Abstract
Research aim: To model the annual value of a novel ready-to-use, room-temperature stable liquid glucagon rescue pen and prefilled syringe (GRP, G-PFS; Xeris Pharmaceuticals, Inc.) for treatment of severe hypoglycemia events (SHE) versus current lyophilized powder glucagon emergency kits (GEK). GRP is a prefilled auto-injector designed to promptly administer concentrated liquid glucagon in a simple two-step process. G-PFS is a stable liquid formulation of glucagon in a prefilled syringe. In simulated emergencies, GRP and G-PFS demonstrated high functional efficacy, where 99% of users successfully administered a full-dose of drug. Studies with currently available injectable GEK suggest very low success rates (6–31%). The high functional efficacy of GRP and G-PFS significantly reduces user errors and may reduce utilization across emergency medical services (EMS), emergency departments (ED), and inpatient and outpatient costs for SHE.
Methods: To estimate the economic impact of GRP and G-PFS, we developed a one-year budget impact model from a US commercial health plan perspective. Cost offsets from successful glucagon administration incorporated EMS, ED, inpatient, and outpatient utilization. Diabetes prevalence and event probabilities were estimated from publicly-available sources and clinical expert opinion. Costs (US$) were obtained from the 2018 Medicare Fee Schedules and adjusted to represent commercial payer costs.
Results: GRP and G-PFS led to fewer EMS, ED, inpatient, and outpatient costs compared to GEK and no kit, resulting in total per-patient SHE costs of $2,564, $3,606, and $3,849, respectively. Costs for 1 million covered lives were 8.2 million following the introduction of GRP and G-PFS compared to almost 9 million before GRP and G-PFS.
Limitations: The model is limited by reliance on assumptions based on expert opinion for key variables, primarily the probability of: (1) ambulance calls, (2) ambulance transport to the ED, and (3) non-ambulance transport to the ED.
Conclusions: A budget impact model suggests GRP and G-PFS can lead to significant annual cost savings for US commercial payers.
Introduction
Large, long-term human clinical trials such as the Diabetes Control and Complications Trial and the United Kingdom Prospective Diabetes Study, provide the foundational evidence to support the strict management thresholds for glycemic levels, that help delay or prevent diabetes-related complicationsCitation1–6. Long-term health outcomes research provides support that optimal glycemic control may lead to fewer microvascular and macrovascular complications in persons with type 1 diabetes (T1D) and type 2 diabetes (T2D). However, as a result of tight glycemic control through intensive insulin therapy in T1D and T2D, the risk of hypoglycemia is increasedCitation6.
Hypoglycemia can result in: (1) physical manifestations such as palpitations, sweating, and neurological impairments; (2) psychological impacts, such as mood disturbance and generalized worry/fear of hypoglycemia)Citation7–9; (3) reduction in quality of life; (4) and increased risk of deathCitation9. It is estimated that 2−6% of all deaths in people with T1D are attributed to severe hypoglycemiaCitation10,Citation11. In T2D, especially when receiving sulfonylurea monotherapy, severe hypoglycemia has been associated with a 9% overall mortality rateCitation12.
In addition to clinical and humanistic detriments, severe hypoglycemia is a contributor to higher costs of care. Williams et al.Citation13 illustrated that diabetes-related total healthcare costs were significantly higher for persons with diabetes (PWDs) with confirmed hypoglycemia compared to PWDs without hypoglycemia ($6,042 vs. $3,760). PWDs with confirmed hypoglycemia also had a 71% increase in annual diabetes-related healthcare costs when adjusted for potential, multiple confounders. Diabetes-related costs of physician office visits were significantly higher in confirmed hypoglycemia cases ($505 vs. $428), with a similar pattern for pharmacy costs ($2,939 vs. $1,583). Hypoglycemia imposes a significant clinical, economic, and humanistic burden on PWDs, caregivers, payers, providers, and health systems.
Despite being an important and effective treatment option for severe hypoglycemia, nearly one-third of adults with T1D report that they do not have a current glucagon prescription. Recent researchCitation14 has suggested that:
85% of adults with T1D said they had been prescribed glucagon by a healthcare provider
68% of adults with T1D said they had a current glucagon prescription
18% of adults with T1D who received glucagon reported that it was administered without problems or errors
Furthermore, Mitchell et al.Citation15 found that both T1Ds and T2Ds who filled glucagon prescriptions, shortly after initiating insulin therapy, had fewer hypoglycemia-related ER visits than non-early glucagon counterparts, indicating the importance of early receipt and filling of glucagon prescriptions in insulin-dependent diabetic patients.
