Abstract
Aims: Allopurinol is the most common urate lowering therapy (ULT) used to treat gout but may cause life-threatening severe cutaneous adverse reactions (SCAR) in a small number of patients. Risk of SCAR is increased for patients with the HLA-B*58:01 genotype. When alternative ULT is required, febuxostat or probenecid are recommended. The aim of this study was to conduct a cost-utility analysis of sequential ULT treatment strategies for gout, including strategies with and without HLA-B*58:01 genotyping prior to treatment initiation, with a view to inform optimal gout management in Singapore.
Materials and methods: A Markov model was developed from the Singapore healthcare payer perspective. Reflecting local practice, 12 different treatment strategies containing at least one ULT (allopurinol, febuxostat, probenecid) were evaluated in adults with gout. Response rates (SUA < 6mg/dL) were derived from an in-house network meta-analysis and from published literature. Incremental cost-effectiveness ratios (ICERs) were calculated over a 30-year time horizon, with costs and benefits discounted at 3% per annum. Sensitivity analyses were conducted to explore uncertainties.
Results: Sequential treatment of allopurinol 300 mg/day-allopurinol 600 mg/day-probenecid (“standard of care”) was cost-effective compared to no ULT, with an ICER of SGD1,584/QALY. Allopurinol300-allopurinol600-probenecid-febuxostat sequence compared to allopurinol300-allopurinol600-probenecid had an ICER of SGD11,400/QALY. All other treatment strategies were dominated by preceding strategies. Treatment strategies incorporating HLA-B*58:01 genotyping before ULT use were dominated by the corresponding non-genotyping strategy.
Conclusions: Current standard of care (allopurinol300-allopurinol 600-probenecid) for gout is cost-effective compared with no ULT in the local context. Febuxostat is unlikely to be cost-effective in Singapore at current prices unless it is used last-line.
Introduction
While gout affects approximately 4.1% of SingaporeansCitation1, patients usually only seek treatment with urate lowering therapy (ULT), such as allopurinol, febuxostat or probenecid, when they experience recurrent acute flares. In local practice, allopurinol is used by 95–99% of patients receiving ULT as it is affordable and generally well tolerated, however, in a small (0.2%) proportion of patients it can cause severe cutaneous adverse reactions (SCAR) including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) or drug reaction with eosinophilia and systemic symptoms (DRESS)Citation2. These adverse events (AEs) can be acute and life threatening, and are associated with a mortality rate of 5–30%Citation3–5. They also carry a risk of long-term sequelaeCitation6.
There is a genetic association between SCAR and the presence of the HLA-B*58:01 allele. In Singapore, the weighted prevalence of HLA-B*58:01 is 18.5% with varying rates occurring across different ethnic groupsCitation7. While there is an association, not all patients with HLA-B*58:01 who receive allopurinol will develop SCAR, suggesting the co-existence of other influences such as immunological factors or drug accumulation may also play a roleCitation7–9. In Singapore, genotyping for HLA-B*58:01 prior to starting ULT is not routinely conducted. Instead, patients are monitored for AEs after initiating allopurinol. For patients who develop mild cutaneous reactions to allopurinol, desensitization with incremental dose escalation can be used to induce toleranceCitation10. This has been adapted successfully in a small series of HLA-B*58:01 positive individuals who were allopurinol naïve, although it is not recommended as standard of careCitation11.
For patients who have an inadequate response or hypersensitivity to allopurinol, alternative ULT (probenecid or febuxostat) is considered. However, febuxostat is currently not subsidized and is considerably more expensive than both allopurinol and probenecid, contributing to its low level of use in Singapore.
There are no price controls on drugs at the point of marketing authorization in Singapore. Any patients who can afford to pay for a drug have access as soon as it is made commercially available by the company. To improve affordability of treatment, subsidies and financial assistance are provided to eligible patients treated at public healthcare institutions, for drugs listed on the Standard Drug List and the Medication Assistance FundCitation12. These subsidy listings are informed by health technology assessments which play an important role in determining the relative value of new treatments and how best to allocate finite health care resources to ensure long-term sustainability of the health care system.
With a view to inform optimal gout management in Singapore, we evaluated the cost-effectiveness of various sequential ULT treatment strategies compared with each other, including strategies with and without HLA-B*58:01 genotyping prior to treatment initiation as part of a health technology assessment to inform national subsidy of ULTs.
