical trials (ISCTs). The free delivery of study medication and treatment services necessary are funded by the pharmaceutical industry within clinical trials, which provides an immediate and tangible benefit to the healthcare system. Literature about the quantification of investments in ISCTs is extremely limited across countries. Before now such information was also not available for AustriaCitation4–10. Yet, in 2016, 259 clinical trials of potential new pharmaceutical products were submitted for authorization to the respective Austrian Agency (AGES). Of these, 199 (76.8%) clinical trials of pharmaceutical products were sponsored by the pharmaceutical industry. The percentage ratio of submitted ISCTs compared to academic investigational clinical trials was constant over the years and amounted to 70:30% in favor of ISCTs, which is higher than compared to the EU (60: 40%)Citation11. Between 2015 and 2017, 102 newly authorized pharmaceuticals were launched in Austria (IQVIA data on file).
Yet, it is the innovative pharmaceutical industry which accounts for the great majority of expenditures in clinical trials. Since the data from AGES covers only newly submitted trials, an overview of new and ongoing ISCTs spanning over a lager time horizon is necessary. In this respect, the Association of the Austrian Pharmaceutical Industry (Pharmig) every year carries out a detailed survey among the member companies on ISCTs. This data collection shows the number of ongoing clinical trials (Phase I to Phase IV). Between 2013 and 2016 on average 463 active (new and ongoing) ISCTs per year were documentedCitation12–15. Based on these surveys ISCTs can be considered in a larger context. Conducting clinical trials involves a range of spillover effects on the quality of healthcare and quality-of-life of patients. Patients participating in clinical trials benefit from early access to innovative medicinal products and enhanced medical care via more frequent and intense clinical visit schedules than in routine medical care. Clinical trials accelerate the transfer of new procedures and products into routine clinical practice. They are also the basis from which one can distinguish effective and life enhancing treatments from ineffective medical treatments. The planning, execution, and evaluation of clinical trials according to international standards requires considerable resources, infrastructure, and the availability of qualified personnel. It is noteworthy that industry sponsorship means that sponsors provide the investigational pharmaceutical products free of charge, cover the costs of the study-specific diagnostics and other treatments, and compensate for the medical and administrative work. The healthcare system benefits from the free treatment of thousands of patients in clinical trials. Yet, ISCTs should also be assessed in a larger context. Thus, the conduct of clinical trials requires the services of external service providers (such as IT and other supply industries). These and others are linkages with the economy as a whole. It is therefore adequate to view ISCTs in such a larger framework to quantify the economic flows between ISCTs and the whole economy. Consequently, one has to determine which monetary transactions are induced by ISCTs. Furthermore, the employment figures related to the monetary transactions are to be estimated. In this way additional turnover, value added, and employment become evident. Calculating these figures substantiates the induced benefits to the Austrian economy and society.
Consequently this study embarks on: (1) collecting the available information on ISCTs in Austria based on a specific survey related to the Pharmig survey. This specific survey provided detailed information which was so far not available. (2) Analyzing this survey data permits us to quantify the monetary value of treatment delivered in ISCTs, which alleviates the burden of reimbursed services in the healthcare system. (3) The final and most important step is a quantification of the impacts due to ISCTs on the Austrian economy and society. The most relevant indicators are gross production, value added, and employment. In this way the foundations are set for exploiting the role of ISCTs like industry policy, location policy, and of course possible reductions in public healthcare finance. It must, however, be reiterated that since the focus of the analysis covers the economic aspects, the purpose of the study is not the investigation of the improvement of health and life expectancy due to marketing authorization of new and innovative pharmaceutical products.
Materials and methods
In order to determine the wide-ranging effects of ISCTs of pharmaceutical products in Austria, encompassing calculations are necessary. To facilitate the understanding, the following stepwise calculations have to be carried out:
A sound database of ISCTs is provided;
The annual value of the medical treatment services performed in ISCTs is calculated; and
In a macroeconomic framework the impact of ISCTs is calculated.