Even among PWDs who have received a glucagon prescription, the persistency rates to refill a prescription remain low. Less than 50% of T1D and less than 1% of T2D patients renew prescriptions after 48 months Mitchell et al.Citation15 Most PWDs who receive glucagon for treatment of severe hypoglycemia reported a host of problems about the experienceCitation14. While these findings may suggest the need for improvements in prescribing practices and patient/caregiver education on administration practices, they also indicate that better glucagon formulations that are simple to use and successfully deliver a full-dose of glucagon (high functional efficacy) may be needed in order to increase the use of glucagon in acute emergency situations.
Currently approved lyophilized injectable glucagon emergency kits (GEKs) for severe hypoglycemia rescue are based on lyophilized formulations that require manual, multi-step reconstitution with a vial and syringe at time of use, and thus are difficult to administer and not well accepted by users. Additional limitations of current GEKs include:
The drug is mixed at the time of useCitation16
Limited stability in solutionCitation16
Multiple steps to injectionCitation16
1 mL intramuscular injectionsCitation16
Many possible failure modesCitation17
Creates fear and anxietyCitation18
Poor user experienceCitation17
Requires dosing measurement for pediatric vs. adult doseCitation16
A ready-to-use, room-temperature stable liquid glucagon rescue pen auto-injector (Gvoke Hypopen; GRP; Xeris Pharmaceutials, Inc.) and prefilled syringe (Gvoke PFS; G-PFS; Xeris Pharmaceuticals, Inc.) has been developed for the rescue of severe hypoglycemia events (SHEs). The GRP and G-PFS overcome current kits’ limitations through:
A premixed solution in SHL Molly auto-injector
Long-term stability in solution at room temperature
0.2 mL subcutaneous injection
Easy, rapid injection
User-friendly
GRP and G-PFS have comparable clinical efficacy to GEKs, but instead utilizes a simple two-step process to administer the full-dose of glucagon. Within a simulated emergency setting 99% of both GRPCitation17 and G-PFSCitation18 users successfully delivered a full-dose of glucagon; conversely, in other studies of marketed injectable GEKs only 6–31% of users were successfulCitation17. Further human factors studies with powder glucagon kits demonstrate that <13% of caregivers can successfully prepare and administer a full-dose of glucagon during simulated emergencies, and from a patient perspective there is a desire for a glucagon format that provides prompt and reliable full-dose drug deliveryCitation19.
Given the higher functional efficacy (successful administration of full-dose glucagon) through the GRP and G-PFS during severe hypoglycemia emergencies, the aim of this analysis was to estimate the health service use and financial impact of the GRP and G-PFS compared to other GEKs (or no kit) through a budget impact analysis from the perspective of a US commercial health plan.
Methods
A 1-year budget impact model with a hypothetical US commercial health plan population of 1 million covered lives was developed with a target population of patients with T1D and insulin-using T2D. This model is designed to illustrate the budget impact of covering GRP and G-PFS compared to currently available alternative GEKs or no kit. The model considers the adoption of the GRP and G-PFS into clinical practice and the subsequent improved successful administrations of glucagon while modeling costs related to the kits and downstream healthcare utilization related to a severe hypoglycemia.