Methods
Model design and study population
A Markov model with a 3-month cycle length was developed from the Singapore healthcare payer’s perspective () to derive costs and quality adjusted life-years (QALYs) incurred by ULT treatment strategies in a cohort of patients with symptomatic gout (SUA ≥ 8 mg/dL). The mean baseline age of the cohort was 50 years and a 30-year time horizon was applied, reflecting average life expectancy in SingaporeCitation13. A subgroup analysis in patients with gout and concomitant moderate to severe renal impairment (chronic kidney disease stage ≥3 b, with estimated glomerular filtration rate <45 mL/min) was also conducted.
Figure 1. Abbreviations. SUA, serum uric acid; ULT, urate lowering therapy. Individuals can die in any of the health states.
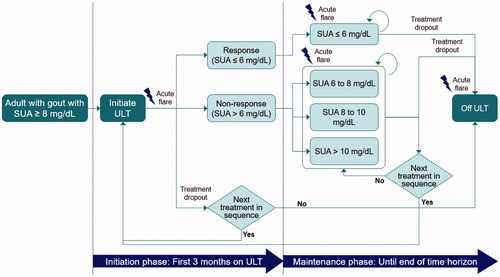
All treatment strategies are shown in . “No ULT” strategy (Strategy 1) describes patients who do not take ULT routinely and only manage acute flare episodes. For all strategies that commenced with allopurinol, an initial dose of 300 mg/day was assumed, which was up-titrated to 600 mg/day in the event of insufficient response. Febuxostat was administered at 80 mg/day (dose escalation was not permitted), in line with the local registered indication. Switching from febuxostat to allopurinol or probenecid following non-response is unlikely in local clinical practice, therefore, it was not permitted in the model. For treatment strategies considered in a subgroup of patients with concomitant moderate to severe renal impairment (Strategies 8–12), allopurinol 600 mg and probenecid were not considered as suitable treatment options, reflecting their registered indications.
Table 1. Treatment sequences included in the model.
It was assumed that patients could receive prophylactic colchicine during initial ULT to prevent ULT-induced flares. After the initiation phase (3 months), response was defined as SUA level <6 mg/dL, in accordance with established clinical guidelinesCitation14,Citation15. Patients who did not achieve a response were categorized into 3 groups: SUA 6–8 mg/dL, SUA 8–10 mg/dL, or SUA ≥10 mg/dL. If patients had an insufficient response to ULT for 6 months, their dose was either increased (if permitted) or they switched to the next line of therapy. Patients who did not respond to last-line ULT continued using it until drop-out.
If SCAR occurred, it was assumed to take place within 60 days of initiation with allopurinol or febuxostat. Patients who survived a SCAR event could experience long-term sequelae, such as severe dry eye syndrome (DES), but were assumed to be able to use probenecid which has not been associated with SCAR.
The model also evaluated the cost-effectiveness of routine HLA-B*58:01 genetic screening before ULT use. For these scenarios, a decision tree for HLA-B*58:01 testing was applied before the Markov model. Patients who tested positive for the HLA-B*58:01 allele were not treated with allopurinol.
Treatment effectiveness and complications
Clinical inputs for the model were derived from numerous sources (). The proportions of patients who achieved target SUA <6 mg/dL with first-line allopurinol or febuxostat were derived by conducting a network meta-analysis (NMA) of randomized controlled trials (RCTs) published up to 1 June 2018Citation16–26. The network plot of available evidence for the endpoint of proportion of patients attaining target SUA <6 mg/dl is provided in Supplementary Figure S1. Details on the methodology and studies included in the NMA are also appended in the Supplementary. Response rates for patients using allopurinol or febuxostat as second- or third-line treatment were assumed to be similar to response rates reported for first-line treatment. In the absence of any trials comparing probenecid with either febuxostat, allopurinol or placebo, probenecid was unable to be included in the NMA. The distribution of patients across the “non-responding” SUA categories were sourced from a published cost-effectiveness analysis by Beard et al, which obtained these values by pooling data from the APEX and FACT trials which compared febuxostat with allopurinol and placeboCitation27.
Table 2. Model inputs: clinical parameters.
The response rate for patients taking allopurinol 600 mg/day was estimated using data from an RCT comparing allopurinol 600 mg/day versus benzbromarone 200 mg/day in patients not attaining target SUA at lower dosesCitation20. The response rate used for probenecid was taken from a study in which probenecid or benzbromarone were administered to patients not achieving SUA <5 mg/dL with allopurinol 300 mg/dayCitation21.
