Abstract
Background: This retrospective analysis evaluates morbidities, outcomes and associated risk factors in patients with myelofibrosis (MF) after ruxolitinib discontinuation, using Truven Health Analytics MarketScan (TR), Optum integrated virtual electronic health records and claims databases (OP), and Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database (SM).
Methods: A total of 290 patients with MF between 2006 and 2015 (using ICD-9 and ICD-O-3 codes), who were treated with and discontinued ruxolitinib were identified. Only patients with ≥90 days of medical history prior to index diagnosis (TR + OP) and Part A, B, and D enrollment at the time of index diagnosis (SM) were included. Morbidities were assessed during the 30-day period each following ruxolitinib initiation, prior to and post ruxolitinib discontinuation. Cumulative incidence of cytopenias and efficacy outcomes were evaluated from baseline.
Results: Median age of patients was 68 years, with equal proportion of either gender. Median time to ruxolitinib discontinuation was 284 days and median follow-up after discontinuation was 70.9 days. The majority of patients were diagnosed with anemia and >30% of the patients received RBC transfusions during 30-day period prior to and the 30-day period post ruxolitinib discontinuation. After ruxolitinib discontinuation, half of the patients developed cytopenias. The median treatment progression-free survival, and overall survival after ruxolitinib discontinuation were 6.0 (4.4, 8.3) months and 11.1 (8.4, 14.5) months, respectively. Age at ruxolitinib discontinuation (HR [95% CI] = 2.071 [1.320, 3.248]), Charlson Comorbidity Index score (HR [95% CI] = 1.172 [1.093, 1.257]) and gender (HR [95% CI] = 1.620 [1.108, 2.369]) increased the risk of treatment progression (start of the subsequent treatment regimen) or death.
Conclusion: Results from this large, retrospective, US population-based outcome analysis of MF patients show an increase in morbidity burden and identifies the risk factors of survival outcomes among real-world patients who have discontinued ruxolitinib.
Introduction
Myelofibrosis (MF), essential thrombocythemia (ET), and polycythemia vera (PV) form a group of clonal hematologic malignancies known as myeloproliferative neoplasms (MPNs), characterized by dysregulated proliferation of the myeloid lineagesCitation1. MF can either be idiopathic, known as primary myelofibrosis (PMF) or occur as the result of ET (Post-ET MF) or PV (Post-PV MF), referred to as secondary myelofibrosis (sMF)Citation2.
Advanced age is a known adverse risk factor for MF and majority of patients are diagnosed after 60 years of ageCitation3,Citation4. According to a 2014 estimate based on a 3-year longitudinal analysis, the age-adjusted prevalence of MF in the US ranged from 3.6 to 5.7 per 100,000 persons. Age-adjusted prevalence of PMF was higher (range: 1.3–2.2) than sMF (range: Post-ET MF, 0.46–1.1; Post-PV MF, 0.29–0.67) per 100,000 personsCitation5. However, overall survival in the PMF population (45 months) and post-PV (48 months) sMF populations tends to be shorter than that observed in the post-ET population (73 months)Citation6.
MPNs are characterized by point mutations in Janus associated kinase 2 (JAK2V617F), CALR and MPL genes which are reported in the majority of patientsCitation7–11. Published guidelines suggest similar treatment approaches for patients with PMF and sMFCitation12. Ruxolitinib, a JAK 1/2 inhibitor, is the only approved therapy for intermediate-to-high risk MF (PMF + sMF) defined by the International Prognostic Scoring System (IPSS)Citation13,Citation14.
Despite significant improvements in disease related symptoms and quality of life, over half of the patients discontinue ruxolitinib treatment within 2–3 years due to adverse events or loss of treatment responseCitation15. Recent studies in MF patients following ruxolitinib discontinuation demonstrated poor survival outcomes, as well as low response rates to salvage therapiesCitation16,Citation17.
Research has been conducted to determine the treatment pattern among PMF and sMF patientsCitation16–18, however, patient outcomes associated with treatment discontinuations or failures in these populations have not been well studied, especially using real-world data sources which provide important evidence to support clinical and regulatory decision-making. To address these gaps in the literature, the present study aims to characterize and determine patient outcomes in the MF (PMF + sMF) population who discontinued ruxolitinib treatment. This is a large US population-based study that identifies an important unmet need in MF patients who discontinue ruxolitinib and provides a comprehensive assessment of morbidities, outcomes and associated risk factors in this population.