The database
To provide a detailed analysis of ISCTs, a specific survey of ISCTs has been compiled by Austrian affiliates of 15 international pharmaceutical companies, covering 82.5% of ISCTs in Austria. The proportions of clinical trials included in the specific survey were consistent with the therapeutic areas and phases of the statistical population of the Pharmig survey. This specific survey included a sample of 574 clinical trials (56% of the total statistical population) conducted between 2012 and 2017 (for more detail see Supplementary Appendix 1).
The specific survey provided the following information:
Name of pharmaceutical company,
Internal number, if needed,
Investigational pharmaceutical product,
Comparator substances—active substances (not trade name)/placebo,
Study design,
Double dummy used,
Therapeutic area (drop down survey),
Indication,
Phase,
International study status (completed/ongoing),
Local study status (AT) (completed/ongoing),
Start Actual Date in AT (first patient visit/screening visit)—dd.mm.yy,
End Actual Date in AT (Last Patient Out – Last patient last visit)—dd.mm.yy,
Number of patients allocated in AT,
Number of patients included in AT—for ongoing studies until the end of 2017, and
Approval date of Investigational Product authorized for the first time in AT or EU (not indication)—until the end of 2017.
Average values calculated for 1 year were estimated based on an observation period of 6 years. The rationale for this approach was that estimates should not be based solely on ongoing clinical trials. This pertains to the moving datasets of clinical trial phases, therapeutic areas, annual number of ISCTs, and patients treated annually. The last available years (2012–2017) were used as the observational period for clinical trial phases, therapeutic areas, and patients treated annually. The representative sample has been drawn from this observational period (2012–2017).
Calculations were carried out using Microsoft Excel 2010.
Study phase (I–IV) and therapeutic areas
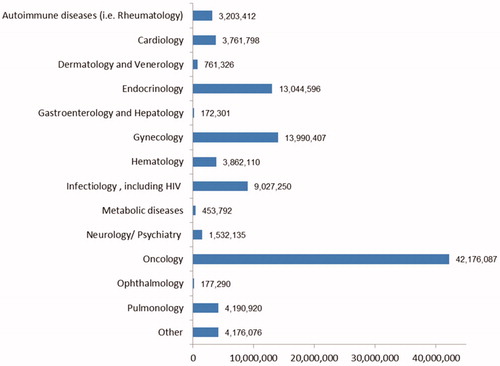
Of the 574 clinical trials, 155 were ongoing and 419 were completed. Oncology accounted for the largest number of clinical trials (222), followed by 54 in cardiology and cardiovascular and 52 in autoimmune diseases (see ). On average, 32 patients were enrolled at a clinical trial site per ISCT. As expected, the numbers vary significantly among therapeutic areas, ranging from an average of 113 patients in the field of vaccinology to an average of eight patients in hematology. There were no patient numbers available for allergology (missing value). This data gap will later be replaced by the average value over all clinical trials of 32 patients (see ).
Figure 1. Average number of patients treated by therapeutic area per industry-sponsored clinical trial. Abbreviation. NA, not available.
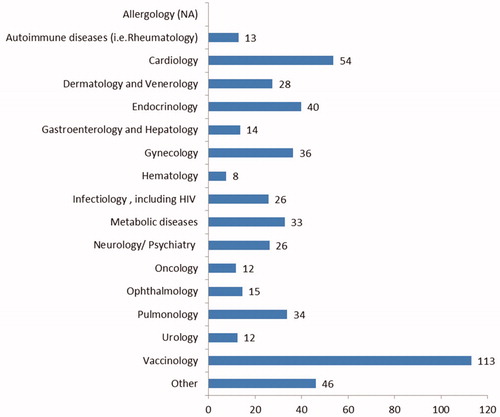
Table 1. Number of industry-sponsored clinical trials by therapeutic area and clinical trial phase (2012–2017).
