Abstract
Aim: To describe the characteristics and medication treatment patterns, healthcare resource utilization (HRU), and associated costs in Japanese patients with systemic lupus erythematosus (SLE).
Methods: Claims data from the Japan Medical Data Center (JMDC) database were used to identify patients with SLE-related claims from 2010 to 2017. Algorithms were developed to retrospectively categorize patients by disease severity, treatment experience, and SLE-related manifestations such as lupus nephritis and central nervous system lupus. Descriptive and multivariate analyses were used to describe treatment pattern and estimate HRU and associated costs for the SLE cohort overall and by disease severity and complications.
Results: Among 4,733 eligible patients, 2,072 (43.8%) were treatment naïve, 2,214 (46.8%) were previously treated for SLE, and 447 (9.4%) did not receive any treatment. Mean (SD) age of the total SLE cohort was 45.2 (13.1) years and mean (SD) follow-up duration was 1,137.3 (758.0) d. Based on disease severity, 1,383 (29.2%) patients had mild, 2,619 (55.3%) patients had moderate, and 731 (15.4%) patients had severe SLE. Patients on glucocorticoids (both oral and parenteral) received add-on medications the most (35.5%, p < .001). Mean annual cost per SLE patient in the post-index period, inclusive of hospitalizations, outpatient visits, and pharmacy was ¥436,836; ¥1,010,772; and ¥2,136,780 for patients with mild, moderate, and severe SLE, respectively.
Limitations: The database only captured information on patients up to 75 years of age. Due to the nature of the database, biases regarding conditions that attribute to the spectrum of SLE severity, flare incidences, or individual physical status cannot be ruled out.
Conclusions: This study describes the treatment patterns and economic burden experienced by Japanese patients with SLE. The results highlight an unmet need to derive better treatment strategies for patients with SLE to effectively address the disease burden in Japan.
Introduction
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disorder characterized by fluctuating clinical manifestations such as recurrent flares (exacerbations) and subsequent remissions, and could progressively inflict organ damage in the bodyCitation1. Although research has substantially added to existing knowledge regarding the pathology of SLE, there is currently no cure for SLE, resulting in life-threatening scenarios when major organs are affectedCitation2.
The estimated global prevalence of SLE varies from 21 per 100,000 in Canada to 25–91 per 100,000 in European countriesCitation3. In Japan, the prevalence of SLE ranges from 3.7 to 37.7 per 100,000Citation4. Globally, SLE has been reported to occur in both males and females; however, females are predominantly affectedCitation5. Moreover, there is evidence of poor health-related quality of life in patients with SLE compared with other rheumatic diseasesCitation6 and this is persistent over long-term follow-upCitation7,Citation8. Although this multifactorial disease is associated with significant morbidity and mortalityCitation9,Citation10, the survival rate of patients with SLE has increased with improvements in diagnosis and therapyCitation11.
The British Society for RheumatologyCitation12 and The European League Against Rheumatism (EULAR)Citation13 recommend initiating SLE management with hydroxychloroquine and glucocorticoids before moving on to immunosuppressants, and eventually biologics such as rituximab or belimumab for refractory patients. Similar guidelines for the management of SLE do not exist in Japan. However, Tanaka et al.Citation14 after retrospectively analyzing claims data of Japanese patients who were diagnosed with SLE between April 2010 and March 2012 and had follow-up data for 3 years, reported corticosteroids to be the mainstay of SLE therapy in Japan, with other medications – except hydroxychloroquine – being utilized more or less in line with the guidelines of other countries.
The economic impact of SLE is two-fold, including both cost of treatment and productivity losses due to complications of SLE, as 25–60% of patients develop renal disease over time and may require expensive treatments and procedures such as dialysis and a transplantCitation15–17. Tanaka et al.Citation14 reported that all patients with SLE in Japan had at least one outpatient visit, while 39.3% had been hospitalized at least once. The mean total all-cause direct medical cost per patient over 3 years was ¥2,913,509 with costs rising proportionally to disease severity. However, it is difficult to understand long-term outcomes and costs due to the episodic and recurring nature of SLECitation18.
Limited evidence exists in the literature with respect to comorbid health and physical conditions, treatment patterns persistency, and rates of switching to or augmentation with alternative therapiesCitation14,Citation19,Citation20. The latest studyCitation14 evaluating the above outcomes assessed claims data for a maximum of 3 years from the index diagnosis period (2010–2012). Moreover, the number of patients who met the inclusion criteria was small (n = 295). Therefore, to provide an update and address existing knowledge gaps, this retrospective cohort study analyzed insurance claims data to describe the treatment patterns, healthcare resource utilization (HRU), and associated costs in Japanese patients with SLE.
Methods
Study design and data source
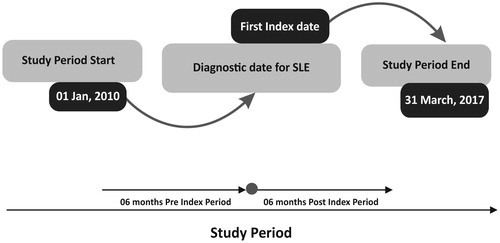
This retrospective observational cohort study analyzed claims data retrieved from the Japan Medical Data Center (JMDC) database between 1 January 2010 and 31 March 2017 (). The database has been capturing patient information and diagnosis from large samples of primary and secondary care physicians in Japan comprising approximately 4% of the total population of Japan (5.6 million) since January 2005Citation21. It contains inpatient, outpatient, and pharmacy administrative claims and has been widely used in health services researchCitation22–25. Patient medical consultation information can be continuously tracked in a chronological order, even if the patient attends multiple medical institutions or transfers to another hospital, as long as the individual belongs to the Health Insurance Association.
