Abstract
Aims: This study assessed the cost-effectiveness of denosumab for treating postmenopausal women with osteoporosis (PMO) at high risk of fracture in Thailand.
Materials and methods: A published Markov cohort cost-effectiveness model was populated with country-specific data as available and other published data as needed. The model used a societal perspective, lifetime horizon, efficacy data from network meta-analysis of trials, and included costs for direct medical and non-medical care, informal care, and osteoporosis treatments to compare denosumab to no pharmacologic treatment (calcium and vitamin D supplements only) and to oral weekly alendronate. The base case (high-risk population) included postmenopausal women with femoral neck T-score ≤−2.5, mean age 65 years at entry, and history of vertebral fracture.
Results: High-risk women with osteoporosis using denosumab had the greatest number of life years and quality-adjusted life-years (QALYs) with higher reductions in hip and vertebral fracture incidence compared with patients with no pharmacologic treatment. The incremental cost-effectiveness ratio (ICER) was 119,575 Thai Baht (THB) per QALY for denosumab versus no pharmacologic treatment and 199,186 THB per QALY for denosumab versus alendronate. Among Thai postmenopausal women with high-risk of fractures, denosumab was cost-effective compared with no pharmacologic treatment at a willingness-to-pay threshold of 160,000 THB per QALY. One-way sensitivity analysis showed models were most sensitive to changes in denosumab pricing.
Limitations: Data from other countries used when country-specific data were unavailable may not accurately reflect the true experience in Thailand. The model focused explicitly on hip, vertebral, and wrist fractures, and therefore provides a conservative estimate of the overall potential impact of osteoporosis-related fracture. The fracture risk was not adjusted to reflect potential changes in risk after denosumab treatment discontinuation.
Conclusions: In Thailand, denosumab offers a cost-effective osteoporosis treatment option versus no pharmacologic treatment in postmenopausal women at high risk of fracture.
Introduction
Postmenopausal osteoporosis is a common disorder associated with significant morbidity and mortality and reduced quality of lifeCitation1,Citation2. In Thailand, the age-adjusted prevalence of osteoporosis in postmenopausal women was 19.8% and 13.6% for lumbar spine and femoral neck, respectively, ranging from 5% with aged less than 50 years to more than 50% among women over age 70 yearsCitation3. As the aged population (60 years and older) in Thailand grows from 14% of the total population in 2015 to nearly 30% by 2050, the number osteoporosis-related fractures, fracture-related healthcare costs, and post-fracture mortality will also riseCitation4–6. The incidence of hip fracture is projected to increase nearly 3-fold between 2018 and 2050, as the number of fractures grows from 30,560 to 86,765 among Thai postmenopausal women (age 50 and older), while, the incidence of vertebral fracture in women ≥50 years of age in a suburb of Bangkok of 32.1/1,000 person-yearsCitation7.
Anti-osteoporotic agents are well established for the treatment of postmenopausal women at a high risk of fractures, and have been shown to reduce the risk of vertebral, nonvertebral and hip fracturesCitation8,Citation9. Besides the clinical profile of a therapy, it is also important to consider consequences of treatment in terms of costs and health effects both in short- and long-term perspectives. The clinical outcome and cost-effectiveness of anti-osteoporotic drugs have been evaluated and proved using health economic models integrating epidemiological, clinical and economic data from both the payer and societal perspectives in several countriesCitation10–17. However, there is a dearth of data to evaluate the cost-effectiveness of osteoporosis treatment in developing countries. This study was undertaken to assess the cost-effectiveness of denosumab compared with calcium and vitamin D supplements only (i.e. no pharmacologic therapy) or alendronate for treating postmenopausal women with osteoporosis at high risk of fractures in Thailand. In clinical trials, over three years, the RANK ligand inhibitor denosumab was associated with a 61–71% relative risk reduction in the incidence of new vertebral fractures and a 20% relative risk reduction for new nonvertebral fracturesCitation18. Denosumab is one of the newest postmenopausal osteoporosis (PMO) treatment agents approved for use in Thailand, while alendronate, an oral bisphosphonate, is the most commonly used osteoporosis treatment.
Methods
Target population
The target population used in the base-case analysis for this study was postmenopausal women with osteoporosis (PMO) who were at high risk of fracture as defined by a mean age of 65 years, a T-score ≤−2.5 at the femoral neck, and a history of vertebral fracture. A separate model scenario was run to evaluate cost-effectiveness in the overall PMO population. In this scenario, the T-score requirement remained the same as in the base-case, but the average starting age was lowered to age 60 years. The prevalence of vertebral fracture was also lowered from 100% to 23.6% based on local epidemiological data for the general Thai population of women age 60 years and olderCitation7.
