Abstract
Aim: Given that rheumatoid arthritis (RA) patients with high anti-citrullinated protein antibodies (ACPA) titer values respond well to abatacept, the aim of this study was to estimate the annual budget impact of anti-cyclic citrullinated peptide (anti-CCP) testing and treatment selection based on anti-CCP test results.
Materials and methods: Budget impact analysis was conducted for patients with moderate-to-severe RA on biologic or Janus kinase inhibitor (JAKi) treatment from a hypothetical US commercial payer perspective. The following market scenarios were compared: (1) 90% of target patients receive anti-CCP testing and the results of anti-CCP testing do not impact the treatment selection; (2) 100% of target patients receive anti-CCP testing and the results of anti-CCP testing have an impact on treatment selection such that an increased proportion of patients with high titer of ACPA receive abatacept. A hypothetical assumption was made that the use of abatacept would be increased by 2% in Scenario 2 versus 1. Scenario analyses were conducted by varying the target population and rebate rates.
Results: In a hypothetical health plan with one million insured adults, 2,181 patients would be on a biologic or JAKi treatment for moderate-to-severe RA. In Scenario 1, the anti-CCP test cost was $186,155 and annual treatment cost was $101,854,295, totaling to $102,040,450. In Scenario 2, the anti-CCP test cost increased by $20,684 and treatment cost increased by $160,467, totaling an overall budget increase of $181,151. This was equivalent to a per member per month (PMPM) increase of $0.015. The budget impact results were consistently negligible across the scenario analyses.
Limitations: The analysis only considered testing and medication costs. Some parameters used in the analysis, such as the rebate rates, are not generalizable and health plan-specific.
Conclusions: Testing RA patients to learn their ACPA status and increasing use of abatacept among high-titer ACPA patients result in a small increase in the total budget (<2 cents PMPM).
Introduction
Biomarkers are objective indications of physiological or pathogenic processes that can be measured and evaluated accurately and reproduciblyCitation1,Citation2. In rheumatology, biomarkers contribute to various clinical objectives such as earlier diagnosis, risk identification to prognosis, disease activity monitoring, assessment of therapy response, and evaluation of drug toxicityCitation3,Citation4.
Anti-citrullinated protein antibodies (ACPA) are an important diagnostic and prognostic biomarker for rheumatoid arthritis (RA), a chronic heterogenous autoimmune diseaseCitation3–5. ACPA are a group of autoantibodies that are directed against citrulline, a nonessential amino acid that triggers immunogenicity. In 2010, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) included ACPA as part of the serology classification criteria to assist in early RA diagnosis, since ACPA are present before the onset of RA symptomsCitation6. Studies have found that patients with ACPA may initially present with similar clinical presentations as patients with no ACPA, but have worse disease course over time, with higher rates of swollen joints, radiological damage, and even mortalityCitation7,Citation8. Due to the worse prognosis associated with the concentration of ACPA, ACPA is associated with higher healthcare resource utilization and thus higher RA-related cost burdenCitation9. Current guidelines in rheumatologist practice, however, do not incorporate ACPA status in treatment managementCitation10. The rationale for the exclusion of ACPA in treatment selection, specifically for first-line methotrexate, is that some studies have reported that disease activity did not differ between serologic subgroupsCitation11–13.
