Abstract
Aims: To estimate the budgetary impact of providing additional reimbursement for long acting injections for schizophrenia patients in psychiatric hospital settings in Japan to improve patient outcomes in schizophrenia.
Methods: Budget impact analysis of change in reimbursement policy using a prevalence-based model over a five-year time horizon. The results are reported as net change in expenditure and consequent cost/savings in Japanese yen at the time of analysis.
Results: The budget impact analysis shows that an increase in reimbursement for LAIs could lead to cumulative savings of an estimated 36.6 billion JPY over five years. These savings result from a decrease in hospitalization costs and an increased usage of LAI (assumed to be 10%). Based on the sensitivity analysis, the saving estimates are most sensitive to change in market share of generic and branded oral antipsychotics.
Limitations: Historical data were used to estimate the future costs of drug and hospitalization; however, it is not the best predictor of future, hence a source of potential bias. A good level of treatment adherence with oral antipsychotics was assumed, which is generally not the case; therefore, we might have overestimated the effectiveness of oral atypical antipsychotics. Additionally, the drug cost due to reimbursement might have also been overestimated because in clinical setting, the increase of LAI use may not have reached 10% of the market share. Lastly, patients’ behavior was derived from models, which may have loosely approximated the reality.
Conclusions: An additional reimbursement for the use of LAI in schizophrenia patients is likely to be cost neutral/cost saving and should be considered as a policy option to improve patient outcomes and budget sustainability.
Introduction
Symptom control and relapse prevention remains the main treatment goal in schizophrenia, which is associated with direct high treatment costCitation1. Despite various treatment options available and used in clinical practice, frequent relapse and lack of symptom control remains an issue that contributes to the chronicity of schizophreniaCitation2 leading to poor health outcomes for patients in Japan. Low adherence to oral medications has been reported as a major risk factor contributing to frequent relapse and poor symptom controlCitation3,Citation4, including reduction of violent or aggressive behavior leading to incarcerationCitation5,Citation6.
Kishimoto et al.Citation5 observed that discontinuation of medication increases the risk of relapse in schizophrenia patients by up to six times compared to the patients on continuous treatment for acute or chronic schizophrenia. Long acting injectable (LAI) antipsychotics for schizophrenia are intramuscular injections that are administered once every two or four weeks and help maintaining an effective and safe drug concentration in bloodCitation3. Evidence shows that the use of LAIs lowers the risk of relapse in schizophrenia patientsCitation2 and leads to fewer emergency room visits and hospitalizationsCitation7. Studies have reported a significantly higher treatment continuation with LAIsCitation8, longer time in remission compared to oral antipsychoticsCitation9, and better functioning in communitiesCitation10,Citation11. In addition, patients on LAI treatment in Japan have favorable attitudes toward use of LAIsCitation12. However, despite all the evidence and long history of LAI usage (more than 30 years) in JapanCitation13, use of LAIs remains relatively lowCitation14 potentially because of the reimbursement system in JapanCitation15 as well as lack of knowledge regarding LAI or physician preferenceCitation16.
In 2017, nearly 154,000 patients with schizophrenia were hospitalized and another 639,000 were treated in outpatient settingsCitation14 in Japan. The associated medical costs accounted for 80% of all cost incurred in psychiatric hospitalsCitation17. Furthermore, a study shows that nearly half (47%) of these in-patients with schizophrenia had more than five years of hospital stayCitation18, and that nearly half of all schizophrenia patients in chronic wards had no complications (i.e. their symptoms were in control)Citation19. This brings into question the prevailing practice of long hospital stays that puts high pressure on healthcare budget and makes it unsustainable from a healthcare cost perspective in the long run. A more sustainable approach would be to shift patients to lower cost settings such as outpatient or community-based care once they achieve symptom control and are no longer required clinically to be hospitalized. This shift in practice may help improve the sustainability of healthcare expenditure on psychiatric illnesses in Japan and would align well with Government’s policy intended to reduce overall length of hospitalization, integration of patients into community, and reduce the number of psychiatric beds in Japan, which is currently 2.62 per 1,000 habitants, the highest among Organisation for Economic Co-operation and Development (OECD) countriesCitation20.