Methods
Data sources
Data were obtained from three claims databases, the Truven Health Analytics MarketScan (Commercial Claims and Encounters and Truven Medicare) (TR), the Optum (Optum, Inc, Eden Prairie, MN, USA) integrated virtual electronic health records and claims databases (OP), and the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database (SM). OP and TR databases were used to identify patients with MF diagnosed between 2006 and 2015; the SM database was used to identify patients with MF diagnosed between 2007 and 2014.
The OP database combines adjudicated claims data with Humedica’s Electronic Health Record data, which covers over 10 million lives and includes historical, linked administrative claims data, pharmacy and physician claims, facility claims, with clinical information, inclusive of prescribed and administered medications, lab results, vital signs, body measurements, diagnoses, procedures, and information derived from clinical notes. The TR database comprises inpatient medical, outpatient medical, and outpatient prescription drug claims, diagnosis and encounter data on 70 million lives. The SM database reflects the linkage of two large population-based sources of data that provide detailed information about elderly persons with cancer, which can be used for an array of epidemiological and health services research. Clinical and diagnosis information from the SEER cancer registry (covering approximately one-quarter of the total US populationCitation19) is combined with medical and pharmacy claims for Medicare enrollees. Medicare Part A covers hospital stays, hospice care, and some skilled nursing care; Part B covers services and supplies that are medically necessary to treat a patient’s health condition, such as outpatient care, preventive services, ambulance services, and durable medical equipment; and Part D covers prescription drugs. For each incident cancer diagnosis reported in SEER, patient-level information is captured on demographics, specific International Classification of Diseases for Oncology, Third Revision (ICD-O-3) codes, and survival.
Patient cohort
Patients ≥65 years (categorized as elderly) were included from the SM database, as it is the minimum eligible age to be enrolled in the Medicare database. Patients ≥18 years and <65 years (categorized as non-elderly) were included from the TR and OP databases. Including different age groups across the data sources ensured both adequate representation of all age groups and elimination of possible duplication patients. Also, those patients who had ≥90 days of medical history prior to index diagnosis (TR and OP) and who were enrolled in Part A, B, and D at the time of index diagnosis (SM) were included in the analysis. Patients who had PMF diagnosis in Medicare database but did not have a PMF diagnosis in the SEER registry were required to have a minimum of 6 months of prior enrollment to ensure index diagnosis.
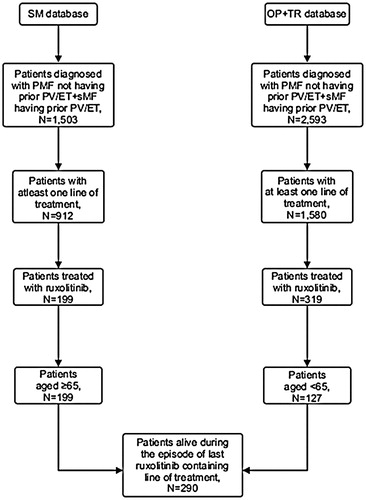
Patients with MF were identified according to ICD-9 (TR, OP, SM) and ICD-O-3 (SM) codes. Patients with MF diagnosis (ICD-9: 238.76, 289.83; ICD-O-3: 9961/3) without prior history of PV (ICD- 9: 238.4, ICD-O-3: 9950/3) or ET (ICD-9: 238.71, ICD-O-3: 9962/3) were classified as PMF, while those with prior history of PV or ET were classified as sMF patients ().
Figure 1. Cohort selection. Abbreviations. PMF, Primary Myelofibrosis; sMF, Secondary Myelofibrosis; PV, Polycythemia Vera; ET, Essential Thrombocythemia; SM, SEER Medicare; TR, Truven; OP, Optum.
Among PMF and sMF patients, lines of treatments (LOTs) were identified from index diagnosis date on the basis of business rules centered on continuity and concurrence of usage of relevant drugs (chemotherapy, erythropoiesis stimulating agents, hydroxyurea, ruxolitinib).
Patient characteristics and outcomes of interest were evaluated in the PMF + sMF population who experienced ruxolitinib discontinuation.
The study protocol was approved by Institutional Review Board (Fox Commercial IRB, Ltd). Because no information was collected on human subjects (all data were retrospective, de-identified, and non-interventional), the study was exempted from patient consent requirements. The data and protocol of this study is not accessible publicly, hence please contact [email protected] for the original data or protocol.
