Abstract
Background: Previous studies support operational benefits when moving insertable cardiac monitor (ICM) insertions outside the cardiac catheterization/electrophysiology laboratories, but this has not been directly assessed in a randomized trial or when the procedure is specifically moved to the office setting. To gain insight, the RIO 2 US study collected resource utilization and procedure time intervals for ICM insertion in-office and in-hospital and these data were used to calculate costs associated with staff time and supply use in each setting.
Methods and results: The Reveal LINQ In-Office 2 US study (randomized [1:1], multicenter, unblinded) included 482 patients to undergo insertion of the ICM in-hospital (in an operating room or CATH/EP laboratory) (n = 231) or in-office (n = 251). Detailed information on resource utilization was collected prospectively by the study and used to compare resource utilization and procedure time intervals during ICM insertion procedures performed in-office vs. in-hospital. In addition, costs associated with staff time and supply use in each setting were calculated retrospectively. Total visit duration (check-in to discharge) was 107 min shorter in-office vs. in-hospital (95% CI = 97−116 min; p < 0.001). Patient preparation and education in-office were more likely to occur in the same room as the procedure, compared with in-hospital (91.6% vs. 34.2%, p < 0.001 and 87.3% vs. 22.1%, p < 0.001, respectively). There was a reduction in registered nurse and cardiovascular/operating room technologist involvement in-office, accompanied by higher physician and medical assistant participation. Overall staff time spent per case was 75% higher in-hospital, leading to 50% higher staffing costs compared to in-office.
Conclusions: ICM insertion in a physician’s office vs. a hospital setting resulted in reduced patient visit time and reduced overall staff time, with a consequent reduction in staffing costs.
Clinical trial registration: ClinicalTrials.gov NCT02395536
Introduction
Insertable cardiac monitors (ICMs) allow for continuous, long-term monitoring of cardiac arrhythmias and other physiologic parametersCitation1. Several studies have shown the safety and operational benefits of moving ICM insertions to less resource intensive hospital settingsCitation2–5. However, the Reveal LINQ In-Office (RIO) 2 randomized US study was the first to demonstrate that insertion of the ICM could also be safely performed in an office setting outside the hospital. RIO 2 compared in-office vs. EP/CATH laboratory insertions and reported no infections and a similarly low rate of complications related to the ICM or the procedure (0.8% and 0.9%, respectively)Citation6. These safety data have contributed to a change in US Medicare reimbursement rules allowing the insertion or removal procedure outside a hospital-based facility or ambulatory surgical center.
One of the primary driving factors for expansion of office-based procedures is to increase the cost-effectiveness of medical careCitation7,Citation8. Additional benefits of moving ICM procedures out of the hospital may include increased availability of operating rooms, CATH/EP laboratories, improved continuity of care, increased patient satisfaction, physician and patient convenience, decreased wait times, and prevention of nosocomial infectionsCitation7,Citation9.
While the office environment is safe for ICM insertion, and may afford some patient and provider benefits, it is unknown whether in-office device insertion results in operational efficiencies. To gain insight, the RIO 2 US study assessed resource utilization and procedure time intervals for ICM insertion in-office and in-hospital. These data were used to calculate costs associated with staff time and supply use in each setting.
Methods
Study design
The RIO 2 US study (ClinicalTrials.gov NCT02395536) was a randomized (1:1), un-blinded, multicenter, prospective, parallel group trial. Study methods have been reported in detail previouslyCitation6. Briefly, patients indicated for an ICM who were willing to undergo device insertion outside of a traditional hospital setting were enrolled. Patients were randomized in a 1:1 ratio stratified by study site to either undergo insertion of the ICM (Reveal LINQ, model LNQ11, Medtronic Inc., Minneapolis, MN) in-hospital (in an operating room, or CATH/EP laboratory) (n = 231) or in-office (n = 251). The study was conducted in accordance with the Declaration of Helsinki and approved by each site’s Institutional Review Board (26 sites located in the US). All patients provided written informed consent prior to participating.