The rate of allopurinol-induced flare was acquired from pooled data reported in a published cost-effectiveness analysisCitation28. The corresponding probability for febuxostat was obtained using the relative risk of febuxostat versus allopurinolCitation29, with the rate for probenecid assumed to be the same as that for allopurinol. The probability of experiencing acute flares in the maintenance phase was related to SUA levels during this period and was taken from an unpublished overall gout population study conducted by IMS, referenced by Beard et al.Citation27,Citation30 For the non-responders, the probability was calculated as a weighted mean using the distribution across the SUA levels as reported in the FACT and APEX studies.
The NMA also informed discontinuation rates due to AEs for allopurinol and febuxostat during the first 3 months. The discontinuation rate for probenecid was sourced from a retrospective observational study in which a high-prevalence of gout was reportedCitation31. ULT-specific dropout rates after the first cycle of allopurinol and febuxostat were appropriated from Beard et al.Citation17,Citation23,Citation27,Citation32. In the absence of long-term data, the dropout rate for probenecid was assumed to be similar to that for allopurinol. Drop-out rates were equally applied to patients in response states, regardless of treatment line, and in non-response states using last-line ULT. All-cause mortality rate was informed by age- and gender-specific mortality rates from the Department of Statistics SingaporeCitation13.
The incidence of SJS/TEN in people using allopurinol (0.2%) was taken from Saokaew et al.Citation33 who reported rates in a Taiwanese population with a similar ethnic profile to Singapore patientsCitation2. For febuxostat, the model assumed an SJS/TEN incidence of 0.01%, in line with the summary of product characteristics and Plumpton et al. The incidence of DRESS induced by allopurinol was obtained from Dong et al.Citation5. Singapore’s national voluntary adverse drug reaction databases reported that DRESS accounted for 30% of SCAR cases occurring between 1993 and 2014. This was applied to the derived incidence of SJS/TEN, giving an incidence of 0.08% for DRESSCitation5. Out of all the SJS/TEN cases, the breakdown of patients with SJS, TEN and SJS/TEN overlap of 70%, 20% and 10% respectively were sourced from Dong et al.Citation5 The mortality rate of SJS/TEN and DRESS was reported by Roujeau et al.Citation4 The reported proportion of patients with SJS, SJS/TEN overlap and TEN who died were taken from epidemiological studies with reasonable sample sizes (between 100 and 600 patients) in EuropeCitation34,Citation35.
Reflecting local demographics, the model used a prevalence for the HLA-B*58:01 allele based on the weighted prevalence of the 3 predominant ethnicity groups in Singapore (22.3% in Chinese, 7.3% in Malay and 3.5% in Indian Singaporeans)Citation7. The predictive value of the HLA-B*58:01 genotyping test was calculated using the methodology described by Saokaew et al.Citation33,Citation36.
Health-related quality of life
No published studies reporting health-related quality of life (HRQoL) in Singapore patients with gout were identified. Utility values for the base-case analysis were derived from Beard et al., who used unpublished data from IMS evaluating HRQoL in patients with gout in EuropeCitation30. This study found the mean EuroQol-5 utility value for patients with gout (treated and untreated) was 0.710 (95% CI 0.638–0.736) and that HRQoL varied by SUA level, with every incremental increase by 2 mg/dL in SUA above 6 mg/dL corresponding to a decrease in utility value by 0.034. In addition, a disutility value of 0.0097 was applied to acute flare episodes experienced. It bears highlighting that adopting data from the IMS study assumes QALY benefits arise from both the avoidance of gout flares and the association of SUA levels with quality-of-life differences.
Baseline utility for SJS/TEN was sourced from a study that evaluated EuroQol-5 utility in Swedish patients affected by burns. Utility for DRESS was derived from an HRQoL study of patients with sepsisCitation37,Citation38. A utility value of 0.14 was reported for patients with a mean of 24.3% total body surface area affected by burns. This extent of skin detachment in patients with burns closely resembles that of SJS/TEN in which 10–30% of body surface area is affected by detachment of the epithelial skin layer. No studies reporting utilities for patients with gout and long-term SJS/TEN sequelae were identified, thus, the utility values for long-term sequelae from SJS/TEN were calculated using the multiplicative approach described by Ara and BrazierCitation39,Citation40.
Cost
Direct medical costs borne by Singapore healthcare payers including patients, government, insurers and public healthcare institutions (PHIs) were considered (). Cost data were sourced from local PHIs in Singapore dollars (SGD). Costs were weighted by the proportion of patients whose gout is managed in primary care (80%) and tertiary care (20%), with the exception of costs for managing acute flares, which was assumed to be managed in primary care for all patients. The cost of ULT, patient consultation/monitoring costs and costs for the treatment of acute flares were obtained from the PHIs and national public sector drug utilization data.
Table 3. Model inputs: cost parameters.