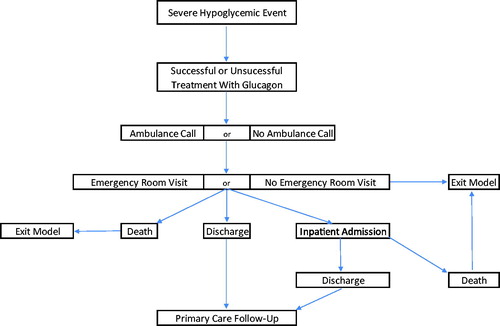
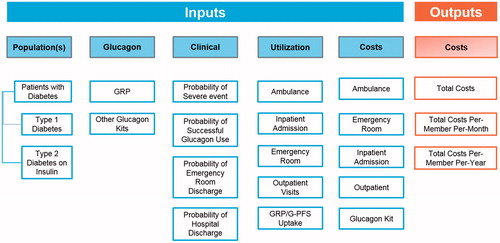
The treatment pathway driving the structure of the model can be found in . The starting patient populations are commercially insured T1D or insulin-using T2D, who are prescribed (or not prescribed) a GEK. PWDs who were prescribed a kit were assumed to have filled the prescription, which has not expired at the time of a given SHE. PWDs either experience or do not experience a SHE; if the former, glucagon is either successfully administered or not. Following successful or unsuccessful administration of glucagon, an ambulance may be called, which may or may not transport the patient to the emergency room (ER), based on their condition. PWDs also have an opportunity to be transported to the ER through non-ambulance means. If transported and treated at the ER the PWD may be admitted to the inpatient ward, discharged alive, or die. All PWDs who are not transported and treated at the ER are assumed to remain alive. If a PWD is admitted to the inpatient ward, they may either be discharged alive or die. If a patient is discharged from the inpatient ward or ER, each patient will accrue an outpatient physician follow-up visit, to ensure comprehensive care coordination, typically current standard of care in the United States. This structure gives rise to several model parameters, encompassing clinical, health resource use, and cost inputs outlined in . Parameter estimates () were drawn from the peer-reviewed literature, health service costs derived from the 2018 Medicare Fee Schedule and guided by a set of assumptions (). Key model inputs with the greatest uncertainty were varied 50% upwards and downwards through one-way sensitivity analyses to test the robustness of their base-case values on model outcomes, including: (1) market uptake assumptions; (2) probabilities of ambulance calls; (3) probabilities of ambulance trips to the ER; (4) probabilities of non-ambulance trips to the ER; (5) probability of inpatient admission following an ER visit; (6) cost of GRP and G-PFS; (7) probability of successful use of GEK; and (8) probabilities of receiving a glucagon prescription.
Table 1. Plan and market assumptions for base case model.
Table 2. Clinical and health resource use outcomes from base case analysis.
Table 3. Health care costs from base case analysis.
Table 4. Key model assumptions.
Results
The base case model results suggest that coverage and reimbursement of the GRP and G-PFS are expected to reduce the plan costs associated with the treatment of SHEs, primarily driven by reductions in ED and inpatient hospitalization costs. Before the introduction of the GRP and G-PFS, total annual plan costs for SHEs was over $8.9 million, and after introduction and uptake of the GRP and G-PFS, total plan costs of treating SHEs dropped to under $8.25 million. The costs per plan member (1 million total members) reduced by $0.73 per year, and $0.06 per plan member per month (PMPM). Therefore, the GRP and G-PFS can lead to significant savings for commercial plans – under this model framework and assuming a 45% market share, plans save over 8% by covering the GRP and G-PFS.
The estimated savings were a function of increasing the rates of successful administration of glucagon, which naturally accompanies a more user-friendly delivery mechanism. The impact of the successful use of glucagon on ambulance costs, as well as ER and inpatient hospitalization, is significant (). Combined, successful administration leads to over $1,500 savings for plans for each serious hypoglycemia event.
Table 5. Base case model results for a commercial plan with 1 million covered lives.
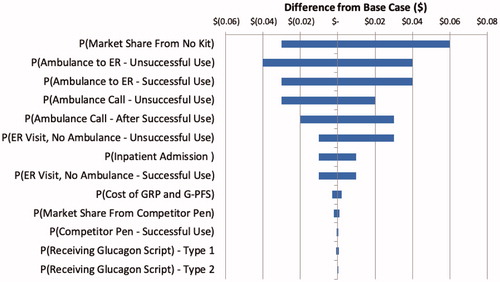
To test the robustness of the model to select model assumptions/parameter estimates, a series of one-way sensitivity analyses were performed (). The parameters adding the most uncertainty to the model were the market share/GRP and G-PFS uptake estimates, probabilities of ambulance transports to the ER following un/successful use of glucagon, and the probabilities of ambulance calls following un/successful use of glucagon. However, while the magnitude of the budget impact varied, the directionality remained consistent – the GRP and G-PFS lead to commercial plan savings over currently marketed injectable GEKs or no kit.
Discussion
A number of different studies, dating back to the 1950s, have evaluated the efficacy and safety of glucagon for severe hypoglycemia, administered either intravenously (IV), intramuscularly (IM), or subcutaneously (SC)Citation20–26. The results have been remarkably consistent in demonstrating that glucagon administered IV, IM, or SC was safe, well-tolerated, and highly effective in restoring blood glucose levels and consciousnessCitation15–17,Citation20–23. Per American Diabetes Association Guidelines for hypoglycemia: “Glucagon should be prescribed for all individuals at increased risk of level 2 hypoglycemia, defined as blood glucose <54 mg/dL (3.0 mmol/L), so it is available should it be neededCitation27. Caregivers, school personnel, or family members of these individuals should know where it is and when and how to administer it. Glucagon administration is not limited to health care professionals.”