Insertion procedure
In-office location was defined as a procedure room, exam room, or office with controlled entry and hard floors outside the hospital walls and not an ambulatory surgery center. The study protocol required use of the Reveal LINQ ICM incision and insertion tools, and the Reveal LINQ ICM supply kit (). Use of individual materials from the supply kit was based on physician preference. At a minimum, all physicians were required to use a surgical hand antiseptic prior to the procedure, wear sterile gloves, gown and mask, and practice a sterile technique. Additionally, at a minimum, all patients were required to be draped or wear a mask during device insertion. Use of prophylactic antibiotics, local anesthesia, and anxiolytic medication were based on physician discretion and patient preference. Sedation was not permitted and use of post-procedure antibiotics was not recommended, unless required due to patient safety. The insertion location was closed using adhesive strips, surgical glue, sutures, staples, or a combination of these methods depending on physician practice. All implanting physicians had prior experience inserting the ICM.
Table 1. Individual supply use from the Reveal LINQ ICM supply kit.
Resource utilization
Detailed information was recorded for time intervals, staff, supply and room utilization during pre-insertion preparation, device insertion, and post-insertion activities. In particular, time intervals were collected in real-time on case report forms, irrespectively of the location as prespecified in the study protocol. Staff costs were calculated utilizing US national average hourly wages plus benefitsCitation10–12. Procedure supply costs were estimated using publicly available data on the retail cost of medical suppliesCitation13 and weighted based on the proportion of procedures utilizing each supply item.
Statistics
The study’s sample size of 476 patients was based on the study’s primary objectiveCitation6. Thus, comparisons of procedure times and resources were not prospectively powered. Total staff time per procedure period were defined as the sum of time across all staff members supporting the procedural period. Procedure and staff times were compared between groups using linear mixed effects models to account for correlation within the study center. Repeated measures logistic regression employing generalized estimating equations were used to compare staffing requirements and to account for multiple patients at each study site. Randomized patients having an ICM insertion attempt were included and analyzed with respect to their randomization assignment. Comparisons were considered statistically significant at the nominal 0.05 level. Analyses were conducted using SAS version 9.4 (SAS Institute) or R (www.r-project.org).
Results
Study population
The study enrolled 525 patients at 26 centers in the US between 30 March 2015 and 19 January 2016. Four patients did not meet inclusion criteria and were excluded prior to randomization. Of the 521 randomized to in-office or in-hospital groups, an ICM insertion attempt occurred in 482, all of which were successful. The present analysis cohort includes 482 patients (251 and 231 allocated to in-office and in-hospital groups, respectively). Exits after enrollment, patient baseline characteristics, ICM insertion site in the body, repositioning attempts, wound closure methods, adverse events, and results from a physician questionnaire completed after each procedure have been previously reportedCitation6. The present analysis cohort includes 251 and 231 patients in the in-office and in-hospital groups, respectively.
Procedure duration
shows procedure time intervals according to procedure location in-office or in-hospital. Definitions of these time intervals are shown in . Total visit duration for the ICM insertion procedure (patient check-in to discharge including all waiting periods) was on average 107 min shorter (95% CI = 97 − 116 min; p < 0.001) when the insertion occurred in-office (81 ± 31 min) rather than in-hospital (188 ± 80 min). Time savings for the overall visit resulted from shorter patient preparation times (the time required for clinical assessment, changing clothes, and surgical site preparation) and process times (the time from the start of the patient preparation to incision closure, including the wait time between patient preparation and the start of device insertion). The reduction in overall procedure duration and process duration associated with moving the procedure in-office was consistent across study centers and there was no association between the number of ICM insertion procedures performed at a study site and total procedure or process duration ().
Figure 1. Time intervals according to procedure location. Box plots represent the duration of different procedure time intervals according to procedure location in-office or in-hospital.