The mean cost of treating SJS/TEN or DRESS during an acute episode of SCAR was obtained from national Casemix and subvention data (2012–2016). In patients who survived SCAR, the probability of developing long-term sequelae was obtained as a mean proportion of patients with ocular complicationsCitation41. The cost of ocular complications was obtained from local ophthalmologists and consisted of one-off costs such as corneal transplants, and recurring costs such as medications. These costs were applied to all patients who experience SJS/TEN or DRESS, including patients who discontinued ULT.
Statistical analysis
Network meta-analysis was performed using Stata Statistical Software Release 15 (StataCorp 2017, College Station, TX: StataCorp LLC). Model simulation was performed in TreeAge Pro 2018 R2. TreeAge software, Williamstown, MA; software available at http://www.treeage.com.
Cost-effectiveness analysis
Strategies were first ranked in order of increasing overall costs, followed by removal of strategies that were both less effective and incurred higher costs than the preceding strategy (that is, dominated strategies). For the remaining strategies, incremental cost-effectiveness ratios (ICERs) were calculated by dividing the incremental cost by the additional benefit, compared with the strategy incurring the next highest cost. Costs and benefits were discounted at a rate of 3% per annum (base-case).
Sensitivity analysis
To identify key model parameters, deterministic one-way sensitivity analysis (OWSA) was conducted for all parameters. Each parameter was varied, if available, within its 95% confidence interval and the extent of consequent changes in ICER plotted as a Tornado diagram. Cost inputs, except for febuxostat and the genotyping test, were considered to be known parameters and hence were not included in the OWSA.
Probabilistic sensitivity analysis (PSA) was performed using second-order Monte Carlo simulation to assess the simultaneous influence of variables on the ICER. Parameters were randomly sampled from the probability distributions 5,000 times, each time generating an estimated value for cost and QALYs. The list of probability distributions for the parameters tested in the PSA has been tabulated as a Supplementary table (Supplementary Table S4). A cost-effectiveness acceptability curve (CEAC) was generated, displaying the probability that a particular strategy is cost-effective at each willingness-to-pay threshold.
Scenario analysis
Two scenario analyses were performed. Considering the low adherence to ULT observed in real-world practice, the base-case analysis applied a risk of treatment discontinuation after the first year of ULT. The first scenario investigated the case where all patients are fully compliant to ULT (no discontinuation). The second scenario involved varying the SJS/TEN incidence rate to determine when genotyping-guided strategies would confer incremental QALYs over the non-genotyping strategies.
Results
Base-case results
The base-case analysis results showed that patients managed with ULT experienced more QALYs, but incurred higher costs than patients on supportive treatment without ULT (). Standard of care (Strategy 2, allopurinol300-allopurinol600-probenecid) was cost-effective compared to supportive treatment without ULT (ICER SGD1,584/QALY). First-line treatment with febuxostat (Strategy 5) was dominated by standard of care (Strategy 2). Febuxostat as third-line treatment after allopurinol and probenecid (Strategy 4) generated an ICER of SGD11,398 per QALY compared to standard of care. This was more favorable than when febuxostat was used second-line after allopurinol (Strategy 3), where the ICER was SGD142,301 per QALY compared to standard of care and was not considered cost-effective in the local context. These findings suggest that febuxostat is likely to be considered cost-effective in Singapore only as last-line therapy after allopurinol and probenecid.
Table 4. Results from the base-case analysis (SGD).
All genotyping-guided treatment strategies were dominated by non-genotyping strategies. In the model, genotyping reduced SJS/TEN incidence from 16 to 1 in 10,000 patients, with 667 patients needing to be tested to avoid one SJS/TEN event. Considering the additional costs, genotyping did not produce sufficient incremental QALYs compared to non-genotyping strategies.
Subgroup analysis of patients with concomitant renal impairment found that treatment with febuxostat after allopurinol (300 mg/day) (Strategy 9) incurred an ICER of SGD33,396/QALY compared to allopurinol 300 mg/day only (Strategy 12). The genotyping-guided treatment strategy was also dominated by the non-genotyping strategy in this subgroup. Despite the model estimating an SJS/TEN incidence reduction from 35 to 1 in 10,000 patients, the associated increase in cost of genotyping was accompanied by a slight decrease in QALYs. The strategy which modelled febuxostat only (Strategy 11) in this subgroup produced fewer QALYs compared to the genotyping-guided treatment strategy, although the SJS/TEN event rate was comparable.
