Abstract
Aims: For this economic analysis, we aimed to model: (1) the cost-efficiency of prophylaxis with biosimilar pegfilgrastim-bmez for chemotherapy-induced (febrile) neutropenia (CIN/FN) compared to reference pegfilgrastim, and (2) the expanded access to CIN/FN prophylaxis and anti-neoplastic treatment that could be achieved with biosimilar cost-savings on a budget-neutral basis.
Methods: In a hypothetical panel of 20,000 cancer patients receiving CIN/FN prophylaxis and using the average sales price (ASP) for the second quarter of 2019 for reference pegfilgrastim, we: conducted an ex ante simulation from the payer perspective of the cost-savings of 10–100% conversion from reference to biosimilar pegfilgrastim-bmez using drug price discounting ranging from 10–35%; estimated the budget-neutral expanded access to biosimilar pegfilgrastim-bmez enabled by these cost-savings; and estimated the budget-neutral expanded access to anti-neoplastic treatment with pembrolizumab. The simulations were replicated using fourth quarter 2019 wholesale acquisition cost (WAC) for reference pegfilgrastim and biosimilar pegfilgrastim-bmez in a post facto analysis.
Results: In ASP simulations, cost-savings of using pegfilgrastim-bmez over reference pegfilgrastim in a 20,000 patient panel range from $1.3 M (at 15% price discount) to $3 M (35%) at 10% conversion rate and from $6.4 M to $14.9 M, respectively, at 50% conversion. These savings could provide prophylaxis with pegfilgrastim-bmez to an additional 352 (15% discount) to 1,076 patients (35%) at 10% conversion or 1,764–5,384, respectively, at 50% conversion. Alternatively, savings could be reallocated for anti-neoplastic treatment with pembrolizumab to 3 (15% discount) to 9 (35%) patients at 10% conversion or 19–45, respectively, at 50% conversion. When utilizing WAC, cost-savings range from $4.6 M (10% conversion) to $23.1 M (50%) which could provide pegfilgrastim-bmez to an additional 1,174 (10% conversion) to 5,873 patients (50%).
Conclusions: Prophylaxis with biosimilar pegfilgrastim-bmez increases the value of cancer care by generating significant cost-savings that could be reallocated to provide expanded access to CIN/FN prevention and anti-neoplastic therapy on a budget-neutral basis.
Introduction
Chemotherapy-induced neutropenia (CIN) predisposes cancer patients to potentially life-threatening infectionsCitation1. The 2019 National Comprehensive Cancer Network (NCCN) has defined neutropenia as an absolute neutrophil count (ANC) <500 neutrophils/mcL or an ANC <1000 neutrophils/mcL and a predicted decline to ≤500 neutrophils/mcL over the next 48 h; and febrile neutropenia (FN) if, in addition, a single temperature ≥38.3 °C orally or ≥38.0 °C over 1 h is observedCitation2. The risk of infection and mortality are dependent on the degree and duration of neutropenia and whether or not fever is presentCitation1. FN, as one of the most serious manifestations of neutropenia, is a major dose-limiting toxicity of chemotherapy that can result in prolonged hospitalization; broad-spectrum antibiotic use; chemotherapy dose reductions, treatment delays, and treatment cancellations in subsequent chemotherapy cycles; and compromised clinical outcomesCitation2,Citation3. Each year in the US, >60,000 cancer patients are hospitalized with neutropenia, with more than 4,000 resulting in deathCitation1. Infections have been documented in approximately 20% to 30% of FN episodes. FN-associated mortality rate ranges from 5% to 13.7%, but may approach or exceed 50% in some high-risk populationsCitation4,Citation5.
Chemotherapy-induced FN places a substantial burden on the healthcare system. The cost of an episode of FN has been estimated to be as high as $34,000Citation1,Citation6–8. A 2012 study by the US Centers for Disease Control and Prevention estimated the cost of a CIN/FN-related hospitalization to average $24,770 per stayCitation9.
Administering granulocyte colony-stimulating factors (GCSF) such as daily injections with biosimilar filgrastim or filgrastim or a single injection with biosimilar pegfilgrastim or pegfilgrastim has become the standard of care for patients at risk of CIN/FN. Prophylaxis with GCSFs aims to correct ANC counts, increase immune response, and decrease the incidence of infection in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of FN.