Recently, however, an increasing number of studies are finding that ACPA is an independent factor for predicting treatment responses to some biologic disease-modifying antirheumatic drugs (bDMARDs), particularly with T-cell activation inhibitor (i.e. abatacept). A post hoc study of data from AMPLE, a randomized control trial of moderate-to-severe RA patients, found that patients with high concentration of ACPA have high response rates to abataceptCitation14. Specifically, 36.4% of patients in the highest quartile (Q4) of ACPA concentration who received abatacept achieved Clinical Disease Activity Index (CDAI) ≤2.8 after one year of treatment. In the same study, 22.7% of Q4 ACPA patients treated with adalimumab achieved CDAI ≤2.8. After 2 years of treatment, 51.2% of the Q4 ACPA patients on abatacept achieved CDAI ≤ 2.8 and 30.8% of those on adalimumabCitation14. These results suggest that abatacept may be an effective treatment choice for patients with high ACPA titer. Consistent findings were observed in a recent real-world data study. Harrold et al.Citation15 used the Corrona RA registry in the United States (US) to compare change in CDAI prior to biologic initiation and 6 months after initiation between patients initiating abatacept and patients initiating a tumor necrosis factor inhibitor (TNFi). While the CDAI response was significantly greater in ACPA positive patients compared to negative patients when treated with abatacept, no difference was observed between ACPA positive and negative groups for TNFi initiatiors. The same study group carried out a follow-up study with an increased number of patients who were also propensity matched on baseline characteristics. The follow-up study found a statistically significantly higher CDAI change with abatacept compared to TNFi among ACPA positive patients who had previously used TNFiCitation16. The differences in treatment response may be related to the treatments’ different mechanism of actions, with abatacept inhibiting the autoantibody-mediated upregulation of cytokinesCitation17. All of these studies suggest that considering ACPA status could inform appropriate treatment selection, as treatment response varied by ACPA status and the treatment’s mechanism of action.
Given the results of the post hoc AMPLE study analysis, we conducted a budget impact analysis (BIA) to estimate the cost impact of using anti-cyclic citrullinated peptide (anti-CCP) testing to guide treatment selection and increasing abatacept uptake for RA patients with high ACPA titer. This analysis informs payers and decision makers of the budget impact of anti-CCP testing and treatment selection based on anti-CCP test results.
Methods
Model structure
The BIA was structured as a simple cost calculator programmed in Microsoft Excel. The analysis was from a hypothetical US commercial payer perspective with one million insured adults (age ≥18 years) and the target population was patients with moderate-to-severe RA on biologic or Janus kinase inhibitor (JAKi). The two market scenarios compared in this analysis were:
Scenario 1: 90% of target patients receive anti-CCP testing and the results of anti-CCP testing do not impact the treatment selection. The current treatment rate of 90% was based on a recent chart review study and an assumption that the test uptake will increase overtimeCitation18.
Scenario 2: 100% of target patients receive anti-CCP testing and the results of anti-CCP testing have an impact on treatment selection such that an increased proportion of patients with high titer of ACPA receive abatacept. A hypothetical assumption that the use of abatacept would be increased by 2% in the overall RA population was made.
The total budget in each market scenario was calculated as the sum of anti-CCP test cost and the total treatment cost for the target patient population. The budget impact of increasing anti-CCP testing and the uptake of abatacept for patients with high ACPA titer was calculated by taking the difference in total annual costs between the two market scenarios. In addition to the total budget impact, findings were also reported on a per member per year (PMPY), per member per month (PMPM), per patient per year (PPPY), and per patient per month (PPPM) basis.
Model parameters
Population
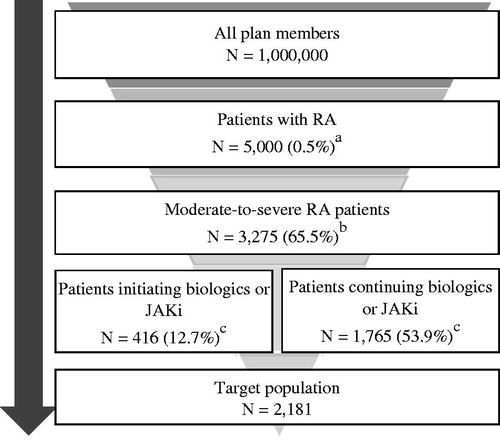
The target population for this analysis was adult US patients with moderate-to-severe RA eligible for the initiation or continuation of treatment with biologic or JAKi. An annual RA prevalence rate of 0.5%Citation19 was applied to the hypothetical 1 million member health plan and estimated that 65.5% of RA patients had moderate-to-severe disease activityCitation20. We estimated 88.4% of patients used disease-modifying anti-rheumatoid drug (DMARD) therapyCitation21, of whom 39% received conventional synthetic DMARD (csDMARD) and 61% received non-csDMARD therapyCitation22. We assumed that non-csDMARD patients had already started a biologic or JAKi therapy whereas patients with inadequate response to csDMARD (36.9% of users)Citation22 would switch to non-csDMARDs for the first time. Thus, we estimated 12.7% of moderate-to-severe RA patients were initiating biologics or JAKi and 53.9% were experienced biologic or JAKi users. summarizes the inputs used to estimate the target population.