The model
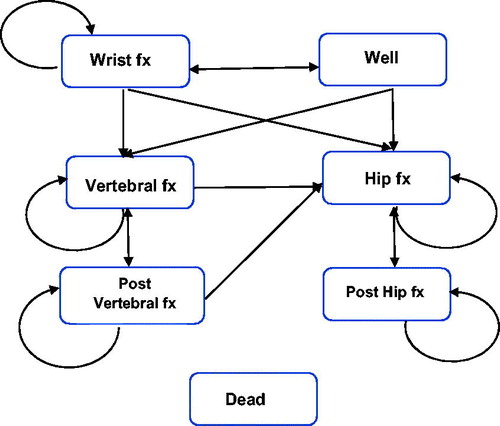
In this study, a previously published Markov cohort cost-effectiveness modelCitation13 was populated with data specific to Thailand. This model was selected for the current study since it employed a societal perspective with a lifetime horizon (i.e. patients were followed from age at treatment initiation to death or a maximum age of 100 years), included costs for direct medical and non-medical care as well as the cost of informal care, and results derived previously from that model will provide useful context for results from the current study. A six-month cycle length was used in alignment with the recommended denosumab dosing frequency, and for each cycle women transitioned into one of seven mutually exclusive health states (). All women entered the model in the well health state, and in each cycle could remain well, sustain a fracture, or enter the dead state. Patients who entered the dead state remained there for the rest of the simulation. Women who sustained a fracture during any cycle would enter either the hip fracture, vertebral fracture, or wrist fracture health state. After one year in any of the fracture health states, the model allowed a woman to either sustain a new fracture or move to either the post-hip or post-vertebral fracture health states or to the dead health state. Women who were in the post-hip fracture health state were only at risk of sustaining new hip fractures or dying whereas women who were in the post-vertebral fracture health state could sustain either new vertebral fractures or hip fractures or die. This approach also means that women who sustained either a hip or vertebral fracture (the two most serious fracture types) were not allowed to have future wrist fractures.
The model compared three osteoporosis treatment options: (1) denosumab (subcutaneous injection, once every six months); (2) no pharmacologic treatment (daily intake of calcium or calcium and vitamin D supplements only); (3) alendronate (oral, once weekly), which is the most commonly prescribed pharmacologic treatment for women with postmenopausal osteoporosis in Thailand. Treatments were intended to be provided for a duration of five years. The “no pharmacologic treatment” comparator provides insights into cost-effectiveness for patients who cannot take alendronate because of contraindications, or adherence or tolerance issues.
The incremental cost effectiveness ratio (ICER) expressed as cost per quality adjusted life year (QALY) gained is the primary outcome from this cost-effectiveness analysis. The number of patients needed to treat (NNT), calculated as the reciprocal of the absolute reduction in fractures, to avoid one hip fracture in 10 years was also determined for both denosumab and alendronate. The ICER threshold for the analysis was obtained from the Thailand National List of Essential Medicines (NLEM) guidance for health economic evaluation, and set at 160,000 Thai Baht (THB) per QALYCitation19. For reference, on 31 December 2018, one THB was valued at 0.03 United States dollarsCitation20 and this threshold is equivalent to ∼5,300 US dollars/QALY.
Model parameters
A literature review, the scope of which was defined by the Population, Intervention, Comparator, Outcome and Study design (PICOS) framework (Supplemental Table 1), was conducted to identify relevant Thai data from publications indexed in PubMed from the inception of the database through July 2018. These published data were supplemented through discussion with local experts in the clinical management of osteoporosis.
Fracture incidence and mortality
Age-specific estimates of fracture incidence in Thailand, derived from the general population were used in the model and are provided in . In the model, the risk of sustaining a fracture depends specifically on three elements: (i) the risk for an individual in the general population of incurring a fracture, (ii) the increased fracture risk associated with osteoporosis (the relative risk), and (iii) risk reduction, if any, attributed to a treatment. The fracture risk in the general population was adjusted upwards to adjust for bone mineral density (BMD) and vertebral fracture history in the osteoporotic population, as described in Jönsson et al.Citation13. The older average age at model entry in the high-risk population compared with the general population (65 years versus 60 years) implies a higher incidence of hip fracture (16 per 10,000 women versus 8 per 10,000 women), although the incidence of vertebral and wrist fracture were assumed to be the same in both populations. Both hip and vertebral fractures have been shown to increase mortality risk above the background risk in the general population in ThailandCitation21. Due to lack of local data, a value of zero was used for all input parameters associated with non-osteoporotic fracture (e.g. risk of event, event-related direct medical and non-medical costs, utility values). With this approach, the “other osteoporotic fractures” arm is still included in the model structure but does not contribute to the model results. For the model, an 18% excess mortality risk was assumed to last for eight years following a hip or vertebral fractureCitation21.