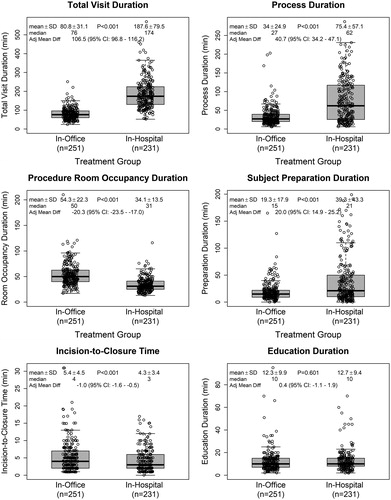
Figure 2. Definitions of time intervals recorded during the study. Total visit duration (time from patient check-in to discharge, including all waiting periods); process duration (time from the start of the patient preparation to incision closure, including the wait time between patient preparation and the start of device insertion); procedure room occupancy duration (time from when patient entered the procedure room to when they exited; variable whether education was delivered in the same room as the procedure or in a different location); patient preparation duration (time required for clinical assessment, changing clothes and surgical site preparation); incision-to-closure time (procedure duration); and patient education (time required to educate patients on the use of their ICM device and care of their insertion wound).
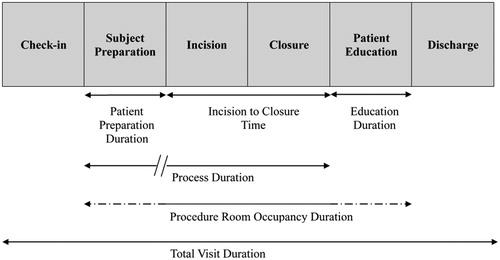
Figure 3. Relationship between number of ICM insertion procedures at each study center and total visit duration (left panel) and process duration (right panel). Point size is proportional to the number of ICM insertion procedures performed. Solid lines indicate predicted average duration and dashed lines display the 95% confidence intervals for the predicted average.
Pre-insertion resource utilization
Patient preparation in-office was more likely to occur in the same room as the procedure (91.6%) compared with the in-hospital group (34.2%, p < 0.001). Prophylactic antibiotics (mainly cephalosporin) were used in 43.8% of patients in the in-office group (all oral) compared to 46.8% in-hospital (41.6% oral; 5.2% intravenous). Pre-procedure anxiolytic medication use was low (4%) and site-specific. Local anesthesia was used in all 482 procedures regardless of location (mainly lidocaine with or without epinephrine).
The surgical site was most commonly prepared by clipping and shaving (if necessary), followed by skin disinfection using an antiseptic. Physicians used a surgical hand antiseptic (e.g. Avagardi) in 79% of procedures, and 24% performed a traditional surgical scrub prior to inserting the ICM in-office. In-hospital, 55% of physicians used a surgical hand antiseptic and 52% performed a traditional surgical scrub. Physicians most commonly wore a mask, gown, surgical head covering, and single layer of sterile gloves regardless of procedure location ().
Table 2. Pre-insertion resource utilization.
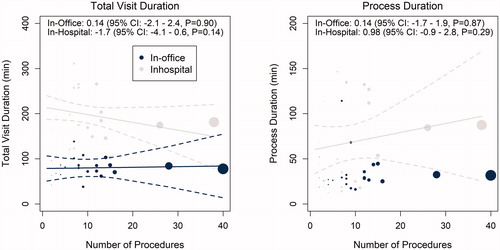
shows that physicians were more likely to be involved in pre-insertion patient preparation in-office (51%) vs. in-hospital (40%) (p = 0.026). Total staff time spent on pre-procedure activities averaged 26.9 min in-office vs. 41.3 min in-hospital (p < 0.001) ().
Figure 4. Staff involvement at each phase of the procedure: pre-insertion (left panel), insertion (center), and post-insertion (right). *p-value < 0.001 calculated by repeated measures logistic regression model using generalized estimating equations to account for multiple patients at each study site. †Nurse practitioners participated in nine insertion procedures at three study sites. ‡Physician assistants participated in nine insertion procedures at one study site. §Nurse practitioners participated in post-procedure education activities following five procedures at one study site. ∥Physician assistants participated in post-procedure education activities following 10 procedures at three sites.