Figure 1. Derivation of target population. aAssuming 0.5% RA prevalenceCitation19. bAssuming 65.5% of RA patients have moderate to severe disease activityCitation20. cAssuming 88.4% of patients use DMARD therapyCitation21, of whom 39% use csDMARD and 61% use non-csDMARDCitation22. 88.4% × 61% = 53.9%; 88.4% × 39% × 36.9% csDMARD non-responderCitation22 = 12.7%. Abbreviations. csDMARD, conventional synthetic disease-modifying antirheumatic drug; DMARD, disease-modifying antirheumatic drug; JAKi, Janus kinase inhibitor; RA, rheumatoid arthritis.
The average weight of a patient was assumed to be 80.4 kg, calculated as the weighted average of US female and male patient weight, with gender distribution sourced from an RA studyCitation19,Citation23.
High ACPA titer
Given the limited understanding on clinical indication of ACPA titers, the threshold of high titer of ACPA is not clearly specified in the practice. Therefore, in this analysis, high ACPA titer was defined as 250 AU/mL or greater, which was the cutoff value of the Q4 ACPA distribution in a chart review study for biomarkersCitation24. Using this definition, 24.9% of the target population would have high ACPA titer and the remainder would be categorized as low ACPA titerCitation24.
Market share
The analysis included both intravenous and subcutaneous forms of abatacept as well as other treatment options including JAKi, TNFi biologics/biosimilars, and other non-TNFi biologics currently available and in use in the US market. lists all treatment options considered in the analysis and the overall market shares in both market scenarios. In Scenario 1, market shares represent the current use of biologics or JAKiCitation25 and are assumed to be the same regardless of ACPA status. In Scenario 2, the low ACPA titer group was assumed to have the same market shares as Scenario 1; for the high ACPA titer group, it was assumed that market share increased for abatacept by 2% overall and decreased for all other medications proportionally to their market shares in Scenario 1. The 2% increase in abatacept in Scenario 2 was applied to both intravenous and subcutaneous abatacept products, proportional to their market shares in Scenario 1.
Table 1. Treatment options and market share scenarios.
Anti-CCP testing costs
A one-time cost of $94.83 was applied to all patients receiving anti-CCP testing, consisted of a $14.39 clinical lab fee (CPT: 86200)Citation26 and a $80.44 physician fee (CPT: 99211-99215)Citation27.
Medication costs
Wholesale acquisition costs (WAC) in the year 2019 were used for medication costsCitation28. The prescribing information for each product was referenced to estimate the number of doses needed separately for newly initiating patients and experienced patients over a 1-year course assuming complete adherence to the medication. Medication costs (in 2019 US dollars) and prescribing information for each drug are presented in . The amount of medication needed was rounded up in the calculation to reflect no vial sharing. Methotrexate and other concomitant drug costs were not considered, since they would be similar across all treatment arms.
Table 2. Medication costs (2019 US Dollars).
In the base case, a 30% discount was applied to WAC cost for adalimumab and etanercept to reflect real-world rebate pricing.
Administration costs
For intravenous medications, administration fees were added. For each initial hour of infusion, there was a cost of $143.08 (HCPCS: 96413) and each additional hour of infusion cost $30.99 (HCPCS: 96415)Citation26. The number of infusions over one year and the number of hours infused at each visit were estimated from the prescribing information. The number of hours infused at each visit was rounded up. Cost of administration or dispensing fee was not considered for subcutaneous or oral products.