Table 1. Fracture incidence per patient-year in Thailand.
Costs
All costs were evaluated from a societal perspective and quantified in THB adjusted to 2018 values using the Thai Consumer Price Index (CPI)Citation22. Direct fracture-related costs included all costs related to managing the fracture in the inpatient, outpatient and long-term care settings (). For analytic purposes, total direct fracture-related costs were split into inpatient (37%) and outpatient (63%) based on results from a prior studyCitation23, although this classification did not impact the total costs. The annual cost of long-term care reported by Khongboon et al.Citation24 was converted into a daily cost of THB 484. Hip and vertebral fractures costs were applied in full for the first year after the fracture, and the cost of four physician visits and one x-ray were assumed for fracture patients in each subsequent year. Wrist fractures were assumed to have an impact only on costs during the first year after the event. Each fracture was assumed to require four physician visits during the first year, regardless of fracture type or patient age. A standard direct non-medical cost (2,929 THB per patient) was assumed for all fracture types with this amount representing the cost of travel and meals associated with the fracture-related physician visitsCitation25. The annual per patient cost of informal care was an average of costs in urban and rural areas and assumed to be 72,150 THB, regardless of patient age or fracture typeCitation24. The annual cost of denosumab was estimated to be 25,550 THB, and annual alendronate costs, based on weekly oral dosing, were assumed to be 4,570 THB (based on median price per unit and dosing per labelCitation26) For patients who sustained a fracture, fracture-specific reductions in utility were applied. Due to the absence of local Thai data, the utility estimates used in this analysis were taken from foreign publications ()Citation27,Citation28. Although gastrointestinal adverse events are associated with the use of oral bisphosphonates, such as alendronate, costs associated with treating these events were not included in the base-case scenario. A discount rate of 3% per annum was applied.
Table 2. Summary of costs.
Table 3. Summary of utility estimates.
Treatment efficacy
Estimates for the treatment efficacy parameters () were obtained from the random effects network meta-analysis of data from randomized controlled trials conducted by Freemantle et al.Citation29 which provides robust data by pharmacologic agent and fracture site. Treatment persistence (i.e. continuing treatment according to medical recommendation/prescribing) varies in real-world use and poor persistence can reduce the clinical benefit that a patient derives from a prescribed osteoporosis medication. Persistent use of osteoporosis medications has been associated with greater reduction in fracture rates, lower healthcare utilization, and lower total healthcare costsCitation7,Citation9,Citation30,Citation31. Therefore, treatment-specific persistence was modelled in each six-month cycle for the duration of the treatment course (). The assumption was made that only persistent patients incur drug costs and receive the full fracture reduction effect of treatment. Since treatment benefit does not stop immediately following discontinuation, it is assumed that the fracture reduction effect declines linearly to 0 over the course of 2 years after treatment ends. For denosumab, per-cycle persistence was implemented as a step function, to reflect the semi-annual dosing schedule, where all patients remain persistent for six months after an injection. Since oral alendronate is delivered weekly, it was assumed that discontinuation could occur at any point in each half-year cycle. As such, persistence was assumed to be derived from a continuous function, with average persistence taken at the midpoint of each six-month cycle. Since persistence data were not available for every six-month period for either treatment, where required, persistence was interpolated by assuming a constant drop-out rate between observed time points, and extrapolated by assuming a drop-out rate equivalent to the last observed interval.
Table 4. Treatment efficacy: relative risk of fracture (treatment versus placebo).
Table 5. Treatment persistence estimates.
Analysis
In addition to producing results for the base-case (high-risk population), results were modelled separately for the general population of postmenopausal women with osteoporosis (overall osteoporosis population). One-way sensitivity analysis was conducted to assess the effect of varying drug and fracture costs, excess mortality risk, and treatment duration by up to 25% from the base-case parameters. Probabilistic sensitivity analysis as described in the Model section above was conducted to assess cost-effectiveness over a range of ‘willingness-to-pay’ values.