The average duration of a clinical trial (time between first patient in and last patient out) is between 6.42 years in the field of vaccinology and 1.08 years in the field of allergology. gives an overview of the total number of ISCT by therapeutic area and clinical trial phase; 63% (355) of the clinical trials are in Phase III, 21% (121) in Phase II, and 8% (48) in Phase IV. The remaining 8% (44) are distributed over Phase I and combined Phase I/II and II/III (see ).
Phase I clinical trials were considered in the calculation of the macroeconomic impact, but not in the calculation of the annual value of the medical treatment services borne by the pharmaceutical industry due to the inclusion of healthy volunteers, as this would not impact the healthcare system.
Population of ISCTs per year
The statistical population was determined based on the average number of ISCTs conducted in Austria each year. At the time of calculation the data from the Pharmig survey (period 2013–2016) was used for this calculationCitation12–15. We assumed that the average number of active ISCTs in this period corresponds to the current situation. In the years before 2013, the average number of active ICSTs was even higher and therefore not suitable to be included in the average calculation. Based on the annual numbers of active ISCTs from 2013 to 2016, an average of 463 active ISCTs per year was calculated.
The duration of clinical trials is generally longer than 1 year. Therefore, to more accurately determine the average duration of a clinical trial by therapeutic area the representative ISCT list of the 574 clinical trials conducted between 2012 and 2017 as provided by Austrian affiliates of 15 international pharmaceutical companies was utilized. The time adjustment is based on the average duration of the clinical trials provided. Over the considered time horizon (2012–2017) a total of 1,029 clinical trials were conducted. The multiplication by the average number of enrolled patients per therapeutic area yielded 23,331 patients treated in industry-sponsored clinical trials. This means that the calculated average of 463 clinical trials a year run on average for 34.7 months (first patient in to last patient out). From an annual perspective, patients were treated on average for 5.8 months within a year.
The annual value of the medical treatment services
The determination of the medical treatment values provided in ISCTs was based on a drawn representative sample from the specific survey of 574 clinical trials. For the selected studies in the sample the study-reports were retrieved to collect the resource consumption of provided treatments.
Drawing a representative random sample of ISCTs
A sample of clinical trials was selected in the form of “cluster sampling”. The cluster sampling method assumes that a population can be divided into more or less “natural” clusters corresponding and resembling in this case to therapeutic areas and the clinical trial phase distribution of the whole list of clinical trials. To determine the sample size, a 95% confidence interval [95% CI] and a standard error of 10% was used. For more details see Supplementary Appendix 3.
The distribution of therapeutic areas and phases of the sample corresponded to the totality of clinical trials (see ).
Table 2. Random representative sample of industry-sponsored clinical trials by therapeutic area and clinical trial phase.
Assessment of medical costs
The financial assessment of treatment services rendered in ISCTs was based on the clinical trial protocols of the 90 representative ISCTs. Each service rendered was quantified and cost-assessed using reimbursement rates (prices, tariffs, and/or lump sums). The reimbursement amounts were derived from official Austrian price lists and represented costs for the year 2018; collected from the payer’s perspective. The assessment of costs was carried out in accordance with the guidelines of the IQWIG (General Methods Version 5.0, Chapter Costs) and the ISPOR Good Research Practices (Chapter Valuing Resource Use)Citation16,Citation17. The procedure for calculating the direct medical costs is descried in Supplementary Appendix 4 in detail.
The average direct treatment costs were determined per therapeutic area for the average clinical trial and the 95% confidence intervals were calculated. The average cost per therapeutic area of the sample was extrapolated to the ISCT population of the therapeutic area. Based on average costs per therapeutic area the total annual costs and the 95% confidence intervals were calculated.
Macroeconomic impact
The macroeconomic impact is measured in terms of value added and jobs created by the ISCTs using Input–Output analysis by applying an extended Leontief-model (see Supplementary Appendix 5).
