Abstract
Background: For novel migraine therapies, economic evaluations will be required to understand the trade-offs between additional health benefit and additional cost. The purpose of this study was to conduct a systematic literature review (SLR) to identify previous economic evaluations in migraine from the United Kingdom or Irish perspective to critically appraise these evaluations and to propose, if necessary, a novel modelling approach that can be used for future economic evaluations of migraine therapies.
Methods: An SLR was conducted to identify previous economic evaluations of preventive migraine treatments. Key opinion leaders were consulted to determine the criteria for a robust migraine economic evaluation. Economic evaluations identified in the SLR were appraised against these criteria, and a novel cost-effectiveness model structure was then proposed.
Results: Eight records reporting on published economic evaluations were identified and critically appraised for general quality. Expert consultation provided 6 recommendations on the ideal model structure for migraine that is both clinically and economically meaningful. A decision-tree plus Markov structure was then developed as a cost-effectiveness model for migraine therapies where each health state is associated with a patient distribution across monthly migraine day (MMD) frequencies.
Conclusions: Future migraine economic evaluations should allow for assessments across the full spectrum of migraine, a response-based stopping rule, and the estimation of benefits and resource costs based on MMD frequency. The approach proposed in this paper captures all of the desired elements for an economic evaluation of migraine therapy and is suitable to assess new migraine therapies.
Introduction
Migraine is a distinct, common neurological disease characterized by recurrent, often unilateral, throbbing head pain of moderate to severe intensity accompanied by symptoms including nausea, vomiting, photophobia, or phonophobia.Citation1 Migraine is a source of significant burden, as it is estimated to affect >10% of the adult population across the world.Citation2
Migraine has a variable burden driven by attack frequency and is sometimes broadly classified as episodic migraine (EM) or chronic migraine (CM) based on the number of migraine days and headache days per month.Citation2 Episodic migraine is defined as fewer than 15 headache days per month, with or without aura, and accounts for more than 90% of persons with migraine.Citation3 Chronic migraine is defined as at least 15 headache days per month (of which ≥8 are migraine days with or without aura), and it affects approximately 5% to 8% of persons with migraine.Citation4
To understand better the value of treatments for migraine, health technology assessment (HTA) agencies and other resource allocation decision makers may utilize economic evaluations. It is important to consider the relevance of existing economic evaluations and modelling approaches to ensure that the costs and benefits are appropriately captured for novel migraine therapies such as those designed to block the trigeminovascular calcitonin gene-related peptide (CGRP) pathway. The purpose of this study was to conduct a systematic literature review (SLR) to identify previous economic evaluations in migraine from the United Kingdom (UK) or Ireland perspective, to critically appraise these evaluations, and finally, to propose a novel modelling approach that can be used for future economic evaluations of new migraine therapies, including recently approved CGRP antagonists.
Methods
SLR overview
An SLR was conducted to identify economic evidence to support the development of a cost-effectiveness model for the prevention of chronic or episodic migraine. The SLR was conducted in July 2017, and subsequently updated in January 2018 and September 2018, to identify all literature published since database inception on any of the following topics:
Economic evaluations of pharmacological interventions for the treatment of chronic or episodic migraine.
Health state utility values for chronic or episodic migraine patients.
Cost and resource use data for chronic or episodic migraine patients.
The SLR and updates were performed in accordance with the methodological principles of conduct for systematic reviews as detailed in the University of York Centre for Reviews and Dissemination’s “Guidance for Undertaking Reviews in Health Care”.Citation5 No ethics review board was required for tertiary research. The focus of this manuscript is the economic evaluations of pharmacologic interventions for the treatment of chronic or episodic migraine that were identified in the SLR.
SLR search strategy
MEDLINE, Embase, The Cochrane Library, and EconLit electronic databases were searched for economic evaluations in migraine over 3 phases during July 2017, with subsequent updates through September 2018 (Appendix Table 1). A list of search terms used in MEDLINE, MEDLINE Daily, MEDLINE In-Process, and Epub Ahead of Print electronic databases for both the original review and the updates is provided in Appendix Table 2. Search terms used in the Embase database, Cochrane Library of databases, and EconLit search for both the original review and the updates are presented in Appendix Table 3, Appendix Table 4, and Appendix Table 5, respectively. Results from the database searches were downloaded into an Endnote database and de-duplicated before being transferred into a bespoke Microsoft Excel-based platform designed to enable record screening. In addition to the electronic database searches, the conference proceedings of the major migraine and neurological congresses held over the prior 3 years (2015–2018) were manually searched (Appendix Table 6).