Results
In the base-case (i.e. high-risk population) analysis, despite having the highest total cost, denosumab produced the lowest fracture-related cost of all modelled treatment options ( and ). This is due to cost savings from the lower incidence of hip and vertebral fractures compared with both no pharmacologic treatment and alendronate. In addition, women treated with denosumab were estimated to have a greater number of life years and QALYs compared to those who received no pharmacologic treatment (0.415 and 0.362) and to those treated with alendronate (0.248 and 0.214) (). The ICER for denosumab versus no pharmacologic treatment was 119,575 THB per QALY (3,587 USD per QALY) and the ICER for denosumab versus alendronate was 199,186 THB per QALY (5,976 USD per QALY). In the model scenario for the total PMO population, the corresponding ICERs were 153,879 THB per QALY (4,616 USD per QALY) (versus no pharmacologic treatment) and 243,983 THB per QALY (7,319 USD per QALY) (versus alendronate).
Table 6. Costs and fracture results for base-case modela.
Table 7. Costs and fracture results for scenario model: total PMO populationa.
Table 8. Incremental cost-effectiveness estimate.
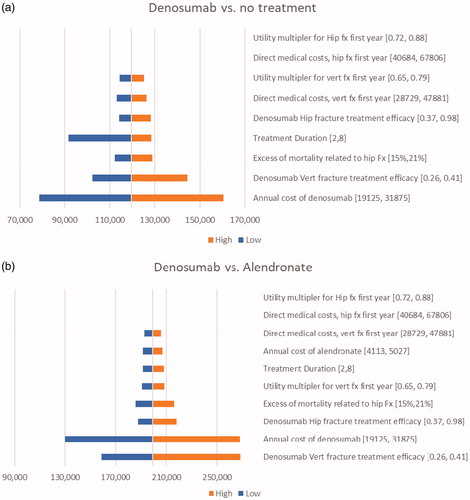
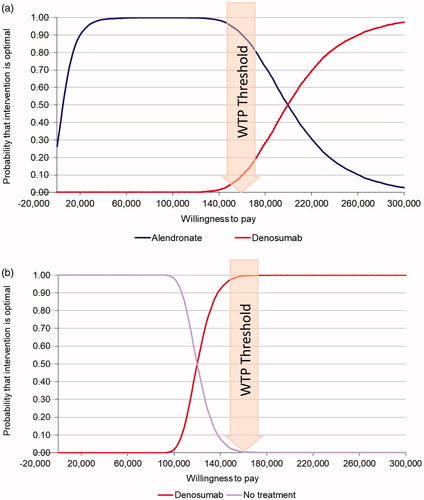
Probabilistic sensitivity analysis for the base-case population indicates that denosumab is cost-effective versus no pharmacologic treatment across a range of willingness to pay (WTP) values. With the WTP threshold set at 160,000 THB per QALY, denosumab had a 99.52% and a 9.32% probability of being cost-effective compared with no pharmacologic treatment and to alendronate, respectively (). One-way sensitivity analysis indicated that the ICER of denosumab versus no treatment was most sensitive to the annual cost of denosumab with a 25% cost reduction lowering the cost per QALY to 78,685 THB (2,361 USD) and a similar increase raising the cost per QALY to 160,466 THB (48,134 USD) (). Denosumab remained cost-effective versus no treatment with changes to treatment duration (reduced to 2 years; increased to 8 years), 25% change in vertebral fracture costs, and changes in both the excess mortality and costs associated with hip fracture. The ICER of denosumab versus alendronate was also most sensitive to changes in the annual cost of denosumab, followed by excess mortality related to hip fracture. Changes in the annual cost of denosumab, denosumab efficacy for hip fracture, treatment duration, and the first-year cost and utility values for vertebral fracture had similarly small relative effects on the ICER. Changes in the first-year cost and utility values for hip fracture had essentially negligible impact (). In the base-case population, the number needed to treat (NNT) to avoid one hip fracture in ten years was 106 for denosumab and 165 for alendronate. In the total PMO population, the corresponding numbers needed to treat (NNT) were 214 for denosumab and 345 for alendronate.
Figure 2. Probabilistic sensitivity analysis: (a) denosumab vs. alendronate and no pharmacologic treatment in high-risk patients: (b) denosumab vs. no pharmacologic treatment in high-risk patients. Abbreviation. WTP: willingness to pay. Postmenopausal women age ≥ 65 years with a femoral neck T-score of ≤−2.5 and history of vertebral fracture.