With rapidly aging population and growing healthcare expenditure as a share of gross domestic product (GDP) in Japan, the Japanese government has introduced policies and incentives to reduce length of hospital stayCitation19. Introduction of differentiated hospital fee based on the patient condition is one such example. Under this approach, psychiatric emergency hospitals receive a higher fee in the first 90 days to incentivize patient discharge after acute treatmentCitation21. However, these government incentives have not been effective for a variety of reasons, as a result the healthcare expenditure on psychiatric conditions continues to rise unabated.
A complexity that is unique to Japan is the fixed fee per patient paid to psychiatric hospitals. This fixed fee includes medication cost as well as accommodation cost, and acts as a “fixed budget” per patient. This “fixed fee” model leaves very little money to cover innovative medicines in psychiatric hospitals that could help reduce the risk of relapse in patients and consequently reduce the overall length of hospital stay for schizophrenia patients.
Universal Health Insurance (UHI) in JapanCitation22 provides Japanese population with good access to primary care and hospital services. The patient co-payment rates vary between 10% for patients above 70 years of age and 30% for those under 70 years. Out-of-pocket payment is capped with ceilings based on the income. For instance, the maximum payment for low income households is JPY 35,400 (USD 307) per month. This essentially means that Government covers most (70–90%) of the total cost of care in Japan. A shorter stay in hospital and a net reduction in re-admission rates would be desirable from the Government’s perspective.
For the purpose of this analysis, we assume that psychiatric hospitals are willing to use LAIs if deemed clinically appropriate but the primary reason for current low usage is the fixed fee per patient. Under the fixed fee model, the cost of hospitalization accounts for most, if not all of the fee, forcing clinicians to use older medicines that maybe less effective in preventing a schizophrenia relapse. This system forces physicians to use only inexpensive (mostly genericized) oral drugs which may not be as effective in reducing relapse risk (unpublished market share) due to lower adherence. We postulate that if hospitals were incentivized with additional reimbursement for LAIs, there is an increased likelihood of direct and tangible benefits for patients and the Japanese healthcare system in form of: (1) better health outcomes for schizophrenia patients with better symptom control, (2) a reduction in rates of re-hospitalization (owing to better relapse prevention), and (3) an overall reduction in healthcare costs in the long run creating more “headroom” for investment in other mental health priorities such as dementia and mood disorders. This approach could also contribute toward a longer-term healthcare sustainability agenda of the government.
Methods
Overview
Budget impact analysis comparing current state (modeled as base case) with low LAI usage vs. a future state (the alternative scenario) where LAI usage is improved due to change in reimbursement policy was conducted. The model used a time horizon of 5 years and included costs of drug, hospitalization, and injection fee from a payer’s perspective, across the two scenarios.
No ethics committee review was required since this research did not include human subject data. Individual patient level information was not used, and the research relies purely on published or simulated data.
Model parameters
Population
The study population comprised of patients that have chronic schizophrenia and a history of relapse and re-admission/hospitalization.
Market forecasting
To build the base case and alternative scenario, a virtual forecast based on historical Japanese patient share data for patients with schizophrenia from 2017 January to 2019 June () was used. For the base case, market related patient share data for risperidone (including LAI/branded oral/generic versions), Paliperidone (including LAI/branded oral versions), aripiprazole (including LAI/branded oral/generic versions), and olanzapine (including branded oral/generic versions) were used. For the alternative scenario, historical patient share data were extrapolated to the future by fitting logarithmic curve to each product’s current patient share. We included atypical antipsychotics in Japan. Typical antipsychotics were not included because it is not recommended by the Japanese Society of Neuropsychopharmacology treatment guidelineCitation23.
Table 1. Forecast of schizophrenia medications.
Key assumptions
For the alternative scenario, it was assumed that an additional 10% patient would receive LAI every year starting from year 2 based on clozapine market share (unpublished) change after additional reimbursement was implemented in 2018Citation24. It was further assumed that the share of patients on oral antipsychotics does not increase after additional reimbursement is introduced for LAIs in hospital settings. Furthermore, it was assumed that the decrease in use of branded oral products will be greater than that for the generic drugs to be consistent with higher use of generics in Japan. No difference in baseline patient characteristics was assumed across the two scenarios.
Cost parameters
To estimate the cost of drug and administration, dose information was obtained from the Pharmaceuticals and Medical Devices Agency (PMDA) prescribing information (PI) for each LAI and oral antipsychotics; cost per dose was estimated using the pricing list published by Ministry of Health, Labor and Welfare (MHLW) (). Hospitalization fee per day for emergency ward was estimated to be 35,570JPY, and the cost of acute ward per day was estimated to be 19,840JPY. Similarly, cost of chronic ward per day was estimated to be 10,900JPY, and injection fee was estimated to be 200JPY per injection based on the clinical fee table published by MHLW (current as of September 2019).