The National Institute for Health and Care Excellence (NICE), Scottish Medicines Consortium (SMC), All Wales Medicines Strategy Group (AWMSG), and National Centre for Pharmacoeconomics (NCPE) websites were manually searched for previous, relevant HTA submissions. In addition, The Cost-Effectiveness Analysis (CEA) Registry, managed by Tufts Medical Center, and EconPapers at Research Papers in Economics (RePEc) websites were also manually searched to ensure that no relevant publications were missed.
Finally, the bibliographies of all relevant SLRs, meta-analyses, HTA submissions, and economic evaluations identified through the electronic database, conference, and HTA agency website searches were also manually searched to identify any additional studies of relevance.
SLR study selection
To be included in the review, economic evaluation articles had to meet predefined eligibility criteria that are detailed using the PICOS method ().
Table 1. Inclusion Criteria for the Economic Evaluations SLR and Updates.
The citations found through the searches were first assessed against the eligibility criteria by 2 independent reviewers based on abstract and title. Where the applicability of the inclusion criteria was unclear, the article was included at this stage in order to ensure that all potentially relevant studies were captured. Full-text copies of publications potentially meeting the eligibility criteria were then obtained and reviewed against the same eligibility criteria by 2 independent reviewers. In cases where the article did not give enough information to be sure it met the inclusion criteria at the full-text screening stage, the article was excluded to ensure that only relevant articles were ultimately included in the review. At both the title/abstract and full-text review stages, any disagreements between the reviewers were resolved by discussion until a consensus was met, with a third independent reviewer making the final decision if necessary. For studies meeting the eligibility criteria after the second (full-text) screening stage, data were extracted by a single reviewer into a prespecified data extraction grid and verified by a second individual.
Quality appraisal of economic evaluations
Critical appraisals of each published economic evaluation included in the SLR were conducted using the checklist adapted from Drummond et al.Citation6. The checklist for quality appraisal of economic evaluations includes 25 items in the categories of study design, data collection, and analysis and interpretation of results.
Expert opinion consultation of Migraine-Specific economic evaluation approaches
Three key opinion leaders, within both the medical and health economics and outcomes research (HEOR) fields, were consulted in order to provide the criteria for a suitable migraine economic evaluation. Key opinion leaders were also consulted for recommendations and insights on the ideal model structure for migraine that is both clinically and economically meaningful. Recommendations for a model structure were further informed by relevant clinical guidelines (e.g. British Association of the Study of HeadacheCitation7 and NICECitation8) and previous economic evaluations in order to understand clinical practice and previous modelling approaches in migraine prevention.
Results
SLR findings
A total of 3,914 unique articles were identified (3,410 in the original SLR, 187 more in the January 2018 update, and 317 more in the September 2018 update) from the electronic database searches and reviewed at the title/abstract review stage. After title/abstract review in the second update, 238 articles were reviewed for the full-text stage and 37 articles ultimately met the inclusion criteria. An additional 6 articles to those captured through the database searches were identified through congress, website, and hand searching of bibliographies in the original review. No extra articles were identified through hand searches in the first update, but 4 were identified in the second update. The flow of studies through the systematic review process is presented in Appendix Figure 1.
Identified economic evaluations
In total, 8 records reporting on published economic evaluations were identified in the original SLR (see ), and no further economic evaluations were identified during the update. Importantly, several of the identified publications were published on a similar core model.Citation8–12 All of the publications were cost-utility analyses, and the majority utilized a state-transition (Markov) model structure. Models commonly included health states stratified by the number of migraine or headache days and allowed for treatment discontinuation or treatment on/off periods. The economic evaluations most commonly utilized a 2-year time horizon, and length varied from 1 year to 3 years.