As we have shown previously in our program of pharmacoeconomic analyses of biosimilar growth factors in EuropeCitation10–13 and the USCitation14–16, conversion from reference to biosimilar agents in supportive cancer care affords significant cost-savings. Moreover, these savings can be reallocated on a budget-neutral basis to fund expanded patient access to anti-cancer treatment with expensive therapeutic regimens or to additional CIN/FN prophylaxis. For instance, in the US, savings from prophylaxis with biosimilar filgrastim-sndz (Zarxioi) in a hypothetical panel of 20,000 cancer patients receiving one cycle of GCSF support could provide, on a budget-neutral basis, between 169 and 544 patients with relapsing/refractory follicular lymphoma with treatment with obinutuzumab or an additional 60–192 patients with non-small cell lung cancer with access to pembrolizumabCitation15. Alternatively, the savings could provide CIN/FN prophylaxis with biosimilar filgrastim-sndz to 2,801–9,017 cycles of myelosuppressive chemotherapy, again on a budget-neutral basis. Novel in these analysesCitation12,Citation13,Citation15 is the concept of number-needed-to-convert (NNC): the number of patients that need to be converted from a reference biological to its biosimilar to purchase budget-neutrally one unit of expanded access to a target treatment.
With the expiration of the Neulasta patent, several biosimilar alternatives of pegfilgrastim have been developed and approved in the US; yet, to date, no economic evaluations of these biosimilars have been published. The most recent biosimilar pegfilgrastim to be approved in the US, pegfilgrastim-bmez (Ziextenzo, Sandoz/Novartis AG, Holzkirchen, Germany), was launched in November 2019. We report here on an economic simulation of the cost-efficiency of pegfilgrastim-bmez and the associated expanded treatment access that these savings may enable—and this from the payer perspective. Importantly, the average sales price (ASP) for pegfilgrastim-bmez has not yet been set due to the short market exposure of this biosimilar, therefore we conducted an ex ante economic simulation. Specifically, as applied to a hypothetical panel of 20,000 covered cancer patients receiving CIN/FN prophylaxis, we aimed to: simulate the cost-savings of conversion from reference to biosimilar pegfilgrastim-bmez; estimate the budget-neutral expanded access to biosimilar pegfilgrastim-bmez enabled by these cost-savings; estimate the budget-neutral expanded access to anti-neoplastic treatment with pembrolizumab; and determine the number-needed-to-convert (NNC) to purchase one additional biosimilar pegfilgrastim-bmez injection for one chemotherapy cycle or one regimen of pembrolizumab on a budget-neutral basis. In addition, as no ASP is available as of yet, we also include a post facto replication analysis using the published wholesale acquisition cost (WAC).
Methods
Model
For this economic analysis, we developed a sequential set of three simulation models from the payer perspective for 2019 for a hypothetical panel of 20,000 covered cancer patients in need of CIN/FN prophylaxis in one chemotherapy cycle using ASP. A first model simulates the direct savings in drug costs accrued by conversion from reference to biosimilar pegfilgrastim-bmez in the prevention of CIN/FN. This model considered biosimilar conversion rates between 10% and 100% in 10% increments and drug price discounts relative to reference pegfilgrastim between 15% and 35% in 5% increments to simulate every scenario generated from cross-tabulating both variables. We applied these data in a second model that estimated the incremental number of cancer patients undergoing myelosuppressive chemotherapy who could be provided with one cycle of prophylaxis with biosimilar pegfilgrastim-bmez on a budget-neutral basis. In parallel, we also used the simulation results in a third model that estimated the number of patients who could be provided with expanded access to pembrolizumab to treat non-small cell lung cancer (NSCLC) on a budget-neutral basis. Next, we applied the output of the latter two models to estimate the NNC to afford one additional CIN/FN prophylaxis with biosimilar pegfilgrastim-bmez and one additional pembrolizumab treatment for NSCLC. With 10 assumed conversion rates, 5 assumed discount rates, one supportive treatment, and one therapeutic treatment, a total of 100 expanded access estimates were simulated. The first two models were then replicated using the WAC in a post facto analysis.
Note that the expanded access estimates reflect additional patients that can be treated beyond the panel of 20,000 cancer patients receiving prophylaxis with reference pegfilgrastim from which the savings are generated. This is particularly important for expanded access to biosimilar pegfilgrastim-bmez. Let k be the size of the savings-generating panel of cancer patients receiving CIN/FN prophylaxis and being converted from reference to biosimilar pegfilgrastim-bmez; let x be the additional patients to whom expanded access to biosimilar pegfilgrastim-bmez prophylaxis can be extended; then the payer can provide such prophylaxis to a total of (k + x) patients in need on a budget-neutral basis.