The model is based on a national concept, e.g. the effects of imports and exports are not observed. It analyses economic effects generated on three different levels:
Level one; direct effects on value added and employment—expressed in FTEs—are created due to the ISCTs. Calculations are based on the gross production value which represents total cost of conducting clinical research at local trials sites by the pharmaceutical industry. Additionally, one can comment that gross production value summarizes the gross production value due to medical service rendered within ISCTs plus the gross production value generated in association with all activities that are necessary for the implementation (before and after) of ISCTs.
Level two; to perform their tasks, operating sites need intermediate goods, medicinal products, IT-services, and electricity, etc. Due to the various interconnections between the operating sites and its supplying industries, ISCTs also generate jobs and value added in these preliminary sectors. As these sectors are linked to other supplying industries, value added, and jobs are also created in preliminary companies. The sum of these economic effects is referred to as ‘indirect effects’.
Level three; additional effects to be analyzed are ‘induced effects’. Induced effects, for instance, occur through employees’ consumption and purchase of goods and services provided by ISCTs and its supplying industries, e.g. they spend their salaries to finance their living, buy clothes and food, etc. Consumption therefore generates value added and jobs in business sectors apart from clinical research, its suppliers and their suppliers. The second source of induced effects comes from investments due to ISCTs and preliminary industries. This approach eliminates and disregards the effects of changing end-demand-induced income and private consumption responses, such as expressed in Keynesian multiplier theory. The neglect of these multiplier-induced effects in the traditional Leontief model is perceived as a lack in empirical input–output analyses. With a model extension it is trying to remedy this situation. The combination of so-called matrix of consumption multipliers with the traditional Leontief inverses results in an extended inverse matrix which, in addition to the production effects in the sense of Leontief, also measures the income effects in the sense of KeynesCitation18. These are so-called secondary or induced effects (see ).
Figure 2. Overview of economic effects of industry-sponsored clinical trials on the overall economy.
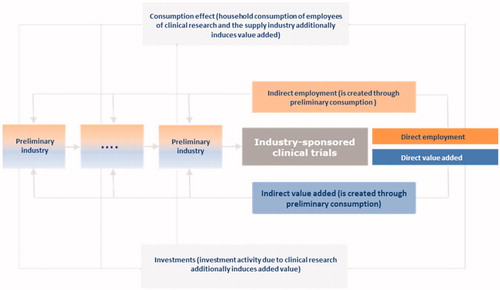
The results of the analysis can be summarized in the form of multipliers. A value-added multiplier describes the ratio of the direct, indirect, and induced value added to the direct value added of the considered industry. The same applies to the employment multiplier.
The above described method of calculation ensures an estimation of the significance of ISCTs as a part of the Austrian economy as a whole in the System of National Accounts (SNA). The calculated value added can be interpreted as part of the gross domestic product.
The calculations are based on the most recent input–output (I-O) tables from 2014. The I-O table covers sectors at NACE two-digit level. The relevant industries for the analysis are:
Healthcare provider NACE 86 or local trial sites, and
Research and development service provider NACE 72 or R&D personnel provided within the pharmaceutic industry and by external consultants.
Healthcare provider NACE 86
According to Statistics Austria (I-O table), 33.3% of the gross production value is spent on intermediate consumption and 66.7% on value addedCitation19.
Analog calculations were carried out for employment. Based on the average gross production value per capita of NACE 86 of €106,000, the average number of employees could be determined.
Research and development service provider NACE 72
For the execution of clinical examinations, highly qualified personnel are required directly at the study sites (see NACE 86 staff). In addition, services of R&D service providers (e.g. IT companies, other business-related services such as statistics, etc.) are necessary and these companies are again employing workers to carry out clinical trials. As a result, these service companies generate revenues and thus create value added for the Austrian economy and jobs for highly qualified personnel. The costs are funded by the pharmaceutical industry as sponsors of the clinical trials.
To calculate the human resources and costs for conducting clinical trials, data from five pharmaceutical companies was analyzed. A detailed description is available in Supplementary Appendix 6.
The R&D service providers require intermediate consumption to carry out the service. According to Statistics Austria (I-O table), 54.9% of the gross production value was spent on intermediate consumptionCitation18.