Table 2. Details of the Relevant Economic Evaluations Identified in the Systematic Review.
Quality appraisal of economic evaluations
Critical appraisals of each published economic evaluation included in the SLR were conducted using the checklist adapted from,Citation6 as recommended by NICE. Economic evaluations were appraised on 35 items across the categories of study design, data collection, and analysis and interpretation of results. The results of these critical appraisals are presented in . All of the economic evaluations fulfilled the items for proper disclosure of the study design. However, several items in the data collection category were missing including the details of the subjects from whom valuations were obtained. Most of the items within the analysis and interpretation of results section were included, but information on the use of a discount rate and rationale and details were often not published.
Table 3. General Quality Appraisal of Economic Evaluations.
Quality appraisal of economic evaluations with respect to evaluating migraine treatments specifically
The economic evaluations identified through the SLR were carefully assessed and provided valuable insights to better understand alternative ways of economically evaluating migraine interventions and to determine if other approaches were needed.
Consultation with medical and HEOR experts identified clinical meaningfulness and scientific robustness as the 2 key areas for the design of economic evaluations. For a migraine model to be clinically meaningful, it should have face validity and ensure each model component reflects current clinical practice and understanding. For migraine interventions, these include:
Capture migraine as a spectrum disease (MMDs between 0 and 28; ie, chronic and episodic together).
Include a discontinuation rule based on treatment response (treatment response being defined by % reduction in MMDs).
Include forms of negative discontinuation (non-responders, adverse event [AE]-related discontinuation, and long-term general discontinuation) and positive discontinuation (patients with well-managed migraine no longer requiring treatment).
HEOR and medical experts also concluded that scientific robustness is essential to have strong internal, external, cross, and predictive validity. The key criteria items for a developing a scientifically robust economic evaluation for migraine treatments are:
The evaluation should closely reflect trial results as a form of validation.
The model should simultaneously track both MMD frequency (in order to estimate quality-adjusted life-years [QALYs] gained and resource utilization incurred) and response status (to facilitate a response-based discontinuation rule).
The model should reflect how patients are distributed across MMD frequencies in order to capture the non-linear impact of MMD frequency change on both QALYs and resource utilization.
Modelling MMD frequency as a continuous outcome, rather than as a series of categories, has several advantages over the use of arbitrary health states which have been previously reported.Citation16,Citation17 The use of continuous MMD frequency distribution retains all of the observed information to accurately estimate the difference in outcomes between groups. This approach also more readily allows for indirect comparisons based on study primary endpoints (e.g. probability of 50% response, or change in MD frequency per 28 days). In addition, the number of MMDs and associated events (e.g. emergency department visits) can be directly quantified.
In consideration of clinical guidelines (International Headache Society [IHS] and NICE), input from the KOLs’ previous economic evaluations were reviewed against the criteria outlined above (). Several modelsCitation8–13 included disease attack frequency via headache days per month but not MMDs. Similarly, these models included a discontinuation rule based on reduction in headache days or rates derived from clinical trials, but not explicitly defined as percentage reduction in MMDs. Previous economic evaluations were parameterized with clinical trial data, but reviewers identified areas of concern. In the 2012 appraisal of botulinum toxin, reviewers noted that the model and trial datasets for headache days per month at week 24 corresponded poorly and that this overestimated the net impact of botulinum toxin type A by 53% and there were no clinical data to support the use of the 2 stopping rules. A modelling approach in which patients are distributed across MMD frequencies may more closely reflect migraine trial results as a form of validation. None of the identified evaluations had a structure that allowed accurate and simultaneous tracking of both MMD frequency and response status.
Table 4. Migraine-Specific Quality Appraisal of Economic Evaluations.