Assumptions
First, as approval of biosimilar pegfilgrastim-bmez was just granted in November 2019, the product is only now being launched and the ASP price schedule has not been publicized, making the modeling with ASP an ex ante economic evaluation. Second, Blackwell et al.Citation17 and Harbeck et al.Citation18 have demonstrated that biosimilar pegfilgrastim-bmez is clinically equivalent in efficacy, safety and immunogenicity to reference pegfilgrastim. Third, the dose of pembrolizumab regimen considered in this analysis was 200 mg Q3W for 2 years. Fourth, our analyses were done from the payer perspective. Fifth, we included only direct medication costs. We did not consider any medical resource utilization variables (e.g. visit costs, patient co-pays, cost for drug administration), and other patient costs (travel, lodging, meals, time; indirect, opportunity, or intangible costs), as these would be the same for both the reference and the biosimilar agent. Lastly, our models assumed assured prophylaxis. Therefore, we excluded Neulasta OnPro, the on-body injector device that is applied at the end of the chemotherapy session and administers pegfilgrastim approximately 27 h later. Device failure rates between 1.7% and 6.9% have been reported, indicating that some patients would not have received prophylaxis or only partially so and therefore would have been at increased risk for CIN/FN and possible hospitalizationCitation19.
Inputs
For reference pegfilgrastim, we used the ASP to estimate the drug cost for one chemotherapy cycle. Specifically, we applied the ASP for the second quarter of 2019 (2Q19) as obtained from the fourth quarter 2019 (4Q19) payment allowance limits set by the US Centers for Medicare and Medicaid Services (CMS)Citation20. The ASP used for Neulasta (6 mg) was $4,249.81 using the current 4.3% CMS reimbursement add-on (6% minus 1.7% sequestration).
For pembrolizumab, we used the 2Q19 ASP to estimate one course of pembrolizumab defined as 200 mg every three weeks for 2 years. The ASP used for pembrolizumab was $9,470.56 per 200 mg dose or $328,312.94 for the two-year regimen.
The ASP for biosimilar pegfilgrastim-bmez will not be available until two quarters of sales have accrued and this justifies using various discount rates. Assuming discounts of 15% to 35% in 5% increments, the assumed ex ante cost of biosimilar pegfilgrastim-bmez as used in this analysis was $3,612.34 at 15%, $3,399.85 at 20%, $3,187.36 at 25%, 2,974.87 at 30%, and $2,762.38 at 35% discount.
The NNC was calculated as the ratio of the cost of treatment (either one cycle of biosimilar pegfilgrastim-bmez or one regimen of pembrolizumab) to the potential cost-savings generated by converting from reference pegfilgrastim to biosimilar pegfilgrastim-bmez.
The cost-efficiency and expanded access to prophylaxis with biosimilar pegfilgrastim-bmez models were replicated in a post facto analysis using the fourth quarter 2019 WAC for reference pegfilgrastim ($6,231.06) and biosimilar pegfilgrastim-bmez ($3,925.53)Citation21.
Results
ASP models
Cost-Savings
As shown in , per-cycle per-patient cost-savings from converting from reference to biosimilar pegfilgrastim-bmez were $637 (at 15% discount), $850 (at 20% discount), $1,062 (at 25% discount), $1,275 (at 30% discount) and $1,487 (at 35% discount). presents the (rounded) potential savings escalated to a hypothetical panel of 20,000 cancer patients receiving CIN/FN prophylaxis with reference versus biosimilar pegfilgrastim-bmez. At a conversion rate of 10%, savings would range from $1,274,944 (15% discount) to $2,974,869 (35% discount). However, at 50% conversion, the corresponding savings would range from $6,374,720 to $14,874,346; rising to respectively $12,749,439 and $29,748,691 if all patients in the panel received prophylaxis with biosimilar pegfilgrastim-bmez.
Figure 1. Cost-savings from biosimilar conversion ($) and expanded access (number of patients) to biosimilar pegfilgrastim at various conversion and discount rates utilizing ASP.