Results
Value of the entire medical treatments
Looking at the total expenditure for medical treatments in ISCTs (phase II–IV) during the 2012–2017 observational period, the figure amounted to €603.93 million. The expenditure for medical treatments comprises all services under the clinical trial protocol and not just the clinical trial investigational pharmaceutical products (see Supplementary Appendix 4). The value of medical treatments was borne by the pharmaceutical industry as sponsors of the clinical trials and thus relieved the healthcare system. During the 2012–2017 observational period, an average of 23,331 patients were treated in 1,029 clinical trials.
Broken down to 1 year, this means that treatments worth €100.53 million are funded annually through ISCTs. This value represents 0.3% of the current yearly health expenditure in Austria. The annual distribution of total value among therapeutic areas is shown in .
The average medical treatment value across all therapeutic areas during 1 observed year amounts to €37,068 per patient. In the therapeutic area, endocrinology costs per patient are the highest (€48,589), followed by oncology (€48,165). provides the estimated value funded per patient.
Table 3. Medical treatment costs per year and per patient by therapeutic area in Euros.
Macroeconomic effects of ISCTs
Direct effects
Total gross production value due to conducting ISCTs in Austria amounts to €116.22 million in 2018, which represents the total cost of conducting clinical research at local trial sites by the pharmaceutical industry. This total gross production is the sum of the share of the gross production product for medical services of €100.53 million (described above) and the gross production value generated, association with all activities that are necessary conducting ISCTs. The latter represents monthly costs (explained in Supplementary Table S2) multiplied per study duration. A gross production value of €15.69 million was calculated.
Based on this a direct value added of the industry-sponsored clinical trials in Austria in 2018 amounted to €74.13 million as the sum of the healthcare providers or NACE class 86 (€67.05 million) and the R&D service providers or NACE class 72 (€7.08 million).
In the year 2018, 950 full-time-equivalents (FTEs) of healthcare providers are directly related with ISCTs. In addition, there are 265 R&D FTEs associated with ISCTs. Overall, ISCTs investment directly secured 1,215 jobs measured in terms of FTEs in 2018 in Austria.
Indirect effects
The indirect value-added effects resulting from ISCTs due to preliminary consumption of intermediate goods amounted to €38.47 million in 2018. By definition, the results only take into account domestic demand for intermediate consumption. Indirect value added is the sum of the indirect value added of the healthcare providers (€30.60 million) and the indirect value added of the R&D service providers (€7.87 million).
Investment in ISCTs secured and created jobs in the supplier companies. The dimension of indirect FTEs due to ISCTs secured and created 475 jobs in 2018. The indirect employment effect was the sum of the indirect employment effect of the healthcare providers (378 FTEs) and the indirect employment effect of the R&D service providers (97 FTEs). Employees of the pharmaceutical industry appeared as a part of the indirect employment of healthcare providers. They provide services that are necessary for conducting the ISCTs in general, and medical therapy during the clinical trial in particular.
Induced effects
The induced value-added effect due to ISCTs, which was generated based on consumer spending of directly and indirectly employed persons as well as the demand for capital goods, results in a value added of €31.60 million for 2018 in Austria. The induced value added is the sum of the induced value-added effects of the healthcare providers (€26.37 million) and the induced value-added effect of the R&D service providers (€5.22 million).
The total number of employment relationships created and secured in this way in 2018 will be 331 FTEs. The induced employment effect is the sum of the induced employment effects of healthcare providers (275 FTEs) and the secondary employment effects of the R&D service providers (56 FTEs) (see ).
Table 4. Economic effects of value added and employment in 2018.
Multiplier effect
The value-added multiplier of ISCTs is 1.95. This means that €1 of value added generated in ISCTs generated €1.95 in the whole Austrian economy, thereby nearly doubling the value added in the overall economy.
When applied to the employment multipliers this similarly provides information on how many employment relationships in the overall economy are created and secured through a job associated with ISCTs. The employment multiplier of ISCTs is 1.66, meaning that one employment relationship, due to clinical trials, creates or secures almost another in the economy as a whole in Austria.