Sensitivity analysis
One-way sensitivity analysis (OWSA)
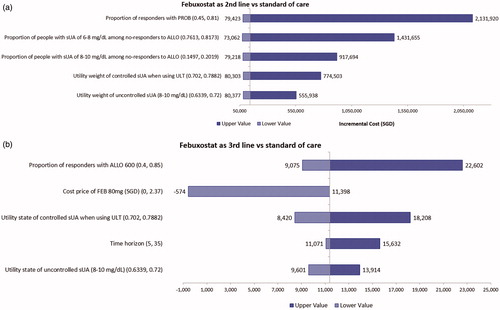
ICERs for febuxostat as second-line ULT were heavily influenced by the probability of response for probenecid and the proportion of non-responders in the SUA 6–8 mg/dL and 8–10 mg/dL categories for allopurinol (). Utility weights for the controlled and uncontrolled SUA states and the cost price of febuxostat exerted moderate impact on the ICER. The efficacy of febuxostat in achieving SUA response had only a minor impact on the ICER. For the strategy investigating febuxostat as third-line ULT, treatment effect of allopurinol 600 mg and cost price of febuxostat were the main drivers of the ICER ().
Figure 2. (a) Top 5 parameters in the one-way sensitivity analysis for second-line febuxostat (Strategy 3) vs standard of care (Strategy 2): Base case SGD142,301/QALY. (b) Top 5 parameters in the one-way sensitivity analysis for third-line febuxostat (Strategy 4) vs standard of care (Strategy 2): Base case SGD11,398/QALY.
Probabilistic sensitivity analysis (PSA)
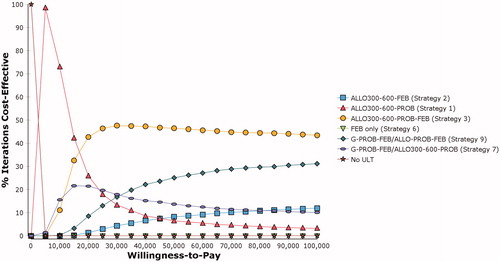
The PSA results were congruent to the deterministic base-case analysis. The CEAC showed that current standard of care (Strategy 2) was the most cost-effective strategy in the willingness-to-pay range SGD1,620 to SGD16,500 per QALY. Above the SGD16,500 per QALY threshold, Strategy 4 became the most cost-effective strategy with a 40% to 50% probability ().
Scenario analysis
Assuming 100% compliance to ULT did not substantially change the ICERs in the base-case analysis. Enhanced compliance led to a better control of SUA levels and lower incidence of acute flares. While this resulted in increased QALYs, costs were correspondingly higher due to increased ULT expenditure.
The threshold SJS/TEN incidence above which the genotyping-guided treatment strategy (Strategy 6) produced incremental QALYs over the non-genotyping strategy (Strategy 2) was 1.12%. Above an incidence of 1.5%, the genotyping-guided strategy dominated the non-genotyping strategy.
Discussion
Our economic model showed that patients with gout who were managed with ULT experienced more QALYs but incurred higher costs than patients on no ULT. The treatment strategy where febuxostat was used first-line was dominated by standard of care. Amongst the sequential ULT strategies, febuxostat as second-line treatment (after allopurinol) generated a high ICER (SGD142,301 per QALY), while febuxostat as third-line treatment (after allopurinol and probenecid) generated a more favorable ICER of SGD11,398 per QALY versus standard of care, which is more likely to be considered a cost-effective use of healthcare resources in Singapore’s context.
Strategies incorporating prior screening of patients for the HLA-B*58:01 allele before ULT to mitigate the risk of SJS/TEN AEs from allopurinol were found to produce smaller QALY benefits at higher costs compared to their respective non-genotyping strategies, and were therefore dominated. This observation is most likely attributed to the low positive predictive value of the genotyping test. Despite the strong association of the HLA-B*58:01 allele with the occurrence of SCAR reported in the literature, the incidence rate of allopurinol SJS/TEN reactions is low in Singapore. Possible reasons for this include patients seeking episodic care for acute gout and not returning for long-term management of symptomatic hyperuricemia; and increasing physician awareness of the risk of SCAR through periodic updates on allopurinol hypersensitivity sent out to all local healthcare professionals. Genotyping essentially removed allopurinol as an effective therapeutic option for the 18.5% of patients who were HLA-B*58:01 carriers in the model. Due to the low incidence of SJS/TEN, the improvement in QALYs from avoiding SJS/TEN did not offset the reduction in QALYs from overall lower probability of achieving the response without allopurinol as a treatment option.