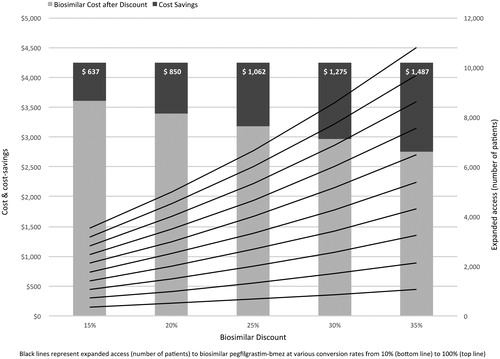
Table 1. Cost savings by conversion from reference pegfilgrastim to biosimilar pegfilgrastim-bmez by scenarios (in US$) utilizing ASP.
Expanded access and Number-Needed-to-Treat
Biosimilar pegfilgrastim-bmez
shows that at a conversion rate of 10% and at the respective discount rates, in a panel of 20,000 patients, an additional 352 (15% discount) to 1,076 patients (35% discount) in need of CIN/FN prophylaxis could be treated with biosimilar pegfilgrastim-bmez on a budget-neutral basis. At a conversion rate of 50%, the corresponding number of additional patients would range from 1,764 to 5,384; increasing to 3,529 and 10,769 if all 20,000 patients were converted (see also ).
Table 2. Expanded access to biosimilar pegfilgrastim-bmez by scenarios (number of patients) utilizing ASP.
Stated differently, holding the discount rate constant at its midpoint of 25% and varying conversion rates, 666 additional patients would gain expanded access to prophylaxis with biosimilar pegfilgrastim-bmez from the savings generated from 10% conversion, rising to 3,333 additional patients at 50% and 6,666 additional patients at 100% conversion. Similarly, holding discount rates constant at each level, on the aggregate between 352 (at 15% discount and 10% conversion) and 10,669 (at 35% discount and 100% conversion) additional patients could receive prophylaxis with biosimilar pegfilgrastim-bmez on a budget-neutral basis.
Alternately, holding the conversion rate constant at 50% and varying discount rates, 1,764 additional patients would gain expanded access to biosimilar pegfilgrastim-bmez prophylaxis from the savings generated at 15% discount. This would increase to 3,333 additional patients at 25% and 5,384 additional patients at 35% discount.
The NNC – that is, the number of patients that need to be converted to prophylaxis with biosimilar pegfilgrastim-bmez to gain expanded access to such prophylaxis for 1 additional patient for 1 cycle – varies across discount rates. As shown in , the NNC is 6 at 15% discount declining to 4 at 25% discount and to 2 at 35% discount.
Table 3. Number-needed-to-convert (number of patients) to gain expanded access for 1 patient to biosimilar pegfilgrastim-bmez or pembrolizumab utilizing ASP.
Pembrolizumab
As summarized in , in a panel of 20,000 patients, at a conversion rate of 10% and at the respective discount rates, an additional 3 (15% discount) to 9 patients (35% discount) needing pembrolizumab treatment would be able to be treated on a budget-neutral basis. At a conversion rate of 50%, the corresponding number of additional patients would range from 19 to 45; rising to 38 and 90 if all 20,000 patients were converted to biosimilar pegfilgrastim-bmez (see also ).
Figure 2. Cost-savings from biosimilar conversion ($) and expanded access (number of patients) to pembrolizumab at various conversion and discount rates utilizing ASP.
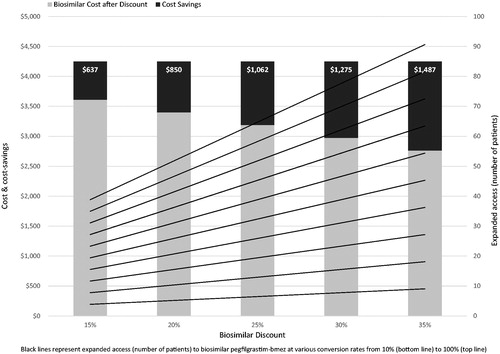
Table 4. Expanded access to pembrolizumab by scenarios (number of patients) utilizing ASP.
Holding the discount rate constant at its midpoint of 25% and varying conversion rates, 6 additional patients would gain expanded access to pembrolizumab therapy from the savings generated from 10% conversion, increasing to 32 additional patients at 50% and 64 additional patients at 100% conversion. Similarly, holding discount rates constant at each level, on the aggregate between 3 (at 15% discount and 10% conversion) and 90 (at 35% discount and 100% conversion) additional patients could be treated with pembrolizumab on a budget-neutral basis.