A novel Cost-Effectiveness model structure
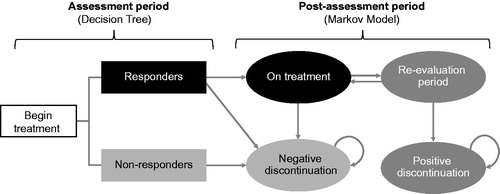
In light of the critical appraisal described above, a novel model structure was developed with the aim of meeting all criteria simultaneously. A decision-tree plus Markov structure was developed as a cost-effectiveness model for migraine therapies. Reflecting clinical practice, the decision-tree component represents an assessment period, allowing for treatment discontinuation based on safety and clinically relevant response criteria. The Markov component represents a post-assessment period, where treatment responders and non-responders follow distinct treatment pathways ()Citation18. Responders to treatment without safety or tolerability issues continue on that treatment over the post-assessment with an optional re-evaluation period, which may lead to positive discontinuation, while non-responders discontinue and do not reinitiate treatment. The underlying assumption of the model is that both costs and QALYs can be estimated based on the MMD frequency being experienced by patients. Thus, each health state in the model is associated with a patient distribution across MMD frequencies, which contrasts with defined ranges of migraine day or headache day frequency used for health states used in previous decision tree or Markov model approaches.
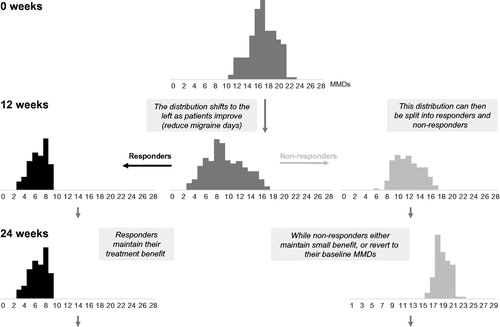
A key component of the model is the expression of treatment efficacy over the assessment period. The assessment period in the model simply replicates, as faithfully as possible, the MMD distributions, per treatment, as observed in the available clinical trial data. Given the selected population of interest, the model estimates (for each treatment):
The MMD distribution for all patients at baseline (Week 0).
The proportion of responders at Week 12 (based on given response threshold).
The MMD distributions for responders and non-responders at Week 12.
The overall MMD distribution (for each treatment) at Week 12 is implied by the combination of the responder and non-responder MMD distributions (weighted by the estimated proportions of responders and non-responders). The response threshold is defined as a specified percentage reduction in MMDs. This process is illustrated in . Note that the MMD distributions displayed here are not based on real data and are for illustrative purposes only.
Discussion
The burden patients experience each day of migraine is meaningful. In a large retrospective, cross-sectional study, an increase of 1 headache-free day (HFD) was associated with an average decrease in absenteeism of 3.9% and presenteeism of 2.1%Citation19. Resource utilization was also reduced, with an increase of 1 HFD being associated with expected decreases in healthcare provider visits and neurologist visits of 1.0% and 4.7%, respectively. The benefits of each additional HFD corresponded to average increases of 0.171, 0.306, 0.003, and 0.008 points for the mental composite summary (MCS) score, physical component summary (PCS) score, SF-6D utility score, and EQ-5D index score, respectively (p < 0.001 for all). The burden of migraine is worse for people who need to change preventive therapy. For these patients, 87% reported that migraine had a negative impact on professional, private, or social domains of lifeCitation20. New therapies for migraines, particularly those for treatment-refractory patients, have the opportunity to bring meaningful improvement for patients, and economic evaluations can be useful tools for assessing their value.
A review of previous modelling approaches of economic evaluations, clinical guidelines, and expert opinion has identified key aspects that should be considered in the development of future economic evaluations for migraine therapies for the UK and Ireland.
To avoid the use of arbitrary established MMD cut-offs, the use of a full MMD distribution is likely to better quantify the impact of associated health benefits and costs. This approach also better reflects the fact that migraine is a spectrum disease with both chronic and episodic characteristics.
A discontinuation rule is required based on treatment response where treatment response is defined as a percent reduction in MMDs. Other forms of discontinuation to cater to tolerability issues (AE-related, positive) should be included to provide flexibility in terms of discontinuation rules, reasons for discontinuation, and consequences for discontinuation. It is particularly important that tolerability be included in any economic evaluation for appropriate risk-benefit assessment where tolerability is the limiting factor for sustained efficacy with prior standard of care in a chronic disorder such as migraine.
Lastly, if comparative data are available, the economic evaluation should include the ability to compare the treatment of interest with other preventive treatments.