Table 2. Drug price (2019 JPY).
Other clinical assumptions and modeling
Duration or length of stay in emergency/acute wards was assumed to be 90 days for both oral and LAI based on clinical fee table published by MHLW. The average duration of hospital stay (non-emergency/acute) with oral antipsychotics in chronic ward was assumed to be 334 days based on a patient survey conducted by the Government of JapanCitation25. The day to relapse for patients on oral medication was assumed to be 226, whereas the time to relapse for patients on LAI was 416 daysCitation6.
Within the model, in the base case, patients were on oral medication while hospitalized (as in-patients) or in outpatient setting. In the alternative scenario, patients on LAIs could also be either hospitalized (in-patient) or in outpatient setting (). The aggregated annual cost of drugs, hospitalization and administration (injection fee) was calculated in the model ( and ). In-patient care was spilt across subcategories of “emergency/acute ward” and “chronic ward”. Dose assumptions for oral and LAIs were based on their approved labels. Owing to the fixed hospitalization fee, only outpatient drug costs were included in the base case. However, in the alternative scenario, drug cost for LAIs was calculated for both in-patient and outpatient settings. Since the injection fee is already included in the fixed hospitalization fee, and it was assumed that this cost will not be covered by additional reimbursement; injection fee was included in outpatient settings only.
Table 3. In-patient–outpatient model.
Table 4. Per patient cost per year for LAI (2019 JPY).
Table 5. Per patient cost per year for oral (2019 JPY).
Lastly, the number of beds in psychiatric emergency ward was 8,012, in psychiatric acute ward was 16,626, and the chronic ward was 94,282, thus the ratio was 1:2:12Citation26.
Budget impact analysis
Method
To compare the net impact of introducing additional reimbursement, total expenditure including cost of hospitalization, drugs (at current and future reimbursement rates), and current injection fees across the healthcare settings was modeled for the base case and the alternative scenario.
Number of schizophrenia patients in a particular year was calculated using the forecasts and the total schizophrenia patient number currently on those products (). The total expenditure on drugs (LAI and oral antipsychotics) across the time period was calculated by adding up the aggregated patient level expenditure on each drug in emergency ward/acute ward/chronic ward for each drug, and the bed number ratio. The total cost of hospitalization, and cost of LAI and oral antipsychotics from year 1 to year 5 was calculated by summing the products of patient number for each drug, hospitalization cost per year in emergency ward/acute ward/chronic ward for each drug, and bed number ratio. The injection fee was calculated by summing the product of patient number, injection cost per year, and bed number ratio ().
Table 6. Number of patients.
Table 7. Drug, hospitalization and injection costs from 2019 to 2024 (2019 JPY).
Results
In the base case, without any additional reimbursement, the total expenditure on schizophrenia patient care was estimated to be 9,566 billion JPY over 5 years (2020–2024). This expenditure is largely driven by hospitalization costs (98% or 9,404 billion JPY over 5 years). The drug expenditure in this time period is 1.7% of the total expenditure (161 billion JPY) over 5 years. The overall trend in expenditure on schizophrenia patient care continued to decline over the time period as the population aged and number of patients decreased in the future.
With additional reimbursement for LAIs under the alternative scenario, the total expenditure in the same time period reduced to 9,530 billion JPY. This was driven largely by a reduction in total hospitalization cost of nearly 118 billion JPY. This was to an extent offset by an increase in expenditure on drugs of 81 billion JPY over 5 years with additional reimbursement for LAIs. Overall, additional reimbursement resulted in net savings of 36.5 billion JPY over 5 years (2020–2024) in direct healthcare costs ().
Table 8. Budget impact of direct healthcare cost (2019 JPY).
Sensitivity analysis
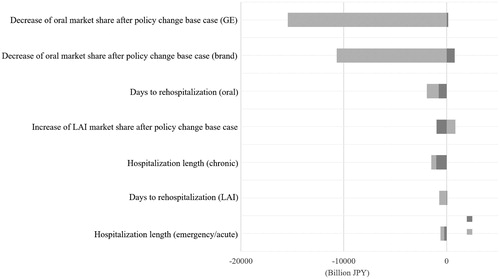
A univariate sensitivity analysis was performed to explore the effect of change in model parameters on the direct medical cost over the time horizon (see ). Sensitivity analysis showed that reduced usage of oral (branded/GE) drugs had greatest impact on direct medical cost, whereas change in market share assumptions (generic oral antipsychotics) impacted the savings estimates most ().