Outcomes of interest
Baseline patient characteristics were summarized at ruxolitinib discontinuation date. Patients were followed up from ruxolitinib discontinuation date to death date, in case of death, or end of follow-up (defined as 31 December 2014 and last claim date in the SM and OP + TR databases, respectively), in others. Descriptive measures of morbidities and red blood cell (RBC) transfusion (codes provided in supplementary file) were assessed during the 30, 60, 90, and 180-day period following ruxolitinib initiation, prior to ruxolitinib discontinuation, and post ruxolitinib discontinuation date. Cumulative incidence of cytopenias, including anemia (ICD-9 code 285.22), neutropenia (ICD-9 code 288.0) and thrombocytopenia (ICD-9 code 287.3, 287.4, 287.5) were evaluated from baseline. Efficacy outcomes including, time to next treatment (TTNT; start of next LOT was defined as an event), treatment progression-free survival (TPFS; start of next LOT or death, whichever occurred first was defined as an event), and overall survival (OS; death was defined as an event) were also evaluated from baseline.
Statistical analysis
Frequencies were used to summarize categorical and, mean (standard deviation, SD) and median (interquartile range, IQR) were used to summarize continuous variables. Cumulative incidence of cytopenias (with death as a competing risk event) was evaluated using Fine and Gray’s methodCitation20. Time-to-event outcomes (TTNT, TPFS and OS) were assessed using Cox proportional hazard models. Median OS was evaluated using Kaplan Meier analysis. All analyses were conducted using SAS® (version 9.4) statistical package (SAS Institute Inc., Cary, North Carolina, USA).
Results
Baseline characteristics
The analysis included 290 MF (PMF = 125, sMF = 165) patients with ruxolitinib discontinuation, from all the three databases (OP + TR [n = 127] + SM [n = 163]). The median (IQR) age of patients at ruxolitinib discontinuation was 68.0 (62.0−77.0) years. Patients from <45–64 years age group comprised the greatest proportion (39%) of the cohort. Half of the patients were men. Median (IQR) time to ruxolitinib discontinuation from ruxolitinib initiation date was 284 (113.0−562.0) days. Median (IQR) follow-up time after ruxolitinib discontinuation was 70.9 (6.9−251.8) days. Median (IQR) number of prior LOTs was 1.0 (0.0−1.0). Majority of patients (83.1%) did not receive any supportive care treatment (that included erythropoietin ± steroids [danazol, fluoxymesterone, prednisolone, or dexamethasone]) after ruxolitinib was discontinued, while a few received recombinant erythropoietin (2.4%), hydroxyurea (4.5%), or chemotherapy (0.3%) ().
Table 1. Baseline characteristics.
Morbidities and transfusions
During the 30-day period following ruxolitinib initiation, 36% had anemia, 12% had thrombocytopenia, and 2% had neutropenia. The proportion of patients diagnosed with these morbidities increased during the 30-day period prior to ruxolitinib discontinuation (anemia [45%], and thrombocytopenia [18%]). The comorbidities increased further in the 30-day period after ruxolitinib discontinuation (AML [7%], anemia [53%], thrombocytopenia [23%], and neutropenia [10%]). Only 19% patients were found to receive RBC transfusion within first 30 days of ruxolitinib initiation, compared to, 33% each during the 30 days prior to and after ruxolitinib discontinuation. The proportion of patients diagnosed with any morbidity or those who received RBC transfusion increased with time at every stage of assessment (following/prior to/or after ruxolitinib initiation) ().
Table 2. Treatment emergent morbidities and transfusions.
Cumulative incidence of cytopenias
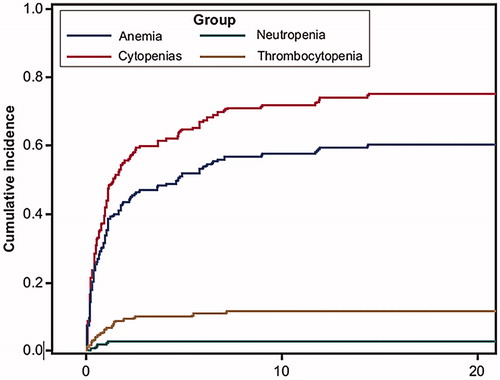
Half of the patients who discontinued ruxolitinib developed cytopenias at some time during their remaining follow-up. Among all patients, 11% had death as the competing event. Cumulative incidence for all the cytopenias (in the presence of death as a competing event) reached 75% in the 18th month after ruxolitinib discontinuation (), after which it remained steady.