Table 3. Staffing costs.
Insertion procedure resource utilization
At least one item from the supply kit was used in 92% and 93% of in-office and in-hospital procedures, respectively (). The cost of supplies used in-office and in-hospital was comparable ($27.5 and $26.3, respectively) (). All procedures were performed by a physician, and physician time spent was not different between locations (p = 0.998). RNs and CV/OR technologists were less likely to participate in procedures performed in-office vs. in-hospital (RNs: 54% vs. 86%; CV/OR technologists: 6% vs. 43%, both p < 0.01) (). When RNs participated they spent on average 9.4 fewer minutes (95% CI = 6.0 − 12.8 min; p < 0.001) on procedure activities in-office relative to in-hospital, where their procedure activity time was 24 ± 20 min. Overall, the average staff time spent in office-based procedures was 21.2 min vs. 39.9 min in hospital-based procedures (p < 0.001) ().
Table 4. Cost of supply use from the Reveal LINQ ICM supply kit.
Post-insertion resource utilization
Patient education on wound care and device use occurred in the same room as the procedure in 87% of in-office and 22% of in-hospital insertions (p < 0.001). Other locations used for patient education in-office were waiting area (7%), dedicated recovery room (3%), or exam room (2%), whereas other locations in-hospital were recovery room (54%), waiting area (16%), or exam room (5%). Physicians tended to participate more frequently in patient education in-office compared with in-hospital (26% vs. 20%, p = 0.065), and the time spent was slightly lower in-office (6 ± 6 vs. 7 ± 6 min, p = 0.055). Staff time spent on post-procedure activities averaged 11.5 min in-office vs. 23.2 min in-hospital (p < 0.001) ().
Total resource utilization and costs
The total staff time spent per ICM insertion was lower in an office setting compared with a hospital setting (59.5 vs. 104.4 min, p < 0.001) (). Correspondingly, the total cost of staff time was lower in an office setting ($72 vs. $108).
Discussion
In the present analysis, we compared resource utilization between inserting an ICM in-office vs. in-hospital in a prospective randomized study. Given that US Medicare reimbursement for in-office insertion of ICMs is currently available, the data reported in this analysis in conjunction with the previous primary RIO 2 publicationCitation6 are of particular relevance for informing site of service decisions. We observed three primary differences in resource utilization when inserting the ICM in-office vs. in-hospital: visit duration, staff involvement, and resource utilization.
When the ICM was inserted in-office, there was a reduction in overall visit time (patient check-in to discharge). This was due in part to a decrease in the time required for patient preparation (clinical assessment, medical history, changing clothes) and may reflect efficiencies that streamline these activities in the office vs. hospital setting. Another key driver of the decreased overall visit time was a reduction in patient wait times. This is supported by the observation that there were no meaningful differences in the time required for device insertion (incision-to-closure) or patient education – key aspects of the overall insertion procedure. Moreover, physicians reported fewer insertion procedures being delayed over 15 min in the office vs. hospital setting, as previously publishedCitation6. Some of the reduction in wait times may have resulted from decreased time spent on patient transport, as it was more common for patient preparation and education to occur in the procedure room when the ICM was inserted in-office compared with in-hospital. The reduction in total visit time has significant implications for improved patient experience and decreased time burden on both patients and caregivers.
Our results are consistent with findings from Kanters et al.Citation14 which compared ICM insertion durations of the previous iteration of the ICM (Reveal XT) in a CATH/EP laboratory to the Reveal LINQ ICM inserted in a hospital procedure room. As seen in the present study, they reported a reduction in total visit duration of ∼100 min for insertions performed in a procedure room, partly achieved through time savings in pre-procedure time (5–10 min decrease). However, the largest driver for the reduction in visit duration was elimination of the post-procedure recovery period, as patients receiving a Reveal LINQ ICM in a procedure room were not admitted to the hospital day care ward. Indeed, the Reveal XT ICM being larger in size and the procedure more invasive, changes in iteration and insertion technique may have led to the observed time savings. Overall, our findings add to evolving evidence that insertion of ICM devices outside of an OR or CATH/EP laboratory can result in operational efficiencies that provide time savings for patients.