Figure 1. Study design. A summary of extracting SLE cohort by applying an algorithm when working with data from claims database. Abbreviation. SLE, systemic lupus erythematosus.
The information retrieved from JMDC database was anonymized to protect patient privacy under the “Act on the Use of Numbers to Identify a Specific Individual in the Administrative Procedure”. This study was approved by the sponsor’s internal approval committee in accordance with Japanese ethical and legal guidelines for Japan. The informed consent of patients was not required as per the Ethical Guidelines for Epidemiological Research issued by the Japanese Ministry of Health, Labor, and Welfare (MHLW). Reporting of claims data was based on International Society for Pharmacoeconomics and Outcomes Research guidelinesCitation26.
Patient selection
The index date was identified as the earliest date observed of the medical claim with a diagnosis of SLE. To categorize SLE patients naive to treatment from the treatment experienced group, a “wash out” period was defined for treatment naive SLE patients by retracing back at least 6 months from the index date to confirm that no treatment was received prior to the index date.
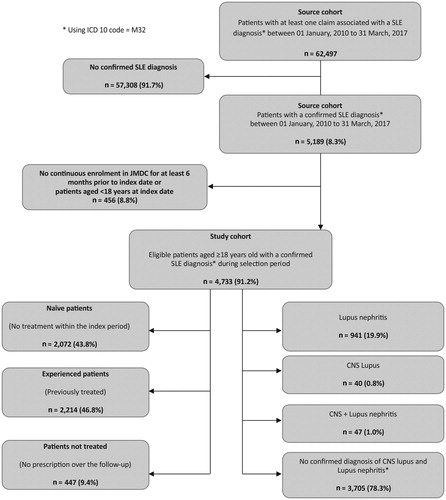
Patients aged ≥18 years at index date with a confirmed SLE diagnosis i.e. at least two International Classification of Diseases, 10th revision diagnosis codes M32.1Citation27, at least 6 months of continuous insurance coverage/enrollment before the index date, and at least 6 months of continuous insurance coverage/enrollment after the index date to ensure adequate follow-up on study measures were included. All eligible patients with or without comorbidities (e.g. mononeuropathy/polyneuropathy, other renal impairment, seizure, pleurisy/pleural effusion, arterial/venous thrombosis, aortitis, stroke/transient ischemic attack, and nephrotic syndrome) during study period were included. Patients who did not have a confirmed diagnosis of SLE, had other complications not related to SLE, were enrolled in the database for less than 6 months prior to or after the index date, or did not have any SLE treatment within the database during follow-up were excluded from the study ().
Study measures
All demographic characteristics in the available patient’s claims data set were documented including age and sex. The SLE cohort extracted from the claims database was divided into three groups: sub-cohort of SLE with organ and system involvement, sub-cohort of SLE prescribed regimen, and sub-cohort of SLE severity.
Sub-cohort of SLE with organ and system involvement included patients with a confirmed i.e. at least two diagnoses of lupus nephritis (but no central nervous system [CNS] lupus), patients with a confirmed i.e. at least two diagnoses of CNS lupus (but no lupus nephritis), patients with confirmed i.e. at least two diagnoses each of lupus nephritis and CNS lupus, and patients without a confirmed diagnosis of lupus nephritis or CNS lupus.
Sub-cohort of SLE prescribed regimen included patients naive to treatment regimen and experienced patients with treatment regimen of interest. Prescriptions of drugs were retrieved using the JMDC Drug table where all drug prescriptions were recorded and identified according to the Anatomical Therapeutic Chemical (ATC) codes. Treatment naive patients were defined as patients with SLE who did not have any record of treatment claims within the pre-index period. In a given line of treatment (LoT), analyses of treatment-related events, and HRU was performed by type of SLE regimen prescribed by the drug classes. These included topical steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), immunosuppressants/immunomodulators (including methotrexate, azathioprine, and mycophenolate mofetil), glucocorticoids (oral or parenteral), and hydroxychloroquine.
Patients in the sub-cohort of SLE severity were categorized based on disease severity as patients with severe, moderate, or mild disease. Disease severity was determined using a proxy algorithm defined based on steroid dosage, immunosuppressant use, or appearance of SLE-related symptoms. The SLE severity algorithm was determined by using a study by Garris et al.Citation28 and in reference to EULAR recommendations for management of SLECitation13,Citation28,Citation29 along with inputs from Japanese clinicians. Severe SLE patients were those who had at least one prescription for cyclophosphamide or rituximab or oral glucocorticoid with a prednisone equivalent dose higher than 60 mg/d, or at least one claim with a diagnosis listed as ‘severe’; and had severe conditions such as acute confusional state/psychosis, aortitis, arterial/venous thrombosis, aseptic meningitis, cardiac tamponade, cranial neuropathy, intestinal pseudo-obstruction, end-stage renal disease, optic neuritis, pulmonary hemorrhage, and stroke or transient ischemic attack. Moderate SLE patients were those with absence of “severe” disease; at least one filled prescription for an oral glucocorticoid with a prednisone-equivalent dose between 7.5 and 60 mg/d or for an immunosuppressive agent (other than cyclophosphamide), or at least 1 claim with a diagnosis of a condition listed as “moderate”; and had moderate conditions such as acute pancreatitis, chorioretinitis, demyelinating syndrome/acute inflammatory demyelinating polyradiculoneuropathy, episcleritis/scleritis, hemolytic anemia, hepatitis (non-viral), ischemic necrosis of bone, nephritis, renal impairment other than nephritis or end-stage renal disease, lupus enteritis/colitis, mononeuropathy/polyneuropathy, myelopathy, myocarditis, pericarditis, pleurisy/pleural effusion, pseudotumor cerebri, seizure, and uveitis vasculitis (excluding aortitis). Mild SLE patients were those who did not have moderate or severe disease.