Discussion
The results of this analysis demonstrate that denosumab offers a cost-effective treatment option to reduce the risk of fracture in postmenopausal Thai women with osteoporosis. In the high-risk (base-case) population, denosumab achieves superior health outcomes (0.362 and 0.214 QALYs gained versus no pharmacologic treatment and alendronate respectively) and attains ICERs below the cost-effectiveness threshold of 160,000 THB/QALY compared with no treatment. The findings from this study provide important insights into the cost-effectiveness of denosumab versus no treatment and versus alendronate, the most commonly used therapy in Thailand; this information fills an important gap in the literature. In order to provide broadly useful information, the study assessed cost-effectiveness in both an overall osteoporosis population, and in a high-risk population whose characteristics are consistent with the reimbursement strategy for osteoporosis therapy in ThailandCitation32. Women in the high-risk population (i.e. women age 65 years or older with osteoporosis) are very likely to have primary osteoporosis and, therefore, good candidates for pharmacologic therapy.
The results from our models are consistent with previous economic evaluations that have found denosumab to be cost-effective in the treatment of postmenopausal osteoporosis in the United States, Canada, various countries in the European Union, and in JAPAC (Japan and Asia Pacific) countries including Japan, Australia, Taiwan and SingaporeCitation10–17. Moreover, denosumab would also provide a unique and cost-effective option for high-risk PMO patients who are transitioning away from alendronate for any reasons including side effect experience or the inability to consistently take alendronate as directed. Non-persistence significantly blunts the fracture risk reduction effect of alendronate and other oral bisphosphonates, leaving many patients at elevated risk of fracture even after initiating pharmacologic PMO therapyCitation33. Effective pharmacologic therapy, which relies on good medication adherence (i.e. compliance with dosing instructions and persistence), is particularly important in countries where support services designed to help patients mitigate non-physiological fracture risk factors (e.g. community fall risk assessments and prevention programs) may be lacking.
Bisphosphonates are contraindicated for use in patients with renal impairment and there was evidence showed that bisphosphonates were associated with deterioration in kidney function until acute kidney failure in some casesCitation34–36. Therefore, routine monitoring of kidney function is advised particularly among patients who are concurrently using other medications that impact kidney functionCitation34–37. The pharmacokinetics and pharmacodynamics of denosumab used at the standard dose, are unaffected by the presence of renal impairmentCitation38. In fact, among patients with chronic kidney disease, denosumab provides greater bone mineral density (BMD) gains than bisphosphonates, although vitamin D supplementation is required to prevent hypocalcemia among denosumab users in this populationCitation38. Impaired renal function, which becomes more common with advancing age, increases the risk of progressive bone loss and osteoporosis. This association between age and renal impairment underscores the importance of having an osteoporosis treatment option available, for patients who cannot use a bisphosphonate therapy because of either existing renal impairment or their use of potentially nephrotoxic medications to treat comorbid conditionsCitation39–42.
In Thailand, the societal importance of osteoporosis and the impact of osteoporosis-related fractures are growing as the population ages. For instance, the incidence of hip fracture, a costly and debilitating fracture, is increasingCitation43, with postmenopausal women and the elderly at greatest risk. Osteoporosis-related fractures of all types (hip, vertebral, and nonvertebral) exert substantial clinical, economic and humanistic burdens with the impact extending from the patient level up to the societal level. In addition to conferring ongoing healthcare costs and disability, osteoporosis-related fractures also increase the risk of mortality. A study of osteoporotic hip fracture patients treated in Chiang Mai in 2006 and 2007, reported a one-year mortality rate of 21%, which was approximately 9 times that of individuals of the same age in the general populationCitation44.