Scenario analyses
We conducted scenario analyses on target population and rebates. In the base case, the target population was all patients on biologics or JAKi regardless of their prior exposure to those medications. As a scenario analysis, we evaluated the budget impact for biologic or JAKi-experienced patients only, as well as for naïve patients only. Market shares by previous use of biologic or JAKi status are shown in Supplementary Table S1.
Additionally, we varied the products for receiving rebates. In the base case scenario, 30% rebates were applied to adalimumab and etanercept. In additional scenario analyses, we evaluated scenarios with (1) 30% rebates for all TNF inhibitors and (2) 30% rebates for all TNF and JAKi.
Results
Based on the epidemiological data, in a hypothetical 1 million-member health plan, 3,275 patients were estimated to have moderate-to-severe RA, of whom 2,181 patients would be on a biologic or JAKi treatment.
In the base case with 30% rebates for adalimumab and etanercept, the total estimated budget for the 2,181 patients in Scenario 1 was $102,040,450 (anti-CCP test cost: $186,155; treatment cost: $101,854,295). In Scenario 2, with additional 2% patients receiving abatacept, the anti-CCP test cost budget increased by $20,684; with a higher number of patients on abatacept (n = 63), treatment cost increased by $160,467 totaling an overall budget increase of $181,151. This was equivalent to a PMPM increase of $0.015 ().
Table 3. Base case results: 30% rebate for adalimumab and etanercept, overall patients (2019 USD).
The budget impact results were consistently negligible across the scenario analyses (). When only biologic or JAKi-naïve patients were considered, the total cost difference was less than $49,000 with a PMPM impact of $0.004. When considering biologic or JAKi-experienced patients only, the PMPM was $0.011.
Table 4. Scenario analyses results (2019 USD).
With a 30% rebate considered for all TNF inhibitors, the total cost difference was $322,504 with a PMPM difference of $0.027. When the same rebate was applied to all TNF and JAKi, the total budget impact difference increased to $396,750 with a PMPM cost difference of $0.033.
Discussion
There has been an effort to discover biomarkers that can effectively guide and improve treatment selection in RA. Studies have shown the clinical benefits of using abatacept compared to other biologics among patients with high ACPA titer, including higher response rate and treatment retentionCitation14–16,Citation29–32. Given such clinical evidence, the goal of this study was to understand the economic impact of increasing the use of anti-CCP testing to guide treatment selection for patients with moderate-to-severe RA on biologics or JAKi and increasing the use of abatacept for those with high ACPA titer. Results from our study showed that the budget impact was a negligible $0.015 PMPM for a hypothetical health plan with one million commercially insured adults in the US. The scenario analyses confirmed the robustness of the results and continued to predict negligible budget impact with varied parameters. Even with higher rebates for other RA agents, the difference in PMPM was consistently less than $0.04. Our results suggest that the budget impact of increasing the use of anti-CCP testing and adding more patients on abatacept will have a minimal budget impact while potentially treating patients more effectively with abatacept.