Subgroup analyses conducted in patients with concomitant renal impairment also revealed that genotyping-guided strategies before ULT were dominated by non-genotyping strategies. In spite of the higher risk of SJS/TEN reactions in this subgroup, relative to the non-renal impaired general population (0.46% vs 0.2%)Citation42, the reduction in SJS/TEN events afforded by pre-emptive HLA-B*58:01 screening was not adequate to confer incremental QALYs.
The deterministic sensitivity analyses (OWSA) for the non-genotyping strategies indicated that results were predominantly influenced by the magnitude of treatment effect for ULT and the proportion of non-responders in the various SUA categories. Parameters such as HRQoL utility weights and cost price of febuxostat were also drivers of the ICER estimates, although to a lesser degree. It should be noted that varying the parameters over their uncertainty ranges did not significantly change the base-case ICERs. In the genotyping-guided strategies, the main parameters impacting the ICER were treatment effect for ULT, annual discount rate, time horizon and cost price of febuxostat. Similarly, the dominance of the non-genotyping strategies over the genotyping strategies was consistent in the OWSAs. Results from the multivariate probabilistic sensitivity analysis were, in general, aligned with the base-case results.
Our model results were consistent with published CEAs which evaluated both the non-genotyping and genotyping-guided strategies. Febuxostat was found to be cost-effective in different settings when prescribed sequentially after first-line allopurinol in three CEAs conducted in overseas jurisdictionsCitation27,Citation43,Citation44, however, the comparator chosen for these evaluations was allopurinol alone. This explains the high ICER we estimated for febuxostat as second-line ULT compared to standard of care. Nevertheless, febuxostat as first-line therapy was not cost-effective in any of the published CEAs, which aligns with our assessment. Recent evidence which suggests that febuxostat is associated with an overall increased risk of cardiovascular and all-cause mortality in comparison to allopurinolCitation45 has further strengthened the case for febuxostat being used as last-line ULT. For the genotyping-guided strategies, our findings were comparable to that of Dong et al.Citation5 who developed a decision tree model to assess the cost–effectiveness of routine genotyping for the HLA-B*58:01 allele from the Singapore health system’s perspective. In contrast, our analysis incorporated a more granular valuation of the management costs of SJS/TEN sequelae and also applied ULT discontinuation rates which provides a more accurate representation of long-term resource consumption and patient behaviours in relation to treatment compliance for goutCitation46,Citation47.
Overseas CEAs that reported HLA-B*58:01 genotyping prior to ULT to be a cost-effective strategyCitation42,Citation48 had short time horizons (1 year), unlike in our analysis, which would have biased the ICERs in favor of genotyping-guided strategies as any QALY benefits would be largely attributable to the avoidance of SJS/TEN events in such a limited time frame.
There are a number of limitations in our current analysis. Our assumption that second- or third-line treatment effect of ULT is equivalent to first-line effects, in the absence of data, is a key limitation that is not supported by evidence from RCTs. With the exception of one trialCitation21, patients enrolled in all other identified RCTs that informed our analysis were not required to have had an insufficient response with maximally tolerated doses of allopurinol prior to switching therapies, alluding to uncertainty in the response rates of febuxostat in the cohort of patients with SUA sub-optimally controlled on allopurinol. This assumption was nonetheless required to be able to address the decision questions within the scope of this evaluation. In addition, the uncertainties in the SUA response rates, specifically for probenecid, were manifested in the small number of published trials evaluating the head-to-head efficacy of probenecid with allopurinol and benzbromarone. This paucity of data led to the exclusion of probenecid studies in the NMA, necessitating reliance on response rates reported in the probenecid and benzbromarone studies. There was also considerable uncertainty in the incidence of allopurinol-induced SCAR events, for which local data was not available for both the general population and subgroup with renal impairment. Studies evaluating the association of the HLA-B*58:01 allele with SCAR would ideally need to be conducted in the Singapore population to provide more accurate estimates of sensitivity, specificity and diagnostic odds ratios associated with genotyping, crucial to the model simulation.
Conclusions
In conclusion, our analyses indicate that current standard of care (allopurinol300-allopurinol600-probenecid) is cost-effective compared with no ULT. Febuxostat used as first or second-line treatment in a ULT sequence is unlikely to be cost-effective in Singapore’s context at its current price. Results will be useful to inform local prescribing behaviors, and ULT national subsidy decisions.
Transparency
Declaration of funding
No sponsorship/funding requires declaration.
Declaration of financial/other interests
The authors have no financial relationships to declare.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship of this manuscript, take responsibility for the integrity of the work as a whole and have given final approval for the version to be published.