Identification of treatment patterns
An algorithm was developed to detect start dates and end dates of LoTs of SLE therapy for each patient based on several assumptions. This algorithm converted all prescriptions of treatment for SLE into a set of treatment sequences using a succession of operations which included selection of prescriptions of interest, defining theoretical end of prescriptions, and identifying treatment sequences and combination therapies.
Prescriptions for SLE were retrieved using the JMDC Drug table where all drug prescriptions were recorded and identified according to ATC codes. The following treatment prescriptions were considered: topical steroids, NSAIDs, glucocorticoids (oral or parenteral), immunosuppressants/immunomodulators (including methotrexate, azathioprine and mycophenolate mofetil, and cyclophosphamide), hydroxychloroquine, and biologic therapies. See Appendix A for a list of drugs identified.
For topical therapy, one prescription was assumed to last for 14 d. If the prescription date was missing, approximation was performed using the date of the related claim. Certain assumptions were made for treatment overlaps. It was assumed that a treatment overlapping between topical therapy and other SLE LoT would not be considered as a period of concomitant therapy. Thus, it was assumed that: (a) patients would have stopped topical therapy when oral therapy would have started and (b) an overlap between topical glucocorticoids and other types of SLE treatment could occur because the discontinuation of glucocorticoid treatment required progressive dose tapering.
Daily defined dosages were used as the reference to detect start and end dates of a LoT i.e. duration of therapy, for each patient. Moreover, for overlap between topical therapy and other SLE therapy, it was assumed that if an overlap period of longer than 14 d was observed between two therapies that would be considered as combination therapy. A LoT was considered as “discontinued” if there was no prescription renewal for a period longer than the maximum allowed gap duration, also termed the “grace period”. A grace period of 60 d was used.
Treatment-related events of SLE therapies
Once the start and end dates of a LoT were determined, patients with LoT duration not exceeding the follow-up period were categorized as (temporarily) discontinued treatment, switched to other treatments, or having an add-on or a reduction in SLE therapy.
Treatment (temporarily) stop/discontinuation – Patients were defined to have (temporarily) stopped treatment at the end of a LoT if no SLE drug category was prescribed within the specified “grace period” after the end of the LoT.
Add-on therapy – The start of a new line was defined as an add-on therapy if SLE drug categories prescribed in the previous line were still prescribed in the new LoT and one or several new SLE drug categories, not prescribed in the previous line, were initiated. In addition, the period between the end of the previous line and the start of the new line was shorter than the specified “grace period”.
Switch to another therapy – The start of a new LoT was defined as switching if one or several new SLE drug categories prescribed in the previous treatment-line were no longer prescribed in the new LoT and one or several new SLE drug categories, not prescribed in the previous line, were initiated in the new line. The period between the end of the previous LoT and the start of the new LoT was shorter than the specified “grace period”.
Treatment reduction – The start of a new LoT was defined as a reduction if one or several new SLE drug categories prescribed in the previous treatment-line were no longer prescribed in the new LoT and no new SLE drug categories were prescribed in the new line. The period between the end of the previous line and the start of the new line was shorter than the specified “grace period”.
Continuous use of the LoT drugs (censored) – Continuous use of LoT SLE drug categories was defined as no switch, stop/discontinuation, add-on or reduction in treatment until end of follow-up.
Healthcare resource utilization
As HRU is influenced by factors such as the local healthcare system, number of specialists, and ease of healthcare access, understanding the existing healthcare system before analyzing HRU is essential. Japan’s healthcare system has been well explained by Zhang et al.Citation30 and Sakamoto et al.Citation31
In addition to the treatment pattern measures, specific HRU measures were assessed for each patient. These measures included outpatient/physician office visit, hospitalization, and pharmacy claims. The proportion of patients with at least one occurrence for each LoT was calculated and reported by type of resource utilized. Each LoT identified had at least one occurrence and the number of occurrences per patient-time, for each of the healthcare resource categories. The numbers of occurrences per patient-time were calculated by dividing the number of occurrences by the duration of the LoT. For the number of resources used per person per month, the average number of occurrences per person-time were calculated during the different LoTs and reported by type of resource utilized and treatment regimen.
These proportions of patients with SLE were estimated using a Kaplan–Meier survival method in terms of measuring the length of time patients continued treatment or medical resource used before discontinuing or without event occurrence at last follow-up (e.g. censored data). First occurrence for the healthcare resource category of interest was the target event and censoring if the patient reached the end of follow-up period without any claim related to this type of resource.
For inpatient stays, the length of stay was described for patients with at least one inpatient stay including number of inpatient days shown by mean and its standard deviation (SD).