Alternately, holding the conversion rate constant at 50% and varying discount rates, 19 additional patients would gain expanded access to treatment with pembrolizumab from the savings generated at 15% discount. This would increase to 32 additional patients at 25% and 45 additional patients at 35% discount.
The NNC to gain expanded access to pembrolizumab therapy for 1 additional patient for a two-year regimen varies across discount rates. As shown in , the NNC is 515 at 15% discount decreasing to 309 at 25% discount and to 220 at 35% discount.
WAC models
presents the results of the post facto simulation models utilizing WAC for cost-efficiency and expanded access to additional prophylaxis with biosimilar pegfilgrastim-bmez. Cost-savings from converting from reference to biosimilar pegfilgrastim-bmez were $4,611,060 at 10% conversion, $23,055,300 at 50%, and $46,110,600 if all patients in the 20,000 patient panel were converted. With these savings, expanded access to prophylaxis with biosimilar pegfilgrastim-bmez could be provided to an additional 1,174 patients at 10% conversion, 5,873 patients at 50%, or 11,746 patients if all patients in the panel were converted. The NNC to provide one additional patient with prophylaxis is 2.
Table 5. Cost savings (A) and expanded access (B) by conversion from reference pegfilgrastim to biosimilar pegfilgrastim-bmez utilizing WAC.
Discussion
In the US healthcare system, market dynamics affect what a buyer pays for a drug and a payer reimburses the provider for a drug. This also applies to biosimilars; therefore, economic evaluations must be interpreted in terms of order of magnitude rather than actual financials – both post facto after market introduction and public pricing and ex ante before finalization of pricing. Using a range of discounts relative to the cost of the reference product, as in this study comparing reference and biosimilar pegfilgrastim-bmez, makes it possible to assess the economic benefits of biosimilar conversion across a range of order-of-magnitude results.
Using a hypothetical panel of 20,000 cancer patients undergoing myelotoxic chemotherapy and therefore at risk for CIN/FN, our ASP conversion-by-discount scenarios yielded marked results in terms of cost-savings and expanded access. Cost-savings ranged from ∼$1.3 million in the 10% conversion and 15% discount scenario to ∼$10.6 million in the 50% conversion/25% discount scenario and ∼$29.7 million in the 100%/35% scenario. In the high-cost analysis of pembrolizumab at $328,313 for a two-year regimen, the corresponding expanded access ranged from 3 additional patients in the 10%/15% scenario, to 32 in the 50%/25% scenario, rising further to 90 in the 100%/35% scenario.
Further, with reference pegfilgrastim costing $4,249.81 and considering the biosimilar discount rates that were applied, expanded access to prophylaxis with biosimilar pegfilgrastim-bmez would be possible for an additional 352 patients in the 10%/15% scenario, an additional 3,333 patients in the 50%/25% scenario, and an additional 10,769 patients in the 100%/35% scenario. Importantly, the additional patients benefiting from biosimilar conversion would be other cancer patients at risk for CIN/FN. The high-end estimate of 10,769 additional patients means that 54% of a payer’s next panel of 20,000 patients needing prophylaxis could be treated from the accrued savings. Stating the 100%/35% scenario more pragmatically: in the end, not 20,000 but 30,769 patients at risk for CIN/FN could receive prophylaxis with biosimilar pegfilgrastim-bmez on a budget-neutral basis (i.e. at no additional cost to the payer)—and this with equivalent efficacy.
The results of the post facto WAC-based models reveal even greater cost savings compared to the models using ASP due to the higher cost input for reference pegfilgrastim. The WAC for biosimilar pegfilgrastim-bmez is 63% of that for reference pegfilgrastim, enabling expanded access to even greater numbers of additional patients at all conversion rates relative to the ASP models. The NNC to provide prophylaxis with biosimilar pegfilgrastim-bmez to one additional patient for one cycle is only 2.