Several studies have incorporated the clinical benefits of abatacept among ACPA positive patients who had inadequate response to methotrexate to evaluate the cost-effectiveness of abatacept compared to adalimumab. In a cost per responder/remission study, the monthly cost per responder/remission was always lower for ACPA positive patients using abatacept compared to adalimumab regardless of the definition used for responder (i.e. ACR [American College of Rheumatology]20, ACR50, ACR70, ACR90, HAQ-DI [Health Assessment Questionnaire-Disability Index]) or remission (i.e. DAS[disease activity score]28, CDAI, SDAI [Simple Disease Activity Index]) in Germany (−€394 versus −€9,682 per month) and Spain (−€169 versus – €5,422)Citation33. The same study found similar results for Italy, Canada, and the US, favoring abatacept for ACPA positive patients, except for ACR50 response and DAS28 remission, in which there was a small increase in the monthly cost per responder/remission compared to ACPA positive patients treated with adalimumab (ranging from €5 in Italy to $488 in the US). This study included costs for drugs, concomitant drugs, disease monitoring, and adverse events. Another study developed a decision tree model to compare the cost-effectiveness of abatacept to adalimumab over 2 years in Germany among the same target population as the previous cost per responder/remission studyCitation34. The model allowed switching to second-line and third-line of biologic therapy for patients who do not respond to the first line abatacept or adalimumab. The study found that the selection of first-line biologic (i.e. abatacept or adalimumab) impacted the cost per day in remission, with a lower average cost with abatacept compared to adalimumab (€330/day versus €384/day) whereas the choice of second- or third-line biologic had insignificant economic impact. Alemao et al. conducted a cost-effectiveness study comparing abatacept to adalimumab that incorporated HAQ-DI score to calculate quality-adjusted life years (QALYs) from a United Kingdom (UK) payer perspective and stratified the results by ACPA quartile. In all quartiles, the treatment cost with abatacept was higher than adalimumab but QALY was higher with abatacept treatment. The authors concluded that with incremental cost effectiveness ratio of £6,200/QALY for the highest ACPA titer group, abatacept is an effective alternative to adalimumab in patients with high ACPA levels. It is important to note that all these studies referenced the AMPLE trial as the efficacy data source. All studies had different study designs and endpoints but consistently found that abatacept is a cost-effective treatment choice despite the higher drug cost, due to its superior clinical efficacy among ACPA-positive patients.
As a chronic disease, the goal of RA treatment is achieving sustained remission. Due to medical advancement, there are many biological treatment options with different mechanism of actions available today. Given the variety of RA treatments, however, patients are often administered therapies on a trial-and-error basis, with dose escalation, combination therapies, and therapy switch, before finding an optimal treatment therapyCitation35. This can lead to a greater cost of switching medications, lower efficacy of the therapy, extra health care utilization and cost, and potentially lower quality of life for patientsCitation36–39. These negative implications highlight the importance of selecting the optimal treatment early on in the treatment course.
The findings from this study help enhance our understanding of the pharmacy budget impact of increased anti-CCP testing and abatacept use. However, several limitations should be considered when interpreting the results. First, the analysis only considers testing cost and medication costs (i.e. drug cost and intravenous administration fee). Concomitant medications and drug monitoring tests were not considered, which indicate that the total budget in both market scenarios may have been underestimated. However, these costs are typically low and would be similar between the two scenarios; therefore, excluding these costs would not have had a significant impact on the results. Next, some parameters used in the analysis, such as the rebate rates, are not generalizable and health plan-specific. To mitigate this limitation, we conducted scenario analyses around target population and products with rebates, which showed consistent results. In addition, more low-cost biosimilars are being approved in the US for RA, and with higher market shares of biosimilars the results may changeCitation40. Currently, however, not all approved biosimilars are commercialized in the US and the penetration of biosimilars have been limited. Given their slow uptake, it may take some time for biosimilars to have any meaningful impact on the results of this analysis. Finally, the uptake of abatacept was assumed to increase by 2% in Scenario 2; this was an assumption and varying this value will impact the results.
Conclusion
In conclusion, our analysis shows that testing RA patients to learn their ACPA status and increasing use of abatacept among high-titer ACPA patients results in a small increase in the total budget (<2 cents PMPM). Given that patients with high ACPA titer values respond well to abataceptCitation14, considering anti-CCP testing for precision medicine and treating ACPA positive patients with abatacept may be a valuable investment.
Transparency
Declaration of funding
The study was funded by Bristol-Myers Squibb (US), Inc.
Declaration of financial/other relationships
XH and FL are employees of Bristol-Myers Squibb (US), Inc. SP, DP, and DK are employees of Pharmerit International, which received consultancy fees from Bristol-Myers Squibb (US), Inc. for this study. FL is a stock shareholder of Bristol-Myers Squibb (US), Inc.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Supplemental Material
Download MS Word (40.2 KB)Acknowledgements
None reported.