Medical cost evaluation
Cost data were extracted from the JMDC database and were reported as Japanese yen (¥). An overall cost per person-time was described by type of healthcare resource costs, treatment regimen costs, and costs related to SLE severity levels. The average cost per person-month was calculated and reported by type of resource utilized for the follow-up period. Patients contributed to these rates only when they were alive and in the health plan. Costs were not adjusted for inflation as Japan has a low inflation rate and prices have been flat for the last decadeCitation32. The low inflation rate is also one of the drivers for the expansive monetary policy of the Bank of Japan in recent years. Additionally, long-term interest rates (which could be used for discounting) are close to 0Citation33.
Statistical analysis
Data for all study measures were summarized descriptively as mean values, SD, median, and range for continuous variables and frequency distribution for categorical variables. Proportions were compared statistically using one-tailed two-sample proportion Z-test while continuous variables were compared statistically using two-sample T-test. Kaplan–Meier analyses were used to examine time to treatment-related events of interest such as time to switch to different treatment class or time to treatment discontinuation. All statistical analyses were conducted using SAS version 9.3 software (SAS Institute Inc., Cary, NC).
Results
Patient demographics
A total of 62,497 patients had at least one claim for SLE diagnosis in the study period and 4,733 patients among them fulfilled the inclusion criteria. The demographic characteristics of these patients are summarized in .
Table 1. Baseline demographics and comorbidities of patients with SLE (by Severity Level) during selection period.
Among the 4,733 included patients, 2,072 (43.8%) were treatment naïve with no treatment within the pre-index period, 2,214 (46.8%) were previously treated for SLE, and 447 (9.4%) were those who did not receive any treatment. Mean (SD) age of total SLE cohort was 45.2 (13.1) years and mean (SD) follow-up duration was 1,137.3 (758.0) d. Majority of the patients with SLE (78.5%) were females, with the incidence ranging from 21.8% to 24.5% in child bearing age of 18–45 years. Based on insurance status, 58.7% were dependents. In terms of disease severity level, 1,383 (29.2%) of the patients had mild, 2,619 (55.3%) had moderate, and 731 (15.5%) had severe SLE. Among treatment naive patients, 573 (27.7%) had mild, 114 (53.8%) had moderate, and 385 (18.6%) had severe SLE. Based on organ and system involvement, the sub-cohort included 941 (19.8%) patients with lupus nephritis, 40 (0.8%) with CNS lupus, 47 (1.0%) with both lupus nephritis and CNS lupus while 3,705 (78.3%) had no confirmed diagnosis of CNS lupus or lupus nephritis (Appendix B).
When comorbid conditions were determined using Charlson comorbidity index (CCI) as a reference, the mean (SD) CCI was 1.0 (1.6) and 802 (16.9%) patients had at least one comorbidity of interest at the baseline. About 6.1% of the patients were recorded with mononeuropathy or polyneuropathy, 6.0% had nephrotic syndrome, and 2.8% had arterial thrombosis.
Treatment patterns
Japanese patients with SLE were prescribed various types of treatment during the post-index period, as outlined in . The treatment patterns mainly comprised topical steroids, NSAIDs, glucocorticoids, immunosuppressants, and hydroxychloroquine. In treatment naïve patients with SLE, NSAIDs (31.9%) and glucocorticoids (both oral and parenteral; 31.0%) were largely used as the first LoT; with a similar pattern continuing up to the final LoT. Significantly higher NSAID (31.9% vs. 28.5%, p < .05) and glucocorticoid (31.0% vs. 19.0%, p < .001) utilization was observed in the first LoT than the second. However, the next LoTs did not show any significant differences. Parenteral glucocorticoids were administered to patients who had been prescribed a high dose of steroids in the first LoT. More than half of the treatment naive patients had moderate SLE in all the LoTs (Appendix C).
Table 2. Treatment regimen per treatment line in all treatment naïve patients with SLE.
Treatment-related events during therapy
Discontinuation was the most frequent treatment-related event for most of the therapies administered in every LoT. NSAIDs (75.9%) and topical steroids (73.4%) were more significantly discontinued compared with other treatments (p < .001 for all). NSAIDs were used for the shortest duration (median 7 d) while glucocorticoids were used for the longest (median 100.5 d). Switching was the second highest treatment-related event after discontinuation. Switching was significantly higher in topical steroids (16.4%) than in other treatments except hydrochloroquine (p < .001 for all) and in NSAIDs compared with glucocorticoids (11.8% vs. 7.4%, p < .001). Glucocorticoids (35.5%) and immunosuppressants (24.2%) were more significantly used as add-on treatments than others (p < .001 for all, ).
Table 3. Treatment persistence/patterns and treatment-related events according to the treatment regimen dispensed.
Distribution of glucocorticoids
Among the 4,733 patients included in the study, 3,115 (65.8%) received oral or parenteral glucocorticoids during the course of their treatment, while 2,666 (56.3%) were prescribed only oral and 1,510 (31.9%) were prescribed only parenteral glucocorticoids. High-dose glucocorticoids (>60 mg) were more likely to be administered parenterally compared with orally (36.0% vs. 1.3%, p < .001). However, oral high-dose glucocorticoids were prescribed for the longest duration (mean [SD] 13.8 [14.9] d, p < .001 compared with other routes). Low glucocorticoid dosage (<7.5 mg) was highly prescribed to patients with oral glucocorticoids (84.0%, p < .001 compared with other doses) as well as used for the highest cumulative duration per-patient (mean [SD] 1,009 [981.7] d, p < .001 compared with other doses) (). shows the distribution of the duration of oral glucocorticoid doses by calendar year. No significant changes were observed from 2010 to 2016.