Discussion
For the first time, this analysis identifies and quantifies the significant expenditures pharmaceutical companies carry out every year in conducting clinical trial activities in Austria. Clinical trial activity also provides substantial benefits to state and local economies in terms of economic impact generated through activities like development of clinical trial protocols; selection of clinical trial sites; implementation of clinical trials, including the recruitment of staff, contractors, vendors, and patient volunteers; manufacture of small batches of pharmaceutical and other medical products for the clinical trials; care of patients, including laboratory tests and ongoing health monitoring; and analysis of the enormous amount of data generated—just to name some of the activities occurring at particular clinical trial sites which require significant expenditure by pharmaceutical companies and their vendors and contractors.
The positive effect of clinical research not only has an impact on the healthcare system but extends much further into the economic and social levels. The aim of this study was to quantitatively evaluate some of these positive effects. The great importance of carrying out clinical trials for the Austrian healthcare service, economy, and society was confirmed by the present study.
The present calculations have shown that medical treatments amounting to €100.53 million per year are financed through ISCTs. This value of care is borne by the pharmaceutical industry as sponsors of the clinical trials and not by the public health system, whereby the healthcare system is financially relieved of these costs. This value represents 0.3% of current health expenditure in Austria.
Yet beyond that, as outlined, this study has calculated macroeconomic figures derived from the narrower health system effects. Thus, total gross production value due to conducting ISCTs in Austria amounts to €116.22 million in the year 2018 only. In terms of the overall economic context, Austrian ISCTs generated a value added of €144.2 million in 2018. In addition, employment was created and secured over 2,021 FTEs in 2018. In addition to their direct contribution to value creation and the employment situation in Austria, ISCTs must be considered with regard their interaction with other industries. The results of the analysis can be summarized as multipliers. Indeed, €1 invested in ISCTs generates a value added of €1.95 in the Austrian economy. One job created and secured due to ISCTs created or secured almost another in the economy as a whole.
A few other countries have dealt with this economically relevant topic, but to a less detailed extent. An analysis for Hungary has calculated that clinical trial operation and management activities generated 900 jobs and US $166.9 million in revenue among clinical research organizations and pharmaceutical companiesCitation9. For the US it was calculated that the biopharmaceutical industry spent nearly $10 billion directly in 6,199 clinical trials at the site level across the US in 2013Citation10, but no additional economic effects were assessed.
Therefore, policies improving the economic and legal-regulatory framework to conduct more clinical trials would not only lead to the advancement of science and improvement of patients’ healthcare—they would also induce a positive impact on the national and regional economy and create employment for highly qualified professionals.
The improvement in health due to new authorized medicines cannot be isolated across the wide therapeutic areas because of the influence of other health determinants. As an example, cancer survival can be reported: It is based on a number of efforts towards finding new and efficient therapies. Relative 5-year survival has increased significantly in recent decades. While the relative 5-year survival of a cancer patient was 51% after a diagnosis in the period 1989–1993, it rose to 61% within the diagnosis period 2009–2013Citation20.
It is interesting to compare the results with other economic sectors. One outstanding sector in Austria is tourism. For this sector, the Austrian Institute for Economic Research (WIFO) calculated multipliers for the value-added results of tourism amounting to 1.51 and for the employment of 1.85Citation21.
The importance of calculating the overarching monetary effects of ISCT is not confined to assess the importance and merits of ISTCs. It is of pivotal importance of several fields of policy-making. Relying on the indirect economic effects is a strong argument to attract or to keep back pharmaceutical companies in the country. This can be extended on a national or regional scale. The importance of the above calculated effects is therefore a very helpful argument to foster ISTCs for the sake of improving industrial, locational, and regional policies. Moreover, since healthcare expenditures constitute a significant fraction in national budgets one cannot but underline the importance of ISTCs in public finance.