There are a number of limitations to the present findings. First, although preference was given to use recent data specific to Thailand whenever possible to create parameter estimates for the model, older data and data from other countries were used when there were gaps in the available data. For example, the age-specific incidence of wrist fracture was obtained from a Japanese paper by Hagino et al. 1999 cited by Thailand Health Intervention and Technology Assessment Program (HITAP) economic evaluationCitation45. It is possible that these data obtained from outside of Thailand do not accurately reflect the true experience in Thailand, and their inclusion may add uncertainty to the parameter estimates used in the model. Furthermore, the lack of local data on ‘other osteoporotic fractures’ precluded their inclusion in the model, although this omission actually provides more conservative estimates of the ICERs. Second, the cost data used in this analysis were based on a local study published in 2005. While these data were specific to Thailand, they may be outdated, especially given the technology advancements related to hip replacement surgery. Consequently, the costs of hip replacement may be underestimated, favoring the less efficacious treatment option. However, a one-way sensitivity analysis showed that different assumptions for these costs had little effect on the ICER. Third, the model in this study focused explicitly on hip, vertebral, and wrist fractures, and therefore, provides a conservative estimate of the overall potential impact of osteoporosis-related fractures. In addition, the use of a hierarchy of fracture types in designating transitions between health states, such as allowing post-hip fracture patients to only be at risk for additional hip fractures or death, and allowing women with either a hip or vertebral fracture to no longer be at risk of future wrist fractures may have led to more conservative estimates of the overall fracture burden in the modeled populations. An increased risk of multiple vertebral fractures after discontinuation of denosumab has been observed in data from the FREEDOM trial and its extension (incidence 3.4% among discontinuers and 0.7% among all denosumab users)Citation46,Citation47 and in a recent real-world study (incidence 0.8% in discontinuers)Citation48. However, given the low incidence and current lack of strong evidenceCitation49, the fracture risk assumption used in the model was not adjusted to reflect potential changes in risk after denosumab treatment discontinuation. Adverse events associated with alendronate were not considered in the model; a decision informed by the cost-effectiveness analysis conducted by Jonsson et al.Citation13 in which a sensitivity analysis was done to evaluate the impact of gastrointestinal events associated with alendronate. A minimal impact on the ICER was found (€27,090/QALY base case versus €26,595/QALY including gastrointestinal events). Therefore, adverse events were not included in this analysis. Finally, since the ICER for denosumab in the broader postmenopausal osteoporosis population is relatively close to the WTP threshold of 160,000 THB per QALY, it will be important to re-evaluate cost-effectiveness in this population in the future as the Thai economy continues growing and expanding.
An important strength of this study is that it adheres to the guidance recently provided by Hiligsmann et al. for economic evaluations of osteoporosis therapies including our use of local data to the extent possible to ensure that the model reflects country-specific (i.e. Thailand) osteoporosis management strategies and our detailed presentation of the model parameters and results for specific outcomes (e.g. fracture events, life years, costs per QALY)Citation50. Our models examined cost-effectiveness in women with T-score ≤−2.5 at the femoral neck, with separate models provided for a high risk population (mean age of 65 years, a T-score ≤−2.5 at the femoral neck, and a history of vertebral fracture) which served as the base case, and for an overall osteoporosis population consisting of post-menopausal women with mean age of 60 years, a T-score ≤−2.5 at the femoral neck, 23.6% of whom were assumed to have had prior vertebral fracture. The characteristics of this base-case and the inclusion of the additional population with a differing age/risk profile are also consistent with the guidance. In fact, our study adheres to all of the nine items on Hiligsmann et al. checklist, with one exception – the recommendation for reporting transition probabilities. Although, we have not explicitly reported the transition probabilities used in our models, they are described in a previous paperCitation13 which we have cited. We have also included a general description of the health state transitions (including a figure []) to illustrate how women may move between states in each model cycle.
In conclusion, the current study demonstrates that, from the societal perspective, denosumab offers a cost-effective PMO treatment option versus no pharmacologic treatment for high-risk postmenopausal women with a T-score ≤ −2.5 at the femoral neck and age ≥65 years. This study offers important data for consideration by stakeholders and decision-makers as they formulate policies to ensure adequate access to appropriate therapies in the face of Thailand’s growing burden of osteoporosis and fracture risk in the future.
Transparency
Declaration of funding
This study was funded by Amgen Thailand Limited.
Declaration of financial interests
The authors have no conflicts of interest to declare. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
Prof. Chatlert Pongchaiyakul: Study design, provided data and review literature, manuscript writing
All other authors contributed to the study design, interpretation of results, and writing of this manuscript.
Supplemental Material
Download MS Word (47.5 KB)Acknowledgements
The authors would like to thank Sally Wade (Wade Outcomes Research and Consulting, Salt Lake City, Utah, USA) for medical writing.
References
- Bouxsein ML, Karasik D. Bone geometry and skeletal fragility. Curr Osteoporos Rep. 2006;4(2):49–56.
- Recker R, Lappe J, Davies KM, et al. Bone remodeling increases substantially in the years after menopause and remains increased in older osteoporosis patients. J Bone Miner Res. 2004;19(10):1628–1633.
- Limpaphayom KK, Taechakraichana N, Jaisamrarn U, et al. Prevalence of osteopenia and osteoporosis in Thai women. Menopause. 2001;8(1):65–69.
- International Osteoporosis Foundation. Thailand: Regional Asia Audit. 2019 [cited 2019 Feb 4]; Available from: https://www.iofbonehealth.org/sites/default/files/PDFs/Audit%20Asia/Asian_regional_audit_Thailand.pdf.