Figure 3. Distribution of oral glucocorticoid doses per calendar year. Days are represented as mean.
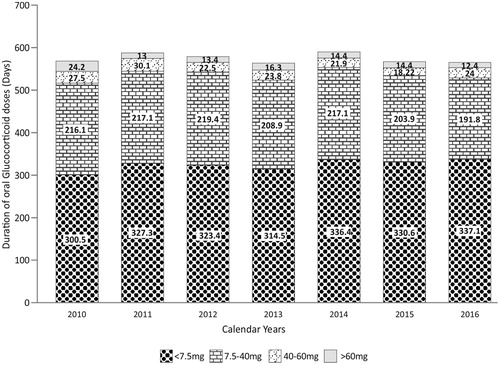
Table 4. Distribution of glucocorticoid doses after index date in patients with SLE.
Healthcare resource utilization and associated costs
At least one hospitalization was reported by 32.4% of patients overall; patients with severe SLE reported higher proportion of hospitalizations (58.1% vs. 33.0% and 17.7%, p < .001 compared with both moderate and mild groups). Irrespective of SLE severity, number of hospitalizations per-patient per-month was similar, with less than two occurrences per year. Almost every patient reported an outpatient visit (99.4%); patients with severe SLE reporting higher number of visits per-patient per-month (mean [SD] 3.7 [5.3]) compared with moderate (3.1 [4.0]) and mild groups (2.6 [2.9]) (p < .001 for both). Magnetic resonance imaging (MRI) was used more than X-rays (34.4% vs. 8.9%; ).
Table 5. Healthcare resource utilization during follow-up period.
Among treatment naïve patients, those who received glucocorticoids were hospitalized the most (21%, p < .001 compared with others), while those who received immunosuppressants reported the highest number of admissions per-patient per-month (mean [SD] 5.1 [12.2], p < .05 compared with others). More than 90% of patients receiving topical steroids, immunosuppressants, or glucocorticoids reported at least one outpatient visit. However, patients receiving NSAIDs reported the highest number of outpatient visits per-patient per-month (mean [SD] 15.2 [20.4], p < .001 compared with others). Similar to the overall cohort, MRI was used more frequently than X-rays by treatment naive patients irrespective of the administered treatment, with patients on NSAIDs reporting the highest number of X-ray visits per-patient per-month (mean [SD] 1.3 [2.2]) and MRI visits per-patient per-month (mean [SD] 1.9 [2.9]) (p < .0.01 for both, ).
Table 6. Healthcare resource utilization in treatment naïve patients by first treatment line and by treatment classes.
A greater proportion of patients with SLE and lupus nephritis were hospitalized compared to naive or patients without CNS lupus and lupus nephritis (45.8% vs. 36.1% vs. 28.2%, p < .001 for both comparisons). However, the number of hospitalizations per-patient per-month was highest in patients without CNS lupus and lupus nephritis (mean [SD] 0.08 [0.13], p < .05 compared with other groups). Almost every patient with SLE reported at least one outpatient visit; patients without CNS lupus and lupus nephritis reported the highest number of visits per-patient per-month (mean [SD] 3.15 [4.05], p < .05 compared with other groups). MRI was utilized more than X-rays in every sub-cohort, with <1 occurrence per-patient per-month for every sub-cohort (Appendix D).
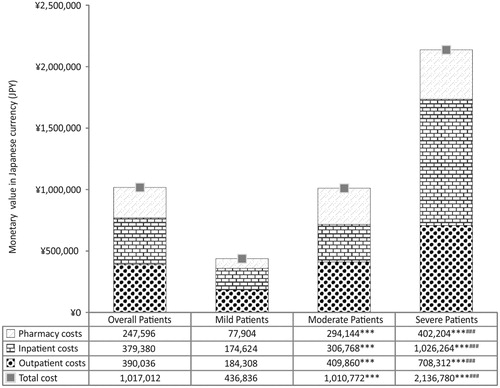
Mean (SD) annual inpatient, outpatient, and pharmacy costs per-patient for the overall cohort were ¥379,380 (¥491,587), ¥390,036 (¥388,814), and ¥247,596 (¥212,336), respectively, resulting in a total annual per-patient cost of ¥1,017,012 (¥707,221). Patients with severe SLE reported the highest expenses regardless of type of cost (p < .001 for all comparisons) while patients with moderate SLE reported higher expenses than patients with mild SLE for all types of costs (p < .001 for all). Outpatient costs were higher than inpatient costs for patients with mild and moderate SLE. However, the reverse was true for patients with severe SLE (). For informational purposes, the costs are also reported in US$ using purchasing power parity for GDP data from 2017Citation34. The reported values are not for comparison as the healthcare markets and financing systems vary across countries and regions. Mean (SD) annual inpatient, outpatient, and pharmacy costs per-patient for the overall cohort were $3,600 ($4,665), $3,701 ($3,690), and $2,350 ($2,015), respectively, resulting in a total annual per-patient cost of $9,651 ($6,711).