Due to entering new territories with this study, the findings have to be considered with the following limitations. The potential financial benefit related to improved health outcomes, which should also reflect the economic multiplier effects due to clinical trial activities, was not included in this analysis. We also did not capture the direct revenues or costs related to capacity building due to clinical research outside the two considered NACE classifications. The analysis included only ISCTs of phase I to IV, which are only a portion of the full economic impact of the R&D supported by the pharmaceutical industry (e.g. support of academic research, etc.). Therefore, in general, this study took a conservative approach to estimate the economic benefits of clinical trials.
Considering these limitations, it has also to be mentioned that using input–output analysis under accounting principles of SNA differs from the Frascati Manual (FM) and the statistical data provided under this framework. The FM is explicitly an input framework. It seeks to capture the cost, source, and nature of inputs (financial and personnel) dedicated to R&D activities only. By comparison, the SNA production accounts can be viewed in this context as an input–output framework, across a wide range of economic activities, and seeks to capture financial flows, which was the primary reason for using it within the present calculationCitation22.
The key strengths of this analysis encompass the robust approach of using representative data from the Austrian setting only, which presupposes a careful procedure to forward sound statistical data. The evaluation of the treatment value provided in ISCTs was calculated based on a micro costing approach, which represents the most detailed way of evaluation. The entire calculations are based on an observational period of 6 years to include the structural shift in our findings (e.g. shift in key research areas, etc.).
Could one carry over such a calculation procedure? To date there seems to exist no similar attempt in Europe. Yet, since assessing healthcare policies very well extends over national borders an international comparison could prove quite helpful. In particular, comparing a range of multipliers would be interesting.
In conclusion, our explorative study showed that clinical trials conducted by the pharmaceutical industry resulted in tangible benefits and a positive macroeconomic impact, which both contribute to the sustainability of the Austrian healthcare system and beyond by complementing its limited resources.
Transparency
Declaration of financial/other relationships
E. W. has received honorarium from Pharmig. G. E. and M. V. have no conflict of interest. C. B., A. C., C. H., B. M., C. O., J. P.-D., B. P., I. P. G. P. H. T., G. W., D. B., W. B., and S. T. K. are members of the Standing Committee Clinical Research.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed that they have received research grants from Boehringer-Ingelheim, Bayer, Onyx-Amgen, Pfizer, Merck, Sanofi, Astrazeneca, Celgene; advisory board fees from Pfizer, BMS, Genentech, EMD Serono, Novartis, Merck, Sanofi, Seattle Genetics/Astellas, Astrazeneca, Exelixis, Janssen, Amgen, Eisai, NCCN; travel costs from BMS, Astrazeneca; speaking fees from Physicians Education Resource (PER), Onclive, Research to Practice, Clinical Care Options; writing fees from Uptodate; and that they have been on the steering committee of trials sponsored by BMS, Astrazeneca, Bavarian Nordic, Debiopharm, QED. The reviewers have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (56.8 KB)Acknowledgements
We thank the participating companies [ABBVIE, ACTELION a Janssen pharmaceutical company of Johnson & Johnson, AMGEN, ASTRAZENECA, BAYER, BRISTOL MYERS SQUIBB (BMS), BOEHRINGER INGELHEIM, CELGENE, ELI LILLY, GILEAD, MERCK SHARP & DOME (MSD), NOVARTIS, PFIZER, ROCHE, SANOFI] for providing data, and individuals in those firms who kindly gave their time when we needed some of the responses clarified.
Additional information
Funding
References
- DiMasi JA, Feldman L, Seckler A, et al. Trends in risks associated with new drug development: success rates for investigational drugs. Clin Pharmacol Ther. 2010;87(3):272–277.
- DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33.
- Earm K, Earm YE. Integrative approach in the era of failing drug discovery and development. Integr Med Res. 2014;3:211–216.
- Bredin C, Eliasziw M, Syme R. Drug cost avoidance resulting from cancer clinical trials. Contemp Clin Trials. 2010;31:524–529.