Efficacy outcomes
After ruxolitinib discontinuation, the median (95% CI) TTNT was NE (11.2, NE), TPFS was 6.0 (4.4−8.3) months, and OS was 11.1 (8.4−14.5) months (). Female gender was associated with increased hazard of progression to next treatment (HR [95% CI] = 1.853 [1.007, 3.408]). Age >65 years at time of ruxolitinib discontinuation (HR [95% CI] = 2.071 [1.320, 3.248], unit increase in Charlson Comorbidity Index (CCI) score (HR [95% CI] = 1.172 [1.093, 1.257]) and female gender (HR [95% CI] = 1.620 [1.108, 2.369]) were associated with increased hazard of treatment progression/death. In addition, age >65 (HR [95% CI] = 3.845 [2.118, 6.981]), and unit increase in CCI score (HR [95% CI] = 1.248 [1.154, 1.349]) were associated with increased hazard of death ().
Figure 3. OS, TPFS and TTNT of patients. Time to event analysis in the sample of myelofibrosis patients that discontinued ruxolitinib (n = 290). X-axis represents the event free probability and y-axis represents the follow-up time after ruxolitinib discontinuation. Event = death (Overall survival [OS] analysis), earlier of treatment progression/death (time to progression free survival [TPFS] analysis) and treatment progression (time to next treatment [TTNT] analysis).
![Figure 3. OS, TPFS and TTNT of patients. Time to event analysis in the sample of myelofibrosis patients that discontinued ruxolitinib (n = 290). X-axis represents the event free probability and y-axis represents the follow-up time after ruxolitinib discontinuation. Event = death (Overall survival [OS] analysis), earlier of treatment progression/death (time to progression free survival [TPFS] analysis) and treatment progression (time to next treatment [TTNT] analysis).](/cms/asset/d7df35d4-1aa9-4a65-b2f8-7a361980e70d/ijme_a_1741381_f0003_c.jpg)
Table 3. Time to event efficacy outcomes.
Discussion
The present retrospective analysis evaluated various outcomes before and after ruxolitinib discontinuation. The results show that after discontinuation of ruxolitinib, a greater proportion of patients experienced morbidities resulting in worsened outcomes. MF patients had poor efficacy outcomes post ruxolitinib discontinuation with median TPFS of <6 months and median OS of <12 months likely due to limited treatment modalities available after ruxolitinib discontinuation. These findings are aligned with the results obtained in two real-world studies demonstrating the unmet need to improve the safety and efficacy outcomes associated with the treatments used after ruxolitinib discontinuationCitation4,Citation21. In this study, median time to ruxolitinib discontinuation was <1 year, which is similar to the results obtained from another study based on US claims databaseCitation22. However, longer median time to ruxolitinib discontinuation (3 years) in COMFORT trialsCitation14,Citation23 could be due to the inclusion of healthier patients in controlled studies as compared to real-world population.
To our knowledge, this is the first population-based study assessing morbidities in a treatment setting following ruxolitinib discontinuation. In the present study, during ruxolitinib treatment, anemia (36%−45%) was the most frequently reported hematological complication morbidity followed by thrombocytopenia (12%−18%) and neutropenia (5%−9%) as assessed by ICD-9 codes. Study findings are aligned with the COMFORT-1 trial, where 45.2% of patients developed anemia, and 12.9% and 7.1% of patients developed thrombocytopenia and neutropenia, respectivelyCitation14. Also, a real-world study that retrospectively reviewed medical records data showed that, only 22.2% patients experienced anemia and 7.4% experienced thrombocytopenia while being treated with ruxolitinibCitation24. However, findings from the current study point to a higher burden of cytopenias after ruxolitinib discontinuation compared to the 30-day period prior to and the 30-day period post ruxolitinib discontinuation. Although differing from the current study, Cervantes et al.Citation23 reported occurrence of cytopenias within 28 days after ruxolitinib discontinuation to be similar to that reported during treatment. The greater cytopenic burden we observed after discontinuation could be attributed to low platelet counts at the time of discontinuation, as such patients often have a poor prognosisCitation17. This outcome could be a result of disease progression as also observed by Newberry et al.Citation17. Therefore, patients who are truly failing ruxolitinib due to progressive disease may not experience a rebound of platelet count on discontinuing JAK inhibitors as this outcome is a function of the disease rather than treatment-related cytopenias.