Figure 4. Direct healthcare cost of Japanese SLE patients. Total healthcare costs of SLE in Japanese patients, estimated during entire follow up period of the study. Data are plotted as mean of the total patient number in the cohort/sub-cohort. Costs of patients with severe, moderate, and mild SLE were compared against each other using two-sample T-test. ***p<.001 compared with mild SLE group and ###p<.001 compared with moderate SLE group.
Discussion
SLE is a complicated and fickle disease that inflicts a considerable humanistic and economic burden on patients, caregivers, and the health services. This retrospective cohort study allowed us to observe longitudinal treatment patterns and HRU in patients with SLE in Japan. Our study identified differences among patients in terms of sex, age, disease duration, and comorbidity at baseline, which were consistent with a previous epidemiology reportCitation35. Both males and females were susceptible during their productive years, with SLE being highly prevalent among females. Based on insurance category, more than half of the overall patients with SLE were identified as dependent household members, suggesting that they were either unemployed or unable to work due to SLE. On the other hand, the majority of claims in the treatment naïve group were employment-related. This observation might imply that these patients were on a treatment break or undergoing a washout period for 6 months wherein they did not experience any symptoms. However, the fact that such patients restarted treatment suggests that these patients experienced SLE-related symptoms which could, hypothetically, negatively impact their ability to work. It should be noted that the existing data are not comprehensive enough to fully support our speculation. Moreover, the difference in type of insurance category claims could also have resulted from a skewed patient proportion due to the treatment naïve selection algorithm.
In our study, the proportion of patients with moderate SLE was greater compared to patients with mild or severe SLE. Due to the complexity of SLE symptom presentation, treatment administration could significantly vary depending on factors such as comorbidities, signs and symptoms, extent of organ involvement, and access to different specialists for careCitation36. Patients with moderate SLE could be at risk of progressing to severe SLE in case of delayed or incorrect management. Moreover, glucocorticoid-induced side effects would add to the clinical burden.
We identified SLE-related claims for five major drug classes (topical steroids, NSAIDs, immunosuppressants, glucocorticoids, and hydroxychloroquine and their combinations). The meagre number of drug classes observed could be explained by the stringent treatment algorithm that defined the LoT in this study as well as by the presence of a standard treatment algorithm for the clinical management of SLECitation12,Citation13. Our treatment pattern assessment was comparable to a previous Japanese studyCitation14 as well with reports from other countriesCitation37,Citation38. Utilization of antimalarials (e.g. hydroxychloroquine) was low in our and Tanaka et al.’sCitation14 study, since hydroxychloroquine for SLE was approved in 2015 and adequate capture of this treatment in the database would require a longer observation period.
In our study, glucocorticoids and NSAIDs were the most used first LoT in treatment naïve Japanese patients with SLE. Oral glucocorticoids (7.5–40 mg) were administered for a cumulative duration of 17 months for each patient. This shows that a large proportion of patients with SLE were unlikely to successfully complete glucocorticoids tapering within the average 17 months period. In the EULAR recommendation on corticosteroid management, long-term use of dosages above 7.5 mg/d can substantially increase the risk of harmful effects (e.g. irreversible organ damage)Citation13. Based on our observation of long-term usage of glucocorticoid doses >7.5 mg, it is suspected that patients who received these doses were likely to suffer from SLE-related flares and to have high risk of organ-threatening conditions. This assumption was made in reference to SLE flare measurement by treatment change as SLE flare has not yet reached a globally accepted definitionCitation13. This could indicate that current option of SLE treatment may not be optimal for patients with SLE to prevent flares or reduce detrimental effects.
HRU in this study was similar to the results reported by Tanaka et al.Citation14 in a retrospective, observational cohort study conducted using claims data of Japanese patients who were diagnosed with SLE between April 2010 and March 2012. These patients were followed-up for 3 years and data on HRU and costs were analyzed. All the patients in both this study and Tanaka et al.’s study had at least one outpatient visit while 30−40% had an inpatient visit. These visits tied into the direct costs as well, as inpatient and outpatient costs were higher than pharmacy costs. The high occurrence of outpatient visits or hospitalizations in patients who received NSAIDs or immunosuppressants could have resulted in the high annual cost observed in this study. At the same time, long duration of treatment (e.g. glucocorticoids) with increasing add-on medications to control frequent flare activity could have increased the costs tooCitation39,Citation18. Although patients with SLE in Japan are eligible for funding support from the intractable disease programCitation40, the costs were quite high in the severe group compared to mild and moderate group. Mean overall direct costs in the current study were lower than Tanaka et al.’sCitation14 results (¥1,017,012 vs. ¥2,975,066). The large difference in mean direct medical costs could have occurred due to the different inclusion criteria. While this study included patients who were enrolled in the JMDC database for 6 months prior to and after the index date, Tanaka et al. only included patients who were enrolled for 6 months prior to and 3 years after the index date. Patients present in the database for such a long time would have been undergoing continuous treatment and hence experienced greater direct costs. Nevertheless, both the studies reflect the massive economic burden inflicted by SLE, especially in patients with severe disease.
Steroid exposure has been associated with unwanted organ damageCitation41 including an increased risk of cataract and osteoporosis in patients with SLE. However, steroids have remained the backbone of SLE therapy in Japan. This scenario could have changed after late 2017 when MHLW approved the use of belimumab as adjunctive therapy in autoantibody-positive patients with SLE who do not respond adequately to existing therapiesCitation42. As novel treatment therapies for SLE – such as biologics – emerge, there would be greater potential for clinicians and patients to expand therapeutic strategies in order to improve prognosis for SLE, reduce treatment burden, and enable maintenance of long-term remission to prevent flares.