- Shen LJ, Chou H, Huang CF, et al. Economic benefits of sponsored clinical trials on pharmaceutical expenditures at a medical center in Taiwan. Contemp Clin Trials. 2011;32:485–91.
- LaFleur J, Tyler LS, Sharma RR. Economic benefits of investigational drug services at an academic institution. Am J Health Syst Pharm. 2004;61:27–32.
- McDonagh MS, Miller SA, Naden E. Costs and savings of investigational drug services. Am J Health Syst Pharm. 2000;57:40–43
- Rogers SD, Lampasona V, Buchanan EC. The financial impact of investigational drug services. Top Hosp Pharm Manage. 1994;14(1):60–66.
- Kaló Z, Antal J, Pénzes M, et al. Contribution of clinical trials to gross domestic product in Hungary. Croat Med J. 2014;55(5):446–451.
- Battelle: Biopharmaceutical Industry-Sponsored Clinical Trials: Impact on State Economies. 2015. [cited 2019 Jan 5]. Available from: http://phrma-docs.phrma.org/sites/default/files/pdf/biopharmaceutical-industry-sponsored-clinical-trials-impact-on-state-economies.pdf
- Bundesamt für Sicherheit im Gesundheitswesen (BASG). Klinische Prüfungen: Statistik 2016. 2016. [cited 2018 Nov 15]. Available from: https://www.basg.gv.at/fileadmin/user_upload/Klinische_Pr%C3%BCfungen_Statistik_2016_dt.pdf
- Pharmig. Daten & Fakten 2018 Arzneimittel und Gesundheitswesen in Österreich. 2018. [cited 2018 Sept 14]. Available from: https://www.pharmig.at/media/1286/duf_2018_web_deutsch_final_22216_de.pdf
- Pharmig. Daten & Fakten 2017 Arzneimittel und Gesundheitswesen in Österreich. 2017. [cited 2018 Sept 14]. Available from: https://www.pharmig.at/media/1285/duf_kompakt_2017_web_21522_de.pdf
- Pharmig. Daten & Fakten 2016 Arzneimittel und Gesundheitswesen in Österreich. [cited 2018 Sept 14]. Available from: https://www.pharmig.at/media/1281/datenundfakten_2016_englisch_web_15623_de.pdf. 2016
- Pharmig. Daten & Fakten 2015 Arzneimittel und Gesundheitswesen in Österreich. Vienna, Austria; 2015.
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWIG). Allgemeine Methoden: Version 5.0 vom 10.07.2017. 2017. [cited 2018 Nov 10]. Available from: file:///C:/Users/ipf/Downloads/Allgemeine-Methoden_Version-5-0.pdf
- Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II – an ISPOR Good Research Practices Task Force report. Value Health. 2015;18:161–172.
- Stäglin R, Pischner R. Darstellung des um den Keynes’schen Multiplikator erweiterten offenen statischen Input-Output-Modells. Mitteilungen aus der Arbeitsmarkt- und Berufsforschung. 1976;9:345–349.
- Statistik Austria. Input-Output-Tabelle. Austria: Statistik Austria; 2018.
- Statistik Austria. Überleben mit Krebs. [cited 2020 Jan 21]. Available from: https://www.statistik.at/web_de/statistiken/menschen_und_gesellschaft/gesundheit/krebserkrankungen/ueberleben_mit_krebs/index.html
- Laimer P, Ostertag-Sydler J, Smeral E. WIFO Austrian Institute of Economic Research2010. Ein Tourismus-Satellitenkonto für Österreich Methodik, Ergebnisse und Prognosen für die Jahre 2000 bis 2011. [cited 2019 Jan 15]. Available from: https://www.ttr.tirol/sites/default/files/2017-10/ein_tourismus-satellitenkonto_fuer_oesterreichbrmethodik_ergebnisse_und_pr-3.pdf
- Ker D, Galindo-Rueda F. Frascati Manual R&D and the System of National Accounts. OECD Science, Technology and Industry Working Papers.2017. No. 2017/06. Paris: OECD Publishing.