Discussion
This is the first budget impact analysis of additional reimbursement for LAIs for schizophrenia in Japan. Based on this analysis, introduction of additional reimbursement for LAIs in hospital (in-patient) settings and consequent increased usage of long acting treatments in psychiatric hospitals, may reduce direct healthcare cost associated with treating schizophrenia patients and may result in net savings for healthcare system in Japan.
From a policy perspective and in the context of the rising healthcare costs in Japan, this budget impact analysis shows that additional reimbursement may be desirable both to decrease financial burden of schizophrenia treatment, which is considered the most expensive psychiatric disorder to treat among all worldwideCitation1, and to ease cost pressure on the healthcare system. In addition to the social and clinical benefit of LAIs such as reduction of violent or aggressive behavior or better community functioningCitation6, which also potentially leads to cost reduction, we showed financial benefit of LAIs through this study.
The analysis included only atypical antipsychotics available in Japan and typical antipsychotics were not included because it is not recommended in treatment guidelinesCitation23. Individuals prescribed with atypical antipsychotics tend to be more adherent compared to typical antipsychoticsCitation27, therefore, not including typical antipsychotics in the analysis may have potentially increased the degree of adherence overestimation in this analysis.
This study has several potential limitations, however, wherever possible, attempts have been made to control for uncertainties using sensitivity analysis. For example, certain key drivers of cost were identified, and sensitivity analysis was performed to estimate the overall impact on costs and savings. Similarly, there was uncertainty around the estimates of the drug and hospitalization costs into the future. Historical data were used as a proxy noting the caveat that history may not be the best predictor of future costs. Also, this analysis may overestimate the effectiveness of oral atypical antipsychotic therapies as it assumes that all patients on oral antipsychotic drugs have good adherence, which is generally not the case. The result might be underestimated because reduced risk of self-harm or failure to comply with treatment which are factors that improve better long-term clinical outcome thus would reduce costCitation6,Citation10. Thus, exclusion of these factors from the assumption or analysis may underestimate the cost benefit of LAI. Patient behavior was based on the models, which may not truly reflect the reality. For example, key assumption of the model that the use of oral medications would be associated with relapse in an average of 226 days while LAIs would extend this to 416 days, may change depending on the referred evidence. We might have overestimated the drug cost due to additional reimbursement because in clinical setting, the increase in LAI use may not have reached 10% of the market share.
Conclusions
The present budget impact analysis shows that LAI use at hospital level to manage schizophrenia patients in Japan, may potentially be cost saving from payer’s perspective. Policy makers should consider this approach to improve patient outcomes and budget sustainability.
Transparency
Declaration of financial/other relationships
MK is an employee of Janssen Pharmaceutical KK. AW and JW are employees of Janssen Pharmaceutical KK and hold stocks in the parent company. AC is an employee of Janssen Asia Pacific and holds stocks in the parent company. AI received speaker’s honoraria from Janssen Pharmaceutical KK, Otsuka Pharmaceutical Co., Ltd., and Meiji Seika Pharma Co., Ltd.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Acknowledgements
No assistance in the preparation of this article is to be declared.
Previous presentations
Partial results of this study were presented in The 29th Annual Meeting of the Japanese Society of Clinical Neuropsychopharmacology (JSCNP).
Additional information
Funding
References
- Rossler W, Salize HJ, van Os J, et al. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol. 2005;15(4):399–409.
- Miyamoto S, Wolfgang Fleischhacker W. The use of long-acting injectable antipsychotics in schizophrenia. Curr Treat Options Psychiatry. 2017;4(2):117–126.
- McCreath J, Larson E, Bharatiya P, et al. Long-acting injectable antipsychotics for schizophrenia: sociodemographic characteristics and treatment adherence. Prim Care Companion CNS Disord. 2017;19(1). DOI:10.4088/PCC.16m02005
- Rubio JM, Taipale H, Correll CU, et al. Psychosis breakthrough on antipsychotic maintenance: results from a nationwide study. Psychol Med. 2019;13:1–12.
- Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192–213.
- Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76(5):554–561.