References
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69(3):89–95.
- Strimbu K, Tavel JA. What are biomarkers? Curr Opin HIV AIDS. 2010;5(6):463–466.
- Oderda GM, Lawless GD, Wright GC, et al. The potential impact of monitoring disease activity biomarkers on rheumatoid arthritis outcomes and costs. Per Med. 2018;15(4):291–301.
- Robinson WH, Mao R. Biomarkers to guide clinical therapeutics in rheumatology? Curr Opin Rheumatol. 2016;28(2):168–175.
- Kurowska W, Kuca-Warnawin EH, Radzikowska A, et al. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent Eur J Immunol. 2017;42(4):390–398.
- Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581.
- van der Helm-van Mil AH, Verpoort KN, Breedveld FC, et al. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res Ther. 2005;7(5):R949–58.
- Ajeganova S, Humphreys JH, Verheul MK, et al. Anticitrullinated protein antibodies and rheumatoid factor are associated with increased mortality but with different causes of death in patients with rheumatoid arthritis: a longitudinal study in three European cohorts. Ann Rheum Dis. 2016;75(11):1924–1932.
- Shafrin J, Tebeka MG, Price K, et al. The economic burden of ACPA-positive status among patients with rheumatoid arthritis. J Manag Care Spec Pharm. 2018;24(1):4–11.
- Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26.
- Dekkers JS, Bergstra SA, Chopra A, et al. Autoantibody status is not associated with early treatment response to first-line methotrexate in patients with early rheumatoid arthritis. Rheumatology (Oxford). 2019;58(1):149–153.
- Jonsson MK, Hensvold AH, Hansson M, et al. The role of anti-citrullinated protein antibody reactivities in an inception cohort of patients with rheumatoid arthritis receiving treat-to-target therapy. Arthritis Res Ther. 2018;20(1):146.
- van den Broek M, Dirven L, Klarenbeek NB, et al. The association of treatment response and joint damage with ACPA-status in recent-onset RA: a subanalysis of the 8-year follow-up of the BeSt study. Ann Rheum Dis. 2012;71(2):245–248.
- Schiff M, Weinblatt ME, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2014;73(1):86–94.
- Harrold LR, Litman HJ, Connolly SE, et al. Effect of anticitrullinated protein antibody status on response to abatacept or antitumor necrosis factor-alpha therapy in patients with rheumatoid arthritis: a US national observational study. J Rheumatol. 2018;45(1):32–39.
- Harrold LR, Litman HJ, Connolly SE, et al. Comparative effectiveness of abatacept versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis who are anti-CCP positive in the United States Corrona registry. Rheumatol Ther. 2019;6(2):217–230.
- Bozec A, Luo Y, Engdahl C, et al. Abatacept blocks anti-citrullinated protein antibody and rheumatoid factor mediated cytokine production in human macrophages in IDO-dependent manner. Arthritis Res Ther. 2018;20(1):24.
- Bapat B, Klink AJ, Kaufman J, et al. ACPA testing and resultant treatment patterns in patients with rheumatoid arthritis: findings from U.S. community rheumatology practices. ACR/ARP Annual Meeting; Nov 8–13; Atlanta, Georgia. 2019.
- Hunter TM, Boytsov NN, Zhang X, et al. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. 2017;37(9):1551–1557.
- Decision Resources Group. Pharmacor immune and inflammatory disease: rheumatoid arthritis; 2015. Available from: https://decisionresourcesgroup.com/report/246098-biopharma-rheumatoid-arthritis-2015/
- National Committee for Quality Assurance. DMARD treatment rate: disease-modifying anti-rheumatic drug therapy for rheumatoid arthritis; 2016. Available from: http://www.ncqa.org/report-cards/health-plans/state-of-health-care-quality/2016-table-of-contents/dmards
- Ferrufino CP, Munakata J, Wei W, et al. Budget impact analysis of sarilumab for the treatment of rheumatoid arthritis in patients with an inadequate response to conventional synthetic DMARD or TNF inhibitor therapies. Clinicoecon Outcomes Res. 2018;10:805–819.