Limitations
The findings of this study should be considered within the context of several limitations inherent to the JMDC data source which could not rule out biases toward SLE patients’ conditions that attribute to the spectrum of SLE severity, flare incidences, or individual physical status (e.g. female hormonal or reproductive condition). The elderly are underrepresented in the JMDC database as enrollment into the database is linked to employment status. Patients who were not represented in the database might be receiving healthcare benefits under other insurance types. Loss of productivity and other indirect costs could not be evaluated in the claims database. Our study was descriptive and not comparative in nature. The algorithm of SLE and disease severity using claims databases has not been previously validated. The patient’s medical history might have influenced a physician’s decision to modify or switch treatments. The database did not have an account of reasons underlying specific treatment-related events such as discontinuation, switching, and augmentation, which could not correlate to the outcomes of the patients. In terms of drug claims capturing, some recently approved drugs for SLE may not reflected in this analysis; for example, hydroxychloroquine for SLE, mycophenolate mofetil for lupus nephritis, and belimumab for SLE were approved in 2015 or later in Japan. In this case, the number of these drug claims may not fully account in the treatment pattern in our follow-up time frame. Hence, interpretation of the treatment patterns and treatment-related events should be made with caution.
Conclusion
This is one of the few Japanese studies that analyzed current treatment patterns, treatment-related events, and economic burden of SLE from an administrative database. Glucocorticoids were the backbone of SLE therapy to which other medications were added on. However, the myriad risks associated with steroids should shift healthcare providers and policy makers toward more effective treatment for SLE, resulting in lower SLE-associated burden.
Transparency
Declaration of funding
Data acquisition, research, and preparation of the manuscript were funded by Janssen Pharmaceutical K.K. The funding body did not have any additional role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Declaration of financial/other interests
CM, RS, JM, and WJ were employees of Janssen Pharmaceutical K.K. at the time the study was conducted.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed that they are a shareholder in a company which has received income for scientific services and expenses from Celgene, Janssen and Novartis, amongst others. Per company policy, employees/shareholders cannot contract individually with sponsor organizations and cannot hold equity in sponsor organizations. The reviewers have no other relevant financial relationships or otherwise to disclose.
Author contributions
CM conceived the idea and drafted the manuscript. RS performed data acquisition. CM and JM analyzed and interpreted the data. WJ also assisted in data interpretation. WJ, RS, and JM reviewed and revised the manuscript. All authors provided final approval for the manuscript.
Supplemental Material
Download ()Acknowledgements
The authors thank Leo J. Philip Tharappel from Siro Clinpharm Pvt. Ltd, Mumbai and Dr. Kiran Saqib for providing medical writing assistance and reviewing the manuscript for final submission.
Data availability
The datasets analyzed during the current study are not publicly available due to copyright reasons but are available from the corresponding author on reasonable request.
References
- Zhu TY, Tam LS, Lee VW, et al. Relationship between flare and health-related quality of life in patients with systemic lupus erythematosus. J Rheumatol. 2010;37(3):568–573.
- Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18(6):871–882.
- Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15(5):308–318.
- Iseki K, Miyasato F, Oura T, et al. An epidemiologic analysis of end-stage lupus nephritis. Am J Kidney Dis. 1994;23(4):547–554.
- Costenbader KH, Feskanich D, Stampfer MJ, et al. Reproductive and menopausal factors and risk of systemic lupus erythematosus in women. Arthritis Rheum. 2007;56:1251–1262.
- Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol. 2005;32(9):1706–1708.
- Kuriya B, Gladman DD, Ibanez D, et al. Quality of life over time in patients with systemic lupus erythematosus. Arthritis Rheum. 2008;59(2):181–185.
- McElhone K, Abbott J, Teh LS. A review of health related quality of life in systemic lupus erythematosus. Lupus. 2006;15(10):633–643.
- Lau CS, Mak A. The socioeconomic burden of SLE. Nat Rev Rheumatol. 2009;5(7):400–404.
- Gironimi G, Clarke AE, Hamilton VH, et al. Why health care costs more in the US: comparing health care expenditures between systemic lupus erythematosus patients in Stanford and Montreal. Arthritis Rheum. 1996;39:979–987.
- Yurkovich M, Vostretsova K, Chen W, et al. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res. 2014;66:608–616.
- Gordon C, Amissah-Arthur MB, Gayed M, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology (Oxford). 2018;57(1):e1–e45.
- Fanouriakis A, Kostopoulou M, Alunno A, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. 2019;78(6):736–745.
- Tanaka Y, Mizukami A, Kobayashi A, et al. Disease severity and economic burden in Japanese patients with systemic lupus erythematosus: a retrospective, observational study. Int J Rheum Dis. 2018;21(8):1609–1618.
- Agrawal N, Chiang LK, Rifkin IR. Lupus nephritis. Semin Nephrol. 2006;26(2):95–104.
- Mok CC. Prognostic factors in lupus nephritis. Lupus. 2005;14(1):39–44.
- Braun L, Sood V, Hogue S, et al. High burden and unmet patient needs in chronic kidney disease. Int J Nephrol Renovasc Dis. 2012;5:151–163.