Central to our simulations is how savings accrued from biosimilar conversion may be re-purposed on the payer side. In first instance, this is a financial decision. As our present and priorCitation12,Citation13,Citation15 expanded access analyses show, additional patients can be treated on a budget-neutral basis, whether with therapeutic cancer treatments, supportive cancer treatments, or both. This benefit extends beyond provider and payer, but also has a societal benefit as it improves the population’s access to cancer care and thus reduces the societal burden of cancerCitation12. However, the ethical dimensions cannot be ignored. Quality-wise, the same level of supportive cancer care can be provided to more patients without additional financial burden. Further, most health care insurance and related coverages are generated by a society’s population: employees through their employment contributions, citizens through taxation, and employers through the productivity of their employees. It makes ethical sense to advocate that the savings of biosimilar conversion be applied to treating more cancer patients, therapeutically or supportively, on a budget-neutral basis. Lastly, the expanded access funding could be targeted specifically to those unable to contribute to health care funding or those with limited or no health care coverage. Collectively, these arguments address the challenge in our current healthcare environment of enhancing the value of therapies—not just in terms of containing costs but also expanding access to care—by deriving the most benefit (clinical, financial, societal) per dollar spent. These simulations illustrate the magnitude of cost-efficiency and potential for expanded access that could be utilized in developing value-based reimbursement models.
As we noted in our simulation study of expanded access from conversion to standard biosimilar filgrastim, combining biosimilar utilization and expanded access impacts the value equation in two important waysCitation15. First, it affects the cost-to-outcome ratio: “the health outcomes achieved per dollar spent or conversely how much it costs to achieve 1 unit of outcome, such as a CIN/FN episode or a CIN/FIN-related hospitalization averted” (p. 2292)Citation15. Second, it influences the cost-to-access ratio: “how much it costs to provide one patient with access” (p. 2292) to supportive or therapeutic cancer therapyCitation15. In the end, both providers and payers may consider biosimilar conversion a cost-responsible way of providing care that lowers both the cost-to-outcome ratio and the cost-to-access ratio.
In this regard, it is helpful to consider the numbers-needed-to-convert metric. To a payer, it quantifies the number of patients that need to be converted to biosimilar pegfilgrastim-bmez to purchase budget-neutral expanded access to a target treatment for one additional patient. A clinician may interpret the NNC from an efficiency and equity angle: for instance, how many more cancer patients can be treated with pembrolizumab if some or all patients at risk for CIN/FN are provided with prophylaxis with (the equivalent) biosimilar pegfilgrastim-bmez?
Our study has some limitations and identifies areas for future research. It is a simulation, not an analysis based on real-world (clinical) data and should be validated with real-world evidence. Only one cycle of chemotherapy was considered, while guidelines recommend that CIN/FN risk be assessed at the start of each cycleCitation2,Citation22 and real-world evidence indicates that some patients experience a delayed CIN/FN episode in later cyclesCitation23,Citation24. The primary focus of our simulation was on the net benefit and therefore excluded clinical or patient-related direct and indirect costs. It was focused on Ziextenzo while other biosimilar pegfilgrastims are in development or have been launched in the US. We did not consider prescriber reluctance to adopt biosimilars, though it can be assumed under the varying conversion rates adopted. Primary data collection initiatives (e.g. ranging from single-center studies to health plan evaluations) as well as claims database assessments could provide evidence of actual experiences in clinical practice including real-world conversion rates. Such real-world evidence on availability of and conversion to biosimilars could inform payers, providers, policy-makers and patients regarding the impact of biosimilars on access to and cost of care in the US..
Conclusions
Our study confirms and extends previous studies on the cost-efficiency of biosimilar conversion and on the budget-neutral reallocation of associated cost-savings to provide expanded access to therapeutic or supportive cancer care. The cost-savings are significant and can be proportionate to the patient populations being served. Linking the savings from conversion from reference pegfilgrastim to biosimilar pegfilgrastim-bmez with expanded access lowers the cost of supportive cancer care, enables additional supportive or therapeutic cancer care at no incremental cost, and may offer patients access to cancer care.
Transparency
Declaration of funding
This work was sponsored by Sandoz, Inc., Princeton, NJ, USA.
Declaration of financial/other interests
AM serves on a Speakers Bureau and Steering Committee for Sandoz, Inc., the study sponsor.
WW and KC are employees of Sandoz, Inc., the study sponsor.
SB was an employee of Sandoz, Inc., the study sponsor. He is now employed at Novartis Pharmaceuticals Corp.
IA and KM are employees of Matrix45, which was contracted by Sandoz, Inc. By company policy, employees are prohibited from owning equity in client organizations (except through mutual funds or other independently administered collective investment instruments) or contracting independently with client organizations. Matrix45 provides similar services to other biopharmaceutical companies on a non-exclusivity basis.
IA is Deputy Editor-in-Chief of the Journal of Medical Economics. He was not involved in any editorial decisions regarding this manuscript.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors met ICMJE and COPE criteria and contributed substantively to the study as follows:
Study concept: AM, WW, KC, SB.