- Lau EMC, Lee JK, Suriwongpaisal P, et al. The incidence of hip fracture in four Asian countries: the Asian Osteoporosis Study (AOS). Osteoporos Int. 2001;12(3):239–243.
- Pongchaiyakul C, Songpattanasilp T, Taechakraichana N. Burden of osteoporosis in Thailand. J Med Assoc Thai. 2008;91(2):261–267.
- Jitapunkul S, Thamarpirat J, Chaiwanichsiri D, et al. Incidence of vertebral fractures in Thai women and men: a prospective population-based study. Geriatr Gerontol Int. 2008;8(4):251–258.
- Barrionuevo P, Kapoor E, Asi N, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab. 2019;104(5):1623–1630.
- Eastell R, Rosen CJ, Black DM, et al. Pharmacological management of osteoporosis in postmenopausal women: an endocrine society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595–1622.
- Agency for Care Effectiveness. Denosumab for the treatment of postmenopausal women with osteoporosis at high risk of fracture. 2017 [cited 2019 March 28]. Available from: http://www.ace-hta.gov.sg/our-guidance/denosumab-for-the-treatment-of-postmenopausal-women-with-osteoporosis-at-high-risk-of-fracture.html.
- Chau D, Becker DL, Coombes ME, et al. Cost-effectiveness of denosumab in the treatment of postmenopausal osteoporosis in Canada. J Med Econ. 2012;15(sup1):3–14.
- Hiligsmann M, Reginster JY. Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. Pharmacoeconomics. 2011;29(10):895–911.
- Jonsson B, et al. Cost-effectiveness of Denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2011;22(3):967–982.
- Karnon J, Shafie AS, Orji N, et al. What are we paying for? A cost-effectiveness analysis of patented denosumab and generic alendronate for postmenopausal osteoporotic women in Australia. Cost Eff Resour Alloc. 2016;14(1):11.
- Mori T, Crandall CJ, Ganz DA. Cost-effectiveness of denosumab versus oral alendronate for elderly osteoporotic women in Japan. Osteoporos Int. 2017;28(5):1733–1744.
- Parthan A, Kruse M, Yurgin N, et al. Cost effectiveness of denosumab versus oral bisphosphonates for postmenopausal osteoporosis in the US. Appl Health Econ Health Policy. 2013;11(5):485–497.
- Strom O, Jonsson B, Kanis JA. Intervention thresholds for denosumab in the UK using a FRAX(R)-based cost-effectiveness analysis. Osteoporos Int. 2013;24(4):1491–1502.
- Highlights of prescribing information: Prolia (denosumab). 2020 [cited 2020 Jan 07]. Available from: https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/prolia/prolia_pi.pdf.
- Teerawattananon Y, Tritasavit N, Suchonwanich N, et al. The use of economic evaluation for guiding the pharmaceutical reimbursement list in Thailand. Z Evid Fortbild Qual Gesundhwes. 2014;108(7):397–404.
- [cited 2019 Aug 29]. Available from: https://www.exchange-rates.org/Rate/THB/USD/12-31-2018.
- Vaseenon T, Luevitoonvechkij S, Wongtriratanachai P, et al. Long-term mortality after osteoporotic hip fracture in Chiang Mai, Thailand. J Clin Densitom. 2010;13(1):63–67.
- Ministry of Commerce. Report for Consumer Price Index. 2018 [cited 2018 Aug 31]. Available from: http://www.indexpr.moc.go.th/price_present/cpi/stat/others/indexg_report2.asp?table_name.
- Werayingyong P. Health resource utilization of osteoporosis patients at phramongkutklao hospital, in pharmacy administration. 2006. Thailand: Mahidol University.
- Khongboon P, Pongpanich S. Estimating long-term care costs among thai elderly: a phichit province case study. J Aging Res. 2018;2018:1–11.
- Kingkaew P, Maleewong U, Ngarmukos C, et al. Evidence to inform decision makers in Thailand: a cost-effectiveness analysis of screening and treatment strategies for postmenopausal osteoporosis. Value Health. 2012;15(1):S20–S8.
- Health MoP. Median Unit Pricing List. 2017.
- Hiligsmann M, Ethgen O, Richy F, et al. Utility values associated with osteoporotic fracture: a systematic review of the literature. Calcif Tissue Int. 2008;82(4):288–292.
- Tosteson ANA, Gabriel SE, Grove MR, et al. Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int. 2001;12(12):1042–1049.