The natural consequence of successfully administering full-dose glucagon during a SHE is the potential for preventing a costly ambulance call, emergency department visit, or hospitalization. The results of the model bear this out; the budget impact analysis demonstrated that the introduction of GRP and G-PFS produced modest plan savings. With pricing parity compared to other GEKs, accompanied by its rates of successful administration, GRP and G-PFS led to an 8% reduction in annual total plan costs, translating to a $0.06 PMPM reduction for a hypothetical commercial plan with one million covered lives. The model is limited by its reliance on a set of assumptions regarding ambulance calls and subsequent transports to the emergency room, as these data were informed by expert opinion and not derived from the peer-reviewed literature. However, these inputs were varied through sensitivity analyses, and while the magnitude of the incremental cost-benefit varied, directionality remained constant, as GRP and G-PFS was cost-saving.
The model’s results for successful intervention and cost savings should not be affected by the demographics of either the person with diabetes or the caregiver. The incidence rate from Ratzki-LeewingCitation28 was derived from aggregated data across T1Ds and T2Ds, for characteristics such as age, diabetes duration, gender, median HbA1c, drug coverage, full-time work status, shift-work status, continuous glucose monitor use, other comorbidities, medication type, type of community (rural, urban), main healthcare provider type, education status, living arrangement, and income. This incidence rate matches the estimates for an overall incidence rate in our budget impact model. Human factors studies from both the glucagon pre-filled syringe and the auto-injector demonstrate that 99% of users (adolescent and adult, trained and untrained caregivers) can successfully prepare and administer the liquid stable glucagon in an emergency setting. In other words, Xeris glucagon should be able to be used successfully when needed, when considering demographic factors such as sex, age, race, severity of the disease for the person with diabetes, and also for considerations of the demographics of the caregiver. Thus, it is believed that the results of this BIM are generalizable to the T1D and T2D community.
The clinical and economic impact of GRP and G-PFS and its efficacy profile should not overshadow its patient-centered, humanistic benefits. Glucagon may be underutilized because of perceived difficulties for both preparation (manual, powdered drug reconstitution) and administration (dose calibration), by parents and other caregivers. Experts recommend that powdered glucagon preparation and administration requires “hands on” practice and “follow-up assessment of skills” as 69% of caregivers have obvious difficultiesCitation29. Data from a summative human factors study with stable liquid glucagon, in both an auto-injector and prefilled syringe formats, observed no instances of difficulty, hesitation, and/or confusion to train and effectively use it within a simulated emergency setting. The lower training burden and ease of use of a ready-to-use stable liquid glucagon may provide significant peace of mind and confidence to patients and their caregivers. As new medications and devices are developed that continue to optimize glycemic control and to minimize or treat severe hypoglycemic episodes, a key goal must be on helping PWDs and caregivers feel safer and more confident in dealing with every day concerns of hypoglycemia. PWDs and their caregivers derive personal strength and comfort from the belief that one has the necessary resources to manage hypoglycemia-related problemsCitation30.
This budget impact analysis must be interpreted in light of its limitations. First, the model was based on several structural (e.g. future SHE not impacted by successful treatment of prior events) and parameter assumptions (e.g. market update) which were a function of a lack of evidence in the peer-reviewed and grey literature, along with other publicly available sources. However, the estimates were vetted through expert interviews among treating clinicians and the parameter assumptions were varied significantly (±50%) through sensitivity analyses to assess the model’s robustness to these assumptions, and these cost-savings were still realized. Next, the model was developed from the commercial payer perspective but utilized reimbursement rates based on the Medicare Fee Schedules to standardize the costs, as commercial health plans reimburse health services at varying rates. The result was a conservative estimate of budget impact, as Medicare rates are typically lower than commercial reimbursement; thus, commercial health plans may experience greater savings than the model suggests as a result of coverage and reimbursement of GRP and G-PFS. Finally, this budget impact model does not utilize real-world evidence for the usability of GRP and G-PFS, as these data have not yet been collected. Simulation testing for research classically seeks to reproduce features of a real-world phenomenon so that it can be studied, in order to improve health care quality and safety. Multiple formative and summative human factors studies have evaluated the use of GRP and G-PFS across adolescent and adult, trained and untrained caregivers (within simulated emergency settings that both evoke and replicate features of real-world severe hypoglycemia) with results (99%) that far exceed the usability of traditional injectable powder glucagon kits (13%)Citation19. Usability of other glucagon formats such as nasal glucagon (NG) have demonstrated human factors usability in excess of 90%, and in completed real-world evidence studies have characterized that “…no external professional emergency medical assistance was needed in any hypoglycemia episode, indicating that caregivers could administer NG and manage the emergency situation themselves”Citation31, and “the real-life effectiveness [of NG] may help reassure parents that their child can be rescued from a severe hypoglycemia situation immediately with an easy-to-use glucagon treatment even outside the home setting”Citation32. It may be reasonable that [GRP and G-PFS] also demonstrate similar success by caregivers, within a real-world setting.”