Although ruxolitinib can worsen anemia in some patients, previous safety studies do not suggest presence of anemia as a contraindication for ruxolitinib use; where it does occur, it can be mitigated by ruxolitinib dose reductions and RBC transfusionsCitation25–27. In a real-world study, 12% of patients received RBC transfusions during ruxolitinib treatment, while the present study showed that 30% of the patients required transfusion post ruxolitinib discontinuation in order to manage anemia. Similar results were observed in a retrospective chart review study, in which the transfusion dependency in MF patients increased from 18% at the start of ruxolitinib to 36% after discontinuationCitation17. These findings are consistent with progressive MF disease and highlight a need for novel treatment approaches to both effectively manage cytopenias post ruxolitinib discontinuation and to extend survival.
A comprehensive review of past literature did not reveal any previous study that sought to evaluate time to next treatment and treatment progression-free survival in MF patients after discontinuation of ruxolitinib treatment. Until now, OS is the only efficacy outcome assessed in real-world populations who discontinued ruxolitinib treatment. The median OS observed in the present study (11.1 months) is consistent with the OS in previously reported real-world single center studies by Kuykendall et al. (13 months)Citation16 and Newberry et al. (14 months)Citation17. However, another real-world study conducted using claims data reported median OS of 30 months in MF patients on ruxolitinib therapy, while in patients who failed or discontinued ruxolitinib was 7 monthsCitation4,Citation17.
Unlike past studies that were separately conducted in elderly and non-elderly cohortsCitation4,Citation21, the current study is generalizable to patients of both age groups as a population-based study. Also, the present study provides the largest data sample that has been available to determine the OS of patients who discontinued ruxolitinib. A majority of the data (∼56%) was drawn from a nationally representative, population-based cancer registry linked to Medicare, which provides health insurance to over 95% of the elderly in the USCitation28. Hence, diagnosis of MF, use of ruxolitinib treatment, and record of mortality can be considered reliable and accurateCitation29. Moreover, variables derived from SM and TR + OP databases were complete hence, the results are not affected by missing values.
The study findings should be considered in light of several limitations. Due to the observational nature of the research, no cause and effect relationship could be determined in the present study. Among patients who received at least one LOT, only 20% received ruxolitinib and were included in this study. The low use of ruxolitinib could be attributed to its approval only during the latter half of the study period (November 2011) as well as the population in which it was approved for use (intermediate or high-risk MF only). Moreover, it reflects the low usage of ruxolitinib in clinical practice. Information regarding important determinants of ruxolitinib discontinuation and OS (e.g. IPSS, cytogenetic and mutational profile) were unknown. The state of the disease (chronic/blast phase) as well as the reasons for ruxolitinib discontinuation were unavailable in the databases. The treatment regimens as identified in our study may only reflect standard of care up to 2014 in the SM population and up to 2015 in the TR + OP population. Mortality data in the administrative claims databases may be incomplete, as these data rely on completeness of linkage to national death registries as well as compliance of reporting of death information. Also, a majority of patients in the study did not receive subsequent therapy post ruxolitinib discontinuation; this may be due to inadequate follow-up, lack of effective treatment options or patient choice to discontinue active treatment.
Conclusions
In summary, we found that morbidities worsened after ruxolitinib discontinuation. With limited treatment options being available in the real-world post ruxolitinib use, our study findings underscore the need for more treatment options to manage MF after ruxolitinib is discontinued. We also found that efficacy outcomes were associated with age at ruxolitinib discontinuation (TPFS, OS), CCI score (TPFS, OS), and gender (TTNT, TPFS). Further research is warranted to not only assess survival over a longer period of time, but to also critically examine potential prognostic variables (i.e. patient and clinical factors) which affect survival. Such information may further assist clinicians to evaluate the optimal care for MF and also determine effective treatment strategies to manage all the MF subtypes in a population which experiences ruxolitinib discontinuation.
Transparency
Declaration of funding
This study was supported by research funding from Janssen Global Services LLC.
Declaration of financial/other relationships
MM, CL and JH are employees of Janssen Global Services, LLC.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the study design, interpretation of data and drafting the manuscript. JH, RP & MM were involved in data collection and statistical analysis.
Ethics approval and consent to participate
The study protocol was approved by Institutional Review Board (Fox Commercial IRB, Ltd). Because no information was collected on human subjects (all data were retrospective, de-identified, and non-interventional), the study was exempted from patient consent requirements.
Supplemental Material
Download MS Word (13 KB)Acknowledgements
The authors acknowledge Rishabh Pandey, PhD (SIRO Clinpharm Pvt. Ltd.) for providing writing assistance and Harry Ma, PhD (Janssen Global Services, LLC) for additional editorial support.