- Fryar CD, Kruszon-Moran D, Gu Q, et al. Mean body weight, height, waist circumference, and body mass index among adults: United States, 1999-2000 through 2015-2016. Natl Health Stat Report. 2018;(122):1–16.
- BMS Internal Study. Real-world distribution of anti-cyclic citrullinated peptide concentrations from a US community rheumatology clinics chart review study. Internal data.
- Bristol-Myers S. Symphony market share research. Internal data.
- CMS Clinical Diagnostic Laboratory Fee Schedule 2019 [cited 2019 Mar 11]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html
- CMS Physician Fee Schedule 2019 [cited 2019 Mar 11]. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
- Micromedex® (electronic version). IBM Watson Health, Greenwood Village, Colorado, USA [cited 2019 Mar 11]. Available from: https://www.micromedexsolutions.com/
- Alten R, Nüßlein HG, Mariette X, et al. Baseline autoantibodies preferentially impact abatacept efficacy in patients with rheumatoid arthritis who are biologic naive: 6-month results from a real-world, international, prospective study. RMD Open. 2017;3(1):e000345.
- Gottenberg JE, Courvoisier DS, Hernandez MV, et al. Brief report: association of rheumatoid factor and anti-citrullinated protein antibody positivity with better effectiveness of abatacept: results from the Pan-European registry analysis. Arthritis Rheumatol. 2016;68(6):1346–1352.
- Gottenberg JE, Ravaud P, Cantagrel A, et al. Positivity for anti-cyclic citrullinated peptide is associated with a better response to abatacept: data from the ‘Orencia and Rheumatoid Arthritis’ registry. Ann Rheum Dis. 2012;71(11):1815–1819.
- Nüßlein HG, Alten R, Galeazzi M, et al. Efficacy and prognostic factors of treatment retention with intravenous abatacept for rheumatoid arthritis: 24-month results from an international, prospective, real-world study. Clin Exp Rheumatol. 2016;34(3):489–499.
- Weijers L, Baerwald C, Mennini FS, et al. Cost per response for abatacept versus adalimumab in rheumatoid arthritis by ACPA subgroups in Germany, Italy, Spain, US and Canada. Rheumatol Int. 2017;37(7):1111–1123.
- Neubauer AS, Minartz C, Herrmann KH, et al. Cost-effectiveness of early treatment of ACPA-positive rheumatoid arthritis patients with abatacept. Clin Exp Rheumatol. 2018;36(3):448–454.
- Lindstrom TM, Robinson WH. Biomarkers for rheumatoid arthritis: making it personal. Scand J Clin Lab Invest Suppl. 2010;242:79–84.
- Gu T, Mutebi A, Stolshek BS, et al. Cost of biologic treatment persistence or switching in rheumatoid arthritis. Am J Manag Care. 2018;24(8 Spec No):SP338–SP345.
- Rendas-Baum R, Wallenstein GV, Koncz T, et al. Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-alpha inhibitors. Arthritis Res Ther. 2011;13(1):R25.
- Vanderpoel J, Tkacz J, Brady BL, et al. Health care resource utilization and costs associated with switching biologics in rheumatoid arthritis. Clin Ther. 2019;41(6):1080–1089 e5.
- Zhang J, Shan Y, Reed G, et al. Thresholds in disease activity for switching biologics in rheumatoid arthritis patients: experience from a large U.S. cohort. Arthritis Care Res. 2011;63(12):1672–1679.
- US Food and Drug Administration. Center for Drug Evaluation and Research list of licensed biological products with (1) reference product exclusivity and (2) biosimilarity or interchangeability evaluations to date [cited 2010 Jan 29]. Available from: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/UCM560162.pdf