Conclusions
GRP and G-PFS allow for easy administration of liquid stable glucagon by caregivers and improves the successful administration of a full-dose of glucagon. The usability advantage of GRP and G-PFS over-marketed GEKs significantly reduces user errors and may further reduce utilization of emergency medical services, emergency department, overall inpatient and outpatient costs for SHE, along with potentially reducing mortality. Introducing GRP and G-PFS to the market is likely to reduce the budget a commercial health care payer spends across the entire SHE treatment pathway, representing an opportunity to optimize commercial payers’ resources. Our budget impact model suggests that significant overall annual cost savings for U.S. commercial payers can be achieved with GRP and G-PFS.
Transparency
Declaration of funding
Research support provided by Xeris Pharmaceuticals.
Declaration of financial/other interests
AN, JM, KJ are employees of Xeris Pharmaceuticals and hold stock in Xeris Pharmaceuticals. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
BL: Conducted research, manuscript development; MJ: Conducted research, manuscript development; AN: Conducted analysis, interpretation, and manuscript development; JM: Conducted analysis, interpretation, and manuscript development KJ: Conducted analysis, interpretation, and manuscript development.
Previous presentations
AMCP Annual Meeting, 25–28 March 2019; San Diego, CA.
Acknowledgements
None reported.
References
- Diabetes Control and Complications Trial (DCCT) Research Group. The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996;45(10):1289–1298.
- The Diabetes Control and Complications Trial (DCCT) Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes. 1997;46(2):271–286.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32(1):187–192.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J. 2000;321(7258):405–412.
- Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341(8856):1306–1309.
- Amiel SA, Dixon T, Mann R, et al. Hypoglycaemia in type 2 diabetes. Diabetic Med. 2008;25(3):245–254.
- Clarke W, Jones T, Rewers A, et al. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2009;10(12):134–145.
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–1912.
- Cryer PE. Hierarchy of physiological responses to hypoglycemia: relevance to clinical hypoglycemia in type I (insulin dependent) diabetes mellitus. Horm Metab Res. 1997;29(03):92–96.
- Laing SP, Swerdlow AJ, Slater SD, et al. The British Diabetic Association Cohort Study, II: cause-specific mortality in patients with insulin-treated diabetes mellitus. Diabet Med. 1999;16(6):466–471.
- Campbell IW. Metformin and the sulphonylureas: the comparative risk. Horm Metab Res Suppl. 1985;15:105–111.
- Williams SA, Shi L, Brenneman SK, et al. The burden of hypoglycemia on healthcare utilization, costs, and quality of life among type 2 diabetes mellitus patients. J Diab Comp. 2012;26(5):399–406.
- Haymond MW, Liu J, Bispham J, et al. Inadequate use of glucagon in patients with type 1 diabetes. Clin Diab. 2018;67(1):383-P.
- Mitchell BD, He X, Sturdy IM, et al. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocrine Pract. 2016;22(2):123–135.
- GlucaGen® (glucagon [rDNA origin] for injection) Hypokit® Instructions for Use. Novo Nordisk A/S. 25 April 2014.
- Valentine V, Newswanger B, Prestrelski S, et al. Human factors usability and validation studies of a glucagon autoinjector in a simulated severe hypoglycemia rescue situation. Diab Tech & Therap. 2019;21(9):522–530.
- Newswanger B, Prestrelski S, Andre AD. Human factors studies of a prefilled syringe with stable liquid glucagon in a simulated severe hypoglycemia rescue situation. Expert Opin Drug Deliv. 2019;16(9):1015–1025.
- Yale JF, Dulude H, Egeth M, et al. Faster use and fewer failures with needle-free nasal glucagon versus injectable glucagon in severe hypoglycemia rescue: a simulation study. Diabetes Technol Ther. 2017;19(7):423–432.