- Li T, Carls GS, Panopalis P, et al. Long-term medical costs and resource utilization in systemic lupus erythematosus and lupus nephritis: a five-year analysis of a large medicaid population. Arthritis Rheum. 2009;61:755–763.
- Ikeda T, Kanazawa N, Furukawa F. Hydroxychloroquine administration for Japanese lupus erythematosus in Wakayama: a pilot study. J Dermatol. 2012;39(6):531–535.
- Minowa K, Amano H, Ando S, et al. Disease flare patterns and predictors of systemic lupus erythematosus in a monocentric cohort of 423 Japanese patients during a long-term follow-up: the JUDE study. Mod Rheumatol. 2017;27(1):72–76.
- JMDC Claims Database: JMDC. 2020. [cited 2020 Jan 17]. Available from: https://www.jmdc.co.jp/en/jmdc-claims-database
- Mahlich J, Tsukazawa S, Wiegand F. Estimating prevalence and healthcare utilization for treatment-resistant depression in Japan: a retrospective claims database study. Drugs-Real World Outcomes. 2018;5(1):35–43.
- Kuwabara H, Saito Y, Mahlich J. Adherence and rehospitalizations in patients with schizophrenia: evidence from Japanese claims data. Neuropsychiatr Dis Treat. 2015;11:935–940.
- Sruamsiri R, Iwasaki K, Tang W, et al. Persistence rates and medical costs of biological therapies for psoriasis treatment in Japan: a real-world data study using a claims database. BMC Dermatol. 2018;18(1):5.
- Davis KL, Meyers J, Zhao Z, et al. High-risk atherosclerotic cardiovascular disease in a real-world employed Japanese population: prevalence, cardiovascular event rates, and costs. J Atheroscler Thromb. 2015;22(12):1287–1304.
- Motheral B, Brooks J, Clark MA, et al. A checklist for retrospective database studies—report of the ISPOR Task Force on Retrospective Databases. Value in Health. 2003;6(2):90–97.
- Stip E, Lachaine J. Real-world effectiveness of long-acting antipsychotic treatments in a nationwide cohort of 3957 patients with schizophrenia, schizoaffective disorder and other diagnoses in Quebec. Ther Adv Psychopharmacol. 2018;8(11):287–301.
- Garris C, Jhingran P, Bass D, et al. Healthcare utilization and cost of systemic lupus erythematosus in a US managed care health plan. J Med Econ. 2013;16(5):667–677.
- Bertsias G, Ioannidis J, Aringer M, et al. EULAR recommendations for the management of systemic lupus erythematosus with neuropsychiatric manifestations: report of a task force of the EULAR standing committee for clinical affairs. Ann Rheum Dis. 2010;69(12):2074–2082.
- Zhang X, Oyama T. Investigating the health care delivery system in Japan and reviewing the local public hospital reform. Risk Manag Healthc Policy. 2016;9:21–32.
- Sakamoto H, Rahman M, Nomura S, et al. Japan health system review. Vol. 8. New Delhi, India: World Health Organization, Regional Office for SouthEast Asia; 2018.
- Measures of Underlying Inflation Tokyo, Japan: Bank of Japan. 2020. [cited 2020 Feb 08]. Available from: https://www.boj.or.jp/en/research/research_data/cpi/index.htm/
- OECD. Long-term interest rates forecast (indicator). OECD. 2020. [cited 2020 Feb 08]. Available from: https://data.oecd.org/interest/long-term-interest-rates-forecast.htm#indicator-chart
- OECD. PPPs and exchange rates: OECD.Stat. 2020. [cited 2020 Feb 08]. Available from: https://stats.oecd.org/Index.aspx?datasetcode=SNA_TABLE4
- Terao C, Yamada R, Mimori T, et al. A nationwide study of SLE in Japanese identified subgroups of patients with clear signs patterns and associations between signs and age or sex. Lupus. 2014;23(13):1435–1442.
- Tunnicliffe DJ, Singh ‐Grewal D, Kim S, et al. Diagnosis, monitoring, and treatment of systemic lupus erythematosus: a systematic review of clinical practice guidelines. Arthritis Care Res. 2015;67:1440–1452.
- Kan H, Nagar S, Patel J, et al. Longitudinal treatment patterns and associated outcomes in patients with newly diagnosed systemic lupus erythematosus. Clin Ther. 2016;38(3):610–624.
- Davis LS, Reimold AM. Research and therapeutics-traditional and emerging therapies in systemic lupus erythematosus. Rheumatology (Oxford). 2017;56(1):i100–i113.
- Doria A, Amoura Z, Cervera R, et al. Annual direct medical cost of active systemic lupus erythematosus in five European countries. Ann Rheum Dis. 2014;73(1):154–160.
- Goldstein DJ, Lu Y, Detke MJ, et al. Duloxetine in the treatment of depression: a double-blind placebo-controlled comparison with paroxetine. J Clin Psychopharmacol. 2004;24(4):389–399.
- Zonana‐Nacach A, Barr SG, Magder LS, et al. Damage in systemic lupus erythematosus and its association with corticosteroids. Arthritis Rheum. 2000;43:1801–1808.
- GSK receives approval for Benlysta in Japan for the treatment of systemic lupus erythematosus London: GlaxoSmithKline; 2017. [cited 2019 Nov 19]. Available from: https://www.gsk.com/en-gb/media/press-releases/gsk-receives-approval-for-benlysta-in-japan-for-the-treatment-of-systemic-lupus-erythematosus/