Compared to other disease areas, the number of economic evaluations in migraine was limited. Many of these evaluations utilized a similar structure, but each approach has unique strengths and limitations. Importantly, some evaluations with perspectives outside of the UK or Ireland were not in the scope of our research and not included. A review of these evaluations may provide additional insights for developing a novel economic evaluation of migraine therapies. In the time since the SLR was completed, we identified an update to the cost-effectiveness model of onabotulinumtoxinA for the prevention of headache in adults with chronic migraineCitation21. However, this update was to incorporate new EQ-5D utility estimates, and there were no novel changes to the model structure.
The proposed modelling approach that estimates both MMD frequencies and response to treatment is reflective of clinical practice in Europe and is appropriate in assessing the cost-effectiveness of preventive treatments of migraine. In this study, the proposed approach is reflective of migraine as a spectrum disorder, and it is likely to appropriately capture the costs and health benefits of migraine therapies vs appropriate comparators.
Similar to previous economic evaluations, there are some limitations inherent with the proposed model. Limitations of this modelling approach include the assumption that treatment response can be based on MMD frequency alone and the assumption that both health-related quality of life and resource utilization can be estimated based on MMD frequency alone. While it is envisioned that response to therapy in the initial version of this model will be based on reduction in MMD frequency alone, response in later versions could be based on a combination of multiple clinically relevant endpoints and efficacy parameters such as frequency, severity, enhanced effect of acute migraine medication, and benefits in the pro-dromal and post-dromal periods.
A reliable cost-effectiveness assessment utilizing the proposed approach will require careful consideration of inputs and definitions to parameterize the model. This includes establishing responder and non-responder definitions, defining the duration of treatment benefit for responders, determining the frequency of patients re-evaluated, and the most appropriate time horizon. Extensive sensitivity analyses will be important for addressing these issues, but the proposed modelling approach provides a basis for transparency regarding these assumptions.
Conclusion
Based on the findings of the SLR, a limited number of economic evaluations for migraine therapies have been published. The design of any economic evaluation should be informed by clinical expert opinion and clinical guidelines. As new therapies are being developed and approved for use in patients with high unmet needs, such as those patients with migraine experiencing at least 4 MMDs, new economic models developed in this disease area should allow for the assessments of cost-effectiveness across the full spectrum of migraine. The approach proposed in this paper captures all of the desired elements to an economic evaluation of migraine therapy and is suitable to assess new migraine therapies such as CGRP antagonists. Future research is needed to better characterize the data to adequately structure and parameterize an economic model to support decision making for migraine therapies.
Transparency
Declaration of funding
This study was sponsored by Novartis Pharma AG.
Declaration of financial/other interests
RM, JH, VH, AD, VU, SV, PV are employees of Novartis and RM, VH and AD owns shares in the company. FM reports personal fees from Novartis, Teva and Allergan outside the submitted work. SP reports personal fees from Amgen, outside the submitted work. PG reports personal fees from Alder Biopharmaceuticals, personal fees from Allergan, grants and personal fees from Amgen, personal fees from Autonomic Technologies Inc., personal fees from Biohaven Pharmaceuticals Inc., grants and personal fees from Eli Lilly and Company, personal fees from Electrocore LLC, personal fees from eNeura Inc, personal fees from Impel Neuropharma, personal fees from MundiPharma, personal fees from Novartis, personal fees from Teva Pharmaceuticals, other from Trigemina, personal fees from WL Gore, outside the submitted work. In addition, PG has a patent Magnetic stimulation for headache licensed to eNeura without fee and fees for publishing from Oxford University Press, Massachusetts Medical Society, Wolters Kluwer, and for medico-legal work.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Appendix Tables
Download MS Word (38.1 KB)Acknowledgements
The authors gratefully acknowledge the contributions of Rose Wickstead, Eleanor Ferns, and Amy Buchanan-Hughes, who conducted the systematic review and performed the initial data extractions for the referenced economic models. The authors acknowledge David Campbell, Clémence Arvin-Berod, and Marie-Josée Martel of Xcenda for their editorial contributions to this work.