- Eldrick H, Witten TA, Arai Y. Glucagon treatment of insulin reactions. N Engl J Med. 1958;258:476–480.
- Vukmir RB, Paris PM, Yealy DM. Glucagon: prehospital therapy for hypoglycemia. Ann Emerg Med. 1991;20(4):375–379.
- Steel JM, Allwinkle J, Moffat R, et al. Use of Lucozade and glucagon by ambulance staff for treating hypoglycemia. Br Med J. 1992;304(6837):1283–1284.
- Patrick AW, Collier A, Hepburn A, et al. Comparison of intramuscular glucagon and intravenous dextrose in the treatment of hypoglycemia coma in an accident and emergency department. Arch Emerg Med. 1990;7(2):73–77.
- Carstens S, Sprehn M. Prehospital treatment of severe hypoglycaemia: a comparison of intramuscular glucagon and intravenous glucose. Prehosp Disaster Med. 1998;13(2–4):44–50.
- Namba M, Hanafusa T, Kono N, et al. Clinical evaluation of biosynthetic glucagon treatment for recovery from hypoglycemia developed in diabetic patients. Diabetes Res Clin Pract. 1993;19(2):133–138.
- Northam EA, Anderson PJ, Jacobs R, et al. Neuropsychological profiles in children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24(9):1541–1546.
- American Diabetes Association. Glycemic targets: standards of medical care in diabetes—2020. Diabetes Care. 2020;43(Suppl 1):S66–S76.
- Ratzki-Leewing A, Harris SB, Mequanint S, et al. Real-world crude incidence of hypoglycemia in adults with diabetes: results of the InHypo-DM study, Canada. BMJ Open Diab Res Care. 2018;6(1):e000503.
- Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes. 2011;4:337–346.
- Polonsky WH, Fisher L, Hessler D, et al. Investigating hypoglycemic confidence in type 1 and type 2 diabetes. Diabetes Technol Ther. 2017;19(1):1–6.
- Seaquist ER, Dulude H, Zhang XM, et al. A prospective study evaluating the use of nasal glucagon for the treatment of moderate to severe hypoglycemia in adults with type 1 diabetes in a real-world setting. Diabetes Obes Metab. 2018;20(5):1316–1320.
- Deeb LC, Dulude H, Guzman CB, et al. A phase 3 multicenter, open-label prospective study designed to evaluate the effectiveness and ease of use of nasal glucagon in the treatment of moderate and severe hypoglycemia in children and adolescents with type 1 diabetes in the home or school setting. Pediatr Diabetes. 2018;19(5):1007–1013.
- Diabetes Age and Sex Composition: 2010. 2010 Census Briefs [Internet]. U.S. Department of Commerce, Economics and Statistics Administration, U.S. Census Bureau; Washington, DC; [cited 2014 Aug 26]. Available from: http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf.
- National Diabetes Statistics Report, 2013. Estimates of Diabetes and Its Burden in the United States [Internet]. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Washington, DC; [cited 2014 Aug 26; 2019 Jun 30]. Available from: http://www.cdc.gov/Diabetes/pubs/estimates11.htm#6; https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
- Li C, Ford ES, Zhao G, et al. Trends of insulin use among US adults with type 2 diabetes: the behavioral risk factor surveillance system, 1995–2007. J Diab Complicat. 2012;26(1):17–22.
- Frier BM. The incidence and impact of hypoglycemia in type 1 and type 2 diabetes. Int Diab Mon. 2009;21(6):210–218.
- Edridge CL, Dunkley AJ, Bodicoat DH, et al. Prevalence and incidence of hypoglycemia in 532,542 people with type 2 diabetes on oral therapies and insulin: a systematic review and meta-analysis of population based studies. PLoS One. 2015;10(6):e0126427.
- Ginde AA, Espinola JA, Camargo CA. Trends and disparities in U.S. emergency department visits for hypoglycemia, 1993–2005. Diabetes Care. 2008;31(3):511–513.
- Parsaik AK, Carter RE, Pattan V, et al. Population-based study of severe hypoglycemia requiring emergency medical service assistance reveals unique findings. J Diabetes Sci Technol. 2012;6(1):65–73.
- 2018 Medicare Fee Schedules. U.S. Centers for Medicare and Medicaid Services; Baltimore, MD; [cited 2018 Apr 9]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/FeeScheduleGenInfo/index.html.