- Freemantle N, Cooper C, Diez-Perez A, et al. Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int. 2013;24(1):209–217.
- Silverman SL, Gold DT, Cramer JA. Reduced fracture rates observed only in patients with proper persistence and compliance with bisphosphonate therapies. South Med J. 2007;100(12):1214–1218.
- Sunyecz JA, Mucha L, Baser O, et al. Impact of compliance and persistence with bisphosphonate therapy on health care costs and utilization. Osteoporos Int. 2008;19(10):1421–1429.
- Songpatanasilp T, Sritara C, Kittisomprayoonkul W, et al. Thai Osteoporosis Foundation (TOPF) position statements on management of osteoporosis. Osteoporos Sarcopenia. 2016;2(4):191–207.
- Imaz I, Zegarra P, González-Enríquez J, et al. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: systematic review and meta-analysis. Osteoporos Int. 2010;21(11):1943–1951.
- Highlights of prescribing information: Actonel (risedronate sodium) tablets. [cited 2019 Aug 15]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020835s035lbl.pdf.
- Highlights of prescribing information: Fosamax (alendronate sodium). [cited 2019 Aug 15]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021575s017lbl.pdf.
- Highlights of prescribing information: Zometa (zoledronic acid). [cited 2019 Aug 15]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/021223s034lbl.pdf.
- Miller PD, Jamal SA, Evenepoel P, et al. Renal safety in patients treated with bisphosphonates for osteoporosis: a review. J Bone Miner Res. 2013;28(10):2049–2059.
- Suzuki HKM, Mano S, Kobayashi T, et al. Efficacy and safety of denosumab for the treatment of osteoporosis in patients with chronic kidney disease. J Clin Exp Nephrol. 2017;02(01):30.
- Jassal SK, von Muhlen D, Barrett-Connor E. Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res. 2006;22(2):203–210.
- Nickolas TL, Leonard MB, Shane E. Chronic kidney disease and bone fracture: a growing concern. Kidney Int. 2008;74(6):721–731.
- Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int. 2008;74(11):1385–1393.
- Toussaint ND, Elder GJ, Kerr PG. Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol . 2009; 4(1):221–233.
- Wongtriratanachai P, Luevitoonvechkij S, Songpatanasilp T, et al. Increasing incidence of hip fracture in Chiang Mai, Thailand. J Clin Densitom. 2013;16(3):347–352.
- Chaysri R, Leerapun T, Klunklin K, et al. Factors related to mortality after osteoporotic hip fracture treatment at Chiang Mai University Hospital, Thailand, during 2006 and 2007. J Med Assoc Thai. 2015;98(1):59–64.
- (HITAP). H.I.a.T.A. Economic evaluation of screening and treatment options for postmenopausal osteoporosis: Ministry of Public Health. 2013 [cited 2020 Feb 17]. Available from: http://hitap.net/costingmenu/.
- Cummings SR, Ferrari S, Eastell R, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled freedom trial and its extension. J Bone Miner Res. 2018;33(2):190–198.
- Brown JP, Roux C, Törring O, et al. Discontinuation of denosumab and associated fracture incidence: analysis from the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial. J Bone Miner Res. 2013;28(4):746–752.
- Tripto-Shkolnik L, Fund N, Rouach V, et al. Fracture incidence after denosumab discontinuation: Real-world data from a large healthcare provider. Bone. 2020;130:115150.
- Tsourdi E, Langdahl B, Cohen-Solal M, et al. Discontinuation of Denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17.
- Hiligsmann M, et al. Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts’ consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the International Osteoporosis Foundation. Osteoporos Int. 2019;30(1):45–57.
- Woratanarat P, Wajanavisit W, Lertbusayanukul C, et al. Cost analysis of osteoporotic hip fractures. J Med Assoc Thai. 2005;88(Suppl 5):S96–S104.
- IQVIA. Data from expert opinion for economic evaluation of denosumab for postmenopausal osteoporosis in Thailand. Durham: IQVIA; 2018.
- HITAP. H.I.a.T.A. Standard Cost List for Health Technology Assessment: Ministry of Public Health. 2009 [cited 2020 Feb 17]. Available from: http://hitap.net/costingmenu/.
- Ministry of Public Health. Median Unit Pricing List. Geneva: Ministry of Public Health; 2017.
- Jonsson E, et al. Systematic review and meta-analysis of persistence with denosumab in patients with osteoporosis. In ISPOR 17th Annual European Meeting. 2014. Amsterdam, The Netherlands.