Availability of data and material
The data and protocol of this study are not publicly available but are available upon contact with [email protected] on reasonable request.
References
- Gabler K, Behrmann I, Haan C. JAK2 mutants (e.g., JAK2V617F) and their importance as drug targets in myeloproliferative neoplasms. JAKSTAT. 2013;2:e25025.
- Vainchenker W, Constantinescu SN, Plo I. Recent advances in understanding myelofibrosis and essential thrombocythemia. F1000Res. 2016;5:700.
- Cancer Network. Myeloproliferative Neoplasms; 2016 [2017 Feb 7]. Available from: http://www.cancernetwork.com/cancer-management/myeloproliferative-neoplasms/page/0/1.
- Mehra M, Potluri R, He J, et al. Characterization of disease, treatment patterns, and outcomes of patients with myelofibrosis: analysis of 2 United States commercial claims databases. Am Soc Hematology. 2016;128(22):4769.
- Mehta J, Wang H, Iqbal SU, et al. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595–600.
- Masarova L, Bose P, Daver N, et al. Patients with post-essential thrombocythemia and post-polycythemia vera differ from patients with primary myelofibrosis. Leuk Res. 2017;59:110–116.
- Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem. 2005;280(24):22788–22792.
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–397.
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–1790.
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–1148.
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061.
- Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis, essential thrombocythemia, and polycythemia vera in the European Union. Eur J Haematol. 2014;92(4):289–297.
- Incyte Corporation. Highlights of prescribing information; 2011 [2018 Aug 12]. Available from: https://www.jakafi.com/pdf/prescribing-information.pdf.
- Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807.
- Pardanani A, Tefferi A. Definition and management of ruxolitinib treatment failure in myelofibrosis. Blood Cancer J. 2014;4(12):e268–e268.
- Kuykendall AT, Shah S, Talati C, et al. Between a rux and a hard place: evaluating salvage treatment and outcomes in myelofibrosis after ruxolitinib discontinuation. Ann Hematol. 2018;97(3):435–441.
- Newberry KJ, Patel K, Masarova L, et al. Clonal evolution and outcomes in myelofibrosis after ruxolitinib discontinuation. Blood. 2017;130(9):1125–1131.
- Spiegel JY, McNamara C, Kennedy JA, et al. Impact of genomic alterations on outcomes in myelofibrosis patients undergoing JAK1/2 inhibitor therapy. Blood Adv. 2017;1(20):1729–1738.
- Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV3–IV18.
- Zhang MJ, Zhang X, Scheike TH. Modeling cumulative incidence function for competing risks data. Expert Rev Clin Pharmacol. 2008;1(3):391–400.
- He J, Mehra M, Bussolari J, et al., editors. Characterization of disease and outcomes of patients with myelofibrosis: A population based study. Haematologica. Pavia, Italy: Ferrata Storti Foundation; 2017.
- Fonseca E, Silver RT, Kazis LE, et al. Ruxolitinib discontinuation in patients with myelofibrosis: an analysis from clinical practice. Blood. 2013;122(21):2833–2833.
- Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–4053.
- Davis KL, Cote I, Kaye JA, et al. Real-world assessment of clinical outcomes in patients with lower-risk myelofibrosis receiving treatment with ruxolitinib. Adv Hematol. 2015;2015:1–9.
- Tefferi A. JAK inhibitors for myeloproliferative neoplasms: clarifying facts from myths. Blood. 2012;119(12):2721–2730.
- Verstovsek S, Gotlib J, Gupta V, et al. Management of cytopenias in patients with myelofibrosis treated with ruxolitinib and effect of dose modifications on efficacy outcomes. Onco Targets Ther. 2013;7:13–21.
- McMullin MF, Harrison CN, Niederwieser D, et al. The use of erythropoiesis-stimulating agents with ruxolitinib in patients with myelofibrosis in COMFORT-II: an open-label, phase 3 study assessing efficacy and safety of ruxolitinib versus best available therapy in the treatment of myelofibrosis. Exp Hematol Oncol. 2015;4:26.
- Cubanski J, Senate U. An overview of the medicare program and medicare beneficiaries’costs and service use; 2013 [2018 Aug 12]. Available from: https://kaiserfamilyfoundation.files.wordpress.com/2013/02/an-overview-of-the-medicare-program-and-medicare-beneficiaries-costs-and-service-use-testimony.pdf.
- Howlader N, Ries LA, Mariotto AB, et al. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–1598.