The other key difference observed between in-office and in-hospital insertions was a shift in staff involvement. Specifically, when the ICM was inserted in-office, there was a reduction in RN and CV/OR technologist involvement during all phases of the procedure. In addition, RNs spent less time during all phases of device insertion when the procedure was performed in-office. In contrast, physician involvement during patient preparation was higher in-office, and medical assistants participated more frequently in insertion procedures and post-insertion activities when these took place in the office setting. The observed variances in staff participation likely reflect differences in the make-up of personnel between insertion environments.
Differences in resource utilization were also observed between the two settings. Overall staff time spent per case was 75% higher in the hospital setting compared to in-office. The longer staff time required, in combination with a shift in personnel involvement, led to 50% higher staffing costs in the hospital setting compared to an office setting. In addition, beyond the obvious difference in facilities cost of inserting ICMs in office vs. in hospital settings, freeing up 34 min in the hospital operating room or CATH/EP laboratory improves patient access for procedures requiring the intensive resources available in those settings.
Other aspects of the procedure, such as patient and physician preparation and use of supplies, were similar between the two settings with few exceptions. Physicians in-office preferred using a surgical hand antiseptic instead of performing a surgical scrub, probably due to the absence of a sink in the procedure or exam room. Moreover, patients in-office were more frequently required to wear a mask instead of a gown, in addition to being draped. The use of prophylactic antibiotics in our study was similar between settings (less than half of the procedures) and no infections were recordedCitation6. Other studies performing the procedure outside the traditional hospital settings have also shown that use of a sterile technique is associated with an overall safety profile for Reveal LINQ ICM insertionsCitation3–6,Citation15,Citation16. Although the use of antibiotics was variable when inserting the ICM in less resource intensive hospital settings (0–100%) it did not impact the infection rate, which remained low (0–1.6%) regardless of the site of serviceCitation2–5,Citation16.
Limitations
While certain aspects of the insertion procedure were constrained by the protocol to ensure patient safety, the care pathway within the office and hospital environments was left to the discretion of each site. The goal of this study was not to design an insertion procedure workflow, but rather observe differences within current systems and environments. Accordingly, the lack of protocol constraints on the care pathway in this study was intentional to capture real world variations in practice management.
Hospital cost data from participating centers were not captured as part of this study. Collecting financial data reflective of the actual hospital cost burden associated with care delivery is a challenge due largely to the inconsistency of cost accounting systems across institutions and departments, leaving results from such cost comparisons unreliable and often inaccurateCitation17. To understand true hospital cost data a standardized costing protocol would be needed, which was outside the scope of the study. Presented cost data were sourced from US nationally representative sources and may not reflect actual costs incurred by the study centers; however, this may contribute to the generalizability of the reported findings.
Conclusions
Insertion of an ICM device in a physician’s office vs. a hospital setting resulted in reduced patient visit time and overall staff time, with a consequent reduction in staffing costs.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Transparency
Declaration of funding
The study was funded by Medtronic, Inc. Medtronic personnel were involved in study design, analysis and interpretation of the data, and writing of this manuscript.
Declaration of financial/other relationships
JDR reports honoraria from Abbott and Medtronic; CP reports honoraria from Medtronic, Abbott, Biotronik, Siemens, and Biosense Webster, and research support from Abbott, Biotronik, and Imricor; MRS reports a research grant from Medtronic and honoraria from Medtronic, Spectranetics, Boston Scientific, and Aziyo Biologics; RA reports honoraria from Medtronic; MK reports honoraria from Medtronic; SR and KS are Medtronic employees and shareholders; PS is supported by a Practitioner Fellowships from the National Health and Medical Research Council of Australia and by the National Heart Foundation of Australia; has received research funding from Abbott, Boston Scientific, Biotronik and Sorin; and has served on advisory boards and received lecture/consulting fees for Biosense Webster, Medtronic, Abbott, Boston Scientific, and Rx (advisory board only).