- Morrato EH, Parks J, Campagna EJ, et al. Comparative effectiveness of injectable paliperidone palmitate versus oral atypical antipsychotics: early postmarketing evidence. J Comp Eff Res. 2015;4(2):89–99.
- Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–134.
- Smeraldi E, Cavallaro R, Folnegovi-Malc V, et al. Long-term remission in schizophrenia and schizoaffective disorder: results from the risperidone long-acting injectable versus quetiapine relapse prevention trial (ConstaTRE). Ther Adv Psychopharmacol. 2013;3(4):191–199.
- Lambert M, De Marinis T, Pfeil J, et al. Establishing remission and good clinical functioning in schizophrenia: predictors of best outcome with long-term risperidone long-acting injectable treatment. Eur Psychiatry. 2010;25(4):220–229.
- Macfadden W, DeSouza C, Crivera C, et al. Assessment of effectiveness measures in patients with schizophrenia initiated on risperidone long-acting therapy: the SOURCE study results. BMC Psychiatry. 2011;11(1):167.
- Sugawara N, Kudo S, Ishioka M, et al. Attitudes toward long-acting injectable antipsychotics among patients with schizophrenia in Japan. Neuropsychiatr Dis Treat. 2019;15:205–211.
- Fujii Y. Jikousei Chuusha seizai no subete (all about long acting treatment). Japan: Seiwa Shoten; 2018.
- Central social insurance medical council. 434th Central Social Insurance Medical Council General Meeting (Chuikyo Soukai) Handout; 2019; [cited 2020 Feb]. Available from: https://www.mhlw.go.jp/content/12404000/000568660.pdf
- Heres S, Hamann J, Kissling W, et al. Attitudes of psychiatrists toward antipsychotic depot medication. J Clin Psychiatry. 2006;67(12):1948–1953.
- Kane JM, Correll CU. Optimizing treatment choices to improve adherence and outcomes in schizophrenia. J Clin Psychiatry. 2019;80(5):IN18031AH1C.
- Ministry of Health Labor and Welfare. Medical economic survey 2017; 2017; [cited 2020 Feb]. Available from: https://www.mhlw.go.jp/bunya/iryouhoken/database/zenpan/jittaityousa/dl/21_houkoku_iryoukikan.pdf
- Oshima I, Mino Y, Inomata Y. How many long-stay schizophrenia patients can be discharged in Japan? Psychiatry Clin Neurosci. 2007;61(1):71–77.
- Ministry of Health Labor and Welfare. 90th Sectional Meeting for the Disabilities in the Social Security Council; 2018; [cited 2020 Feb]. Available from: https://www.mhlw.go.jp/content/12201000/000307970.pdf
- OECD. Hospital beds (indicator); 2020; [cited 2020 Feb 5]. Available from: https://data.oecd.org/healtheqt/hospital-beds.htm
- Nakamura Y, Shibata I, Mahlich J. Modeling the choice between risperidone long-acting injectable and generic risperidone from the perspective of a Japanese hospital. Neurol Ther. 2019;8(2):433–447.
- Ministry of Health Labor and Welfare. An outline of the Japanese medical system; 2019; [cited 2020 Feb]. Available from: https://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken01/dl/01\_eng.pdf
- The Japanese Society of Neuropsychopharmacology. Medication guideline of schizophrenia; 2015; [cited 2020 Mar]. Available from: http://www.asas.or.jp/jsnp/img/csrinfo/togoshiccho_01.pdf
- Central Social Insurance Medical Council. 385th Central Social Insurance Medical Council General Meeting (Chuikyo Soukai) Handout 2018; 2018; [cited 2020 Mar]. Available from: https://www.mhlw.go.jp/file/05-Shingikai-12404000-Hokenkyoku-Iryouka/0000191569.pdf
- Ministry of Health Labor and Welfare. Hospital report 2018; 2018; [cited 2020 Feb]. Available from: https://www.mhlw.go.jp/toukei/saikin/hw/kanja/14/dl/03.pdf
- Leidy NK, Vernon M. Perspectives on patient-reported outcomes: content validity and qualitative research in a changing clinical trial environment. Pharmacoeconomics. 2008;26(5):363–370.
- Dassa D, Boyer L, Benoit M, et al. Factors associated with medication non-adherence in patients suffering from schizophrenia: a cross-sectional study in a universal coverage health-care system. Aust N Z J Psychiatry. 2010;44(10):921–928.