Acknowledgements
No assistance in the preparation of this article to be declared.
Previous presentations
This work was presented in part, at the 8th HTAsiaLink annual conference, 2019.
Supplemental Material
Download MS Word (75.3 KB)References
- Teng GG, Ang LW, Saag KG, et al. Mortality due to coronary heart disease and kidney disease among middle-aged and elderly men and women with gout in the Singapore Chinese Health Study. Ann Rheum Dis. 2012;71:924–928.
- Tassaneeyakul W, Jantararoungtong T, Chen P, et al. Strong association between HLA-B* 5801 and allopurinol-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenet Genomics. 2009;19:704–709.
- Mahar PD, Wasiak J, Hii B, et al. A systematic review of the management and outcome of toxic epidermal necrolysis treated in burns centres. Burns. 2014;40:1245–1254.
- Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331:1272–1285.
- Dong D, Tan-Koi WC, Teng GG, et al. Cost–effectiveness analysis of genotyping for HLA-B* 5801 and an enhanced safety program in gout patients starting allopurinol in Singapore. Pharmacogenomics. 2015;16:1781–1793.
- Duong TA, Valeyrie-Allanore L, Wolkenstein P, et al. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390:1996–2011.
- Pillai NE, Okada Y, Saw WY, et al. Predicting HLA alleles from high-resolution SNP data in three Southeast Asian populations. Hum Mol Genet. 2014;23:4443–4451.
- Phillips EJ, Mallal SA. Pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2010;11:973–987.
- Yun J, Mattsson J, Schnyder K, et al. Allopurinol hypersensitivity is primarily mediated by dose‐dependent oxypurinol‐specific T cell response. Clin Exp Allergy. 2013;43:1246–1255.
- Fam AG, Dunne SM, Iazzetta J, et al. Efficacy and safety of desensitization to allopurinol following cutaneous reactions. Arthritis Rheum. 2001;44:231–238.
- Jung JW, Kim DK, Park HW, et al. An effective strategy to prevent allopurinol-induced hypersensitivity by HLA typing. Genet Med. 2015;17:807–814.
- Drug prices. Singapore: Ministry of Health; 2016. [2020 Mar 3]. Available from: https://www.moh.gov.sg/content/moh_web/home/pressRoom/Parliamentary_QA/2016/drug-prices.html
- Department of Statistics Singapore. Available from: https://www.singstat.gov.sg/find-data/search-by-theme/populatio/death-and-life-expectancy/latest-data
- Khanna D, Khanna PP, Fitzgerald JD, et al. American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res. 2012;64:1447–1461.
- Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42.
- Becker MA, Schumacher HR, Espinoza LR, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther. 2010;12:R63.
- Becker MA, Schumacher HR, Jr Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–2461.
- Becker MA, Schumacher HR, Jr Wortmann RL, et al. Febuxostat, a novel nonpurine selective inhibitor of xanthine oxidase: a twenty‐eight–day, multicenter, phase II, randomized, double‐blind, placebo‐controlled, dose‐response clinical trial examining safety and efficacy in patients with gout. Arthritis Rheum. 2005;52:916–923.
- Huang X, Du H, Gu J, et al. An allopurinol‐controlled, multicenter, randomized, double‐blind, parallel between‐group, comparative study of febuxostat in C hinese patients with gout and hyperuricemia. Int J Rheum Dis. 2014;17:679–686.
- Reinders M, Haagsma C, Jansen TTA, et al. A randomised controlled trial on the efficacy and tolerability with dose escalation of allopurinol 300–600 mg/day versus benzbromarone 100–200 mg/day in patients with gout. Ann Rheum Dis. 2009;68:892–897.
- Reinders M, Van Roon E, Jansen TTA, et al. Efficacy and tolerability of urate-lowering drugs in gout: a randomised controlled trial of benzbromarone versus probenecid after failure of allopurinol. Ann Rheum Dis. 2009;68:51–56.
- Saag KG, Whelton A, Becker MA, et al. Impact of febuxostat on renal function in gout patients with moderate‐to‐severe renal impairment. Arthritis Rheumatol. 2016;68:2035–2043.
- Schumacher HR, Jr Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28‐week, phase III, randomized, double‐blind, parallel‐group trial. Arthritis Rheum. 2008;59:1540–1548.
- White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378:1200–1210.
- Xu S, Liu X, Ming J, et al. A phase 3, multicenter, randomized, allopurinol‐controlled study assessing the safety and efficacy of oral febuxostat in Chinese gout patients with hyperuricemia. Int J Rheum Dis. 2015;18:669–678.