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd Edition. Cephalalgia. 2018;38:1–211.
- GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–976.
- Lipton RB, Manack Adams A, Buse DC, et al. A comparison of the Chronic Migraine Epidemiology and Outcomes (CaMEO) study and American Migraine Prevalence and Prevention (AMPP) study: demographics and headache-related disability. Headache. 2016;56(8):1280–1289.
- Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache. 2012;52(10):1456–1470.
- Centre for Reviews and Dissemination (CRD) [Internet]. University of York. Systematic reviews: CRD’s guidance for undertaking reviews in health care. January 2009. [cited October 23, 2018]. Available from: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ. 1996;313(7052):275–283.
- British Association of the Study of Headache [Internet]. Guidelines for all healthcare professionals in the diagnosis and management of migraine, [cited August 4, 2019]. Available from: https://www.bash.org.uk/guidelines/.
- NICE. Botulinum toxin type A for the prevention of headaches in adults with chronic migraine. [cited May 2, 2019]. Available from: https://www.nice.org.uk/guidance/ta260.
- Royle P, Cummins E, Walker C, et al. Evidence review group report: Botulinum toxin type A for the prophylaxis of headaches in adults with chronic migraine, 2012. [cited August 22, 2019]. Available from: https://www.nice.org.uk/guidance/ta260/documents/migraine-chronic-botulinum-toxin-type-a-evidence-review-group-report2
- Batty AJ, Hansen RN, Bloudek LM, et al. The cost-effectiveness of onabotulinumtoxinA for the prophylaxis of headache in adults with chronic migraine in the UK. J Med Econ. 2013;16(7):877–887.
- SMC [Internet]. SMC advice: botulinum toxin A (Botox) 2011. [cited October 23, 2018]. Available from: https://www.scottishmedicines.org.uk/files/advice/botulinum_toxin_type_A_Botox_FINAL_MARCH_2011_AMENDED_22.03_for_website.pdf
- SMC [Internet]. SMC advice: botulinum toxin A (Botox) 2013. [cited October 23, 2018]. Available from: https://www.scottishmedicines.org.uk/files/advice/botulinum_toxin_type_A_Botox_RESUBMISSION_Final_March_2013_for_website.pdf
- SMC [Internet]. SMC advice: botulinum toxin A (Botox) 2017. [cited October 23, 2018]. Available from: https://www.scottishmedicines.org.uk/files/advice/botulinum_toxin_A_BOTOX_2nd_Resub_FINAL_Jan_2017_for_website.pdf
- SMC [Internet]. SMC advice: topiramate (Topamax) 2006. [cited October 23, 2018]. Available from: https://www.scottishmedicines.org.uk/files/topiramate_Topamax_297_06.pdf
- Brown JS, Papadopoulos G, Neumann PJ, et al. Cost-effectiveness of migraine prevention: the case of topiramate in the UK. Cephalalgia. 2006;26(12):1473–1482.
- Lipton RB, Porter JK, Shah N, et al. A comparison of approaches to model migraine day frequency in migraine. Cephalalgia. 2017;37:102–103.
- Di Tanna GL, Porter JK, Lipton RB, et al. Migraine day frequency in migraine prevention: longitudinal modelling approaches. BMC Med Res Methodol. 2019;19(1):20.
- Mahon R, Vo P, Cooney P, et al. A model concept for assessing the cost-effectiveness of preventive migraine treatments. 12th European Headache Federation Congress. 2018 Sep 28–30; Florence, Italy. J Headache Pain. 2018;19(Suppl1)80:108.
- Doane MJ, Gupta S, Vo P, et al. Associations between headache-free days and patient-reported outcomes among migraine patients: a cross-sectional analysis of survey data in Europe. Pain Ther. 2019; Dec8(2):203–216.
- Martelletti P, Schwedt TJ, Lanteri-Minet M, et al. My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 201819(1):115.
- Hollier-Hann G, Curry A, Onishchenko K, et al. Updated cost-effectiveness analysis of onabotulinumtoxinA for the prevention of headache in adults with chronic migraine who have previously received three or more preventive treatments in the UK. J Med Econ. 2020;23(1):113–123.