Study design: WW, KC, SB.
Model development and simulations: WW, KM.
Review and interpretation of results: AM, WW, KC, SB, KM, IA.
Development of manuscript: IA, KM.
Review of the manuscript for intellectual content: AM, WW, KC, SB, KM, IA.
Previous presentations
This study was presented as a poster at the Annual Meeting of the American Society of Clinical Oncology, 29 May – 2 June 2019 in Chicago, IL.
Acknowledgements
None reported.
Note
Notes
i Zarxio is a registered trademark of Sandoz/Novartis AG, Holzkirchen, Germany.
References
- Caggiano V, Weiss RV, Rickert TS, et al. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103(9):1916–1924.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Hematopoietic growth factors. Version 2.2019-March 27, 2019; [cited 2019 May 21]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf.
- Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6(2):109–118.
- Barnes G, Pathak A, Schwartzberg L. G-CSF utilization rate and prescribing patterns in United States: associations between physician and patient factors and GCSF use. Cancer Med. 2014;3(6):1477–1484.
- Hirsch BR, Lyman GH. Pharmacoeconomics of the myeloid growth factors: a critical and systematic review. Pharmacoeconomics. 2012;30(6):497–511.
- Wang XJ, Lopez SE, Chan A. Economic burden of chemotherapy-induced febrile neutropenia in patients with lymphoma: a systematic review. Crit Rev Oncol Hematol. 2015;94(2):201–212.
- Michels SL, Barron RL, Reynolds MW, et al. Costs associated with febrile neutropenia in the US. Pharmacoeconomics. 2012;30(9):809–823.
- Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266.
- Tai E, Guy GP, Dunbar A, et al. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552–e561.
- Aapro M, Cornes P, Sun D, et al. Comparative cost-efficiency across the European Union G5 countries of originators and a biosimilar erythropoiesis-stimulating agent to manage chemotherapy-induced anemia in cancer patients. Ther Adv Med Oncol. 2012;4(3):95–105.
- Aapro M, Cornes P, Abraham I. Comparative cost-efficiency across the European G5 countries of various regimens of filgrastim, biosimilar filgrastim, and pegfilgrastim to reduce the incidence of chemotherapy-induced febrile neutropenia. J Oncol Pharm Pract. 2012;18(2):171–179.
- Abraham I, Han L, Sun D, et al. Cost savings from anemia management with biosimilar epoetin α and increased access to targeted antineoplastic treatment: simulation for the European G5 countries. Fut Oncol. 2014;10(9):1599–1609.
- Sun D, Andayani TM, Altyar A, et al. Potential cost savings from chemotherapy-induced febrile neutropenia prophylaxis with biosimilar filgrastim and expanded access to targeted antineoplastic treatment across the European G5 countries: a simulation study. Clin Ther. 2015;37(4):842–857.
- McBride A, Campbell K, Bikkina M, et al. Cost-efficiency analyses for the US of biosimilar filgrastim-sndz, reference filgrastim, and pegfilgrastim with on-body injector in the prophylaxis of chemotherapy-induced (febrile) neutropenia. J Med Econ. 2017;20(10):1083–1093.
- McBride A, Balu S, Campbell K, et al. Expanded access to cancer treatments from conversion to neutropenia prophylaxis with biosimilar filgrastim-sndz. Fut Oncol. 2017;13(25):2285–2295.
- McBride A, Campbell K, Bikkina M, et al. Reply: cost-efficiency analyses for the US of biosimilar filgrastim-sndz, reference filgrastim, and pegfilgrastim with on-body injector in the prophylaxis of chemotherapy-induced (febrile) neutropenia. J Med Econ. 2018;21(6):606–609.
- Blackwell K, Donskih R, Jones CM, et al. A comparison of proposed biosimilar LA-EP2006 and reference pegfilgrastim for the prevention of neutropenia in patients with early-stage breast cancer receiving myelosuppressive adjuvant or neoadjuvant chemotherapy: Pegfilgrastim Randomized Oncology (Supportive Care) Trial to Evaluate Comparative Treatment (PROTECT-2), a phase III, randomized, double-blind trial. Oncologist. 2016;21(7):789–794.
- Harbeck N, Lipatov O, Frolova M, et al. Randomized, double-blind study comparing proposed biosimilar LA-EP2006 with reference pegfilgrastim in breast cancer. Fut Oncol. 2016;12(11):