- Yu K, Lai J, Hsu P, et al. Safety and efficacy of oral febuxostat for treatment of HLA-B* 5801-negative gout: a randomized, open-label, multicentre, allopurinol-controlled study. Scand J Rheumatol. 2016;45:304–311.
- Beard SM, von Scheele BG, Nuki G, et al. Cost-effectiveness of febuxostat in chronic gout. Eur J Health Econ. 2014;15:863–897.
- Plumpton CO, Alfirevic A, Pirmohamed M, et al. Cost effectiveness analysis of HLA-B* 58: 01 genotyping prior to initiation of allopurinol for gout. Rheumatology. 2017;56:1729–1739.
- Tayar JH, Lopez‐Olivo MA, Suarez‐Almazor ME. Febuxostat for treating chronic gout. Cochrane Database Syst Rev. 2012;CD008653.
- IMS. A health economic assessment of febuxostat in the management of gout. Data on File. Version No 1.0. 2007.
- Pui K, Gow PJ, Dalbeth N. Efficacy and tolerability of probenecid as urate-lowering therapy in gout; clinical experience in high-prevalence population. J Rheumatol. 2013;40:872–876.
- Becker MA, Schumacher HR, MacDONALD PA, et al. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol. 2009;36:1273–1282.
- Saokaew S, Tassaneeyakul W, Maenthaisong R, et al. Cost-effectiveness analysis of HLA-B* 5801 testing in preventing allopurinol-induced SJS/TEN in Thai population. PloS One. 2014;9:e94294.
- Revuz J, Penso D, Roujeau JC, et al. Toxic epidermal necrolysis: clinical findings and prognosis factors in 87 patients. Arch Dermatol. 1987;123:1160–1165.
- Schöpf E, Stühmer A, Rzany B, et al. Toxic epidermal necrolysis and Stevens-Johnson syndrome: an epidemiologic study from West Germany. Arch Dermatol. 1991;127:839–842.
- Somkrua R, Chaiyakunapruk N, Tassaneeyakul W. PSS14 what cost of HLA-B* 5801 genotyping would be cost efective for the prevention of allopurinol-induced Stevens Johnson syndrome/toxic epidermal necrolysis in THAILAND: analyses using a decision-analytic model. Value Health. 2010;13:A564–A565.
- Hofhuis JG, Spronk PE, van Stel HF, et al. The impact of severe sepsis on health-related quality of life: a long-term follow-up study. Anesth Analg. 2008;107:1957–1964.
- Oster C, Willebrand M, Dyster-Aas J, et al. Validation of the EQ-5D questionnaire in burn injured adults. Burns. 2009;35:723–732.
- Ara R, Brazier J. Comparing EQ-5D scores for comorbid health conditions estimated using 5 different methods. Medical Care. 2012;50:452–459.
- Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–1419.
- Lee H, Walsh S, Creamer D. Long‐term complications of Stevens–Johnson syndrome/toxic epidermal necrolysis (SJS/TEN): the spectrum of chronic problems in patients who survive an episode of SJS/TEN necessitates multidisciplinary follow‐up. Br J Dermatol. 2017;177:924–935.
- Ke CH, Chung WH, Wen YH, et al. Cost-effectiveness analysis for genotyping before allopurinol treatment to prevent severe cutaneous adverse drug reactions. J Rheumatol. 2017;44:835–843.
- Jutkowitz E, Choi HK, Pizzi LT, et al. Cost-effectiveness of allopurinol and febuxostat for the management of gout. Ann Intern Med. 2014;161:617–626.
- Perez-Ruiz F, Díaz-Torné C, Carcedo D. Cost-effectiveness analysis of febuxostat in patients with gout in Spain. Journal of Medical Economics. 2016;19:604–610.
- Choi H, Neogi T, Stamp L, et al. New perspectives in rheumatology: implications of the cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular morbidities trial and the associated food and drug administration public safety alert. Arthritis Rheumatol. 2018;70:1702–1709.
- Chua XHJ, Lim S, Lim FP, et al. Factors influencing medication adherence in patients with gout: a descriptive correlational study. J Clin Nurs. 2018;27:e213–e22.
- Lin LW, Teng GG, Lim AYN, et al. Cost‐effectiveness of an adherence‐enhancing intervention for gout based on real‐world data. Int J Rheum Dis. 2019;22:545–554.
- Park DJ, Kang JH, Lee JW, et al. Cost‐Effectiveness Analysis of HLA–B5801 Genotyping in the Treatment of Gout Patients With Chronic Renal Insufficiency in Korea. Arthritis Care Res. 2015;67:280–287.