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
We would like to thank Lindsay Werder for her outstanding work managing the study as well as Rachelle Kaplon and Noreli Franco for their contribution in result interpretation and writing of the manuscript.
Notes
Notes
1 Avagard is a trademark of 3M Healthcare, St Paul, MN, USA.
References
- Tomson TT, Passman R. The Reveal LINQ insertable cardiac monitor. Expert Rev Med Devices. 2015;12(1):7–18.
- Beinart SC, Natale A, Verma A, et al. Real-world comparison of in-hospital Reveal LINQ insertable cardiac monitor insertion inside and outside of the cardiac catheterization or electrophysiology laboratory. Am Heart J. 2019;207:76–82. Jan
- Diederichsen SZ, Haugan KJ, Hojberg S, et al. Complications after implantation of a new-generation insertable cardiac monitor: results from the LOOP study. Int J Cardiol. 2017;241:229–234.
- Kipp R, Young N, Barnett A, et al. Injectable loop recorder implantation in an ambulatory setting by advanced practice providers: analysis of outcomes. Pacing Clin Electrophysiol. 2017;40(9):982–985.
- Maines M, Zorzi A, Tomasi G, et al. Clinical impact, safety, and accuracy of the remotely monitored implantable loop recorder Medtronic Reveal LINQTM. Europace. 2017;20(6):1050–1057.
- Rogers JD, Sanders P, Piorkowski C, et al. In-office insertion of a miniaturized insertable cardiac monitor: results from the Reveal LINQ In-Office 2 randomized study. Heart Rhythm. 2017;14(2):218–224.
- Balkrishnan R, Hill A, Feldman SR, et al. Efficacy, safety, and cost of office-based surgery: a multidisciplinary perspective. Dermatol Surg. 2003;29(1):1–6.
- Patel N, Hingorani A, Ascher E. Office-based surgery for vascular surgeons. Perspect Vasc Surg Endovasc Ther. 2008;20(4):326–330. Dec
- Hancox JG, Venkat AP, Coldiron B, et al. The safety of office-based surgery: review of recent literature from several disciplines. Arch Dermatol. 2004;140(11):1379–1382.
- Bureau of Labor Statistics USDoL. Occupational Employment Statistics; May 2017 [cited 2019 Apr 26]. Available from: www.bls.gov/oes/.
- Bureau of Labor Statistics USDoL. Employer Costs for Employee Compensation; June 2018 [cited 2019 Apr 26]. Available from: https://www.bls.gov/news.release/pdf/ecec.pdf.
- Reed SD, Li Y, Kamble S, et al. Introduction of the tools for economic analysis of patient management interventions in heart failure costing tool: a user-friendly spreadsheet program to estimate costs of providing patient-centered interventions. Circ Cardiovasc Qual Outcomes. 2012;5(1):113–119.
- Cascade Healthcare Solutions, Renton, WA. 2019 [Accessed 2019 Mar 29]. [cited 2019 Mar 29]. Available from: cascadehealthcaresolutions.com.
- Kanters TA, Wolff C, Boyson D, et al. Cost comparison of two implantable cardiac monitors in two different settings: Reveal XT in a catheterization laboratory vs. Reveal LINQ in a procedure room. Europace. 2016;18(6):919–924.
- Lee JJ, Weitz D, Anand R. Holding Area LINQ Trial (HALT). Indian Pacing Electrophysiol J. 2017;17(6):163–166.
- Wong GR, Lau DH, Middeldorp ME, et al. Feasibility and safety of Reveal LINQ insertion in a sterile procedure room vs. electrophysiology laboratory. Int J Cardiol. 2016;223:13–17.
- Tompkins CP, Altman SH, Eilat E. The precarious pricing system for hospital services. Health Aff (Millwood). 2006;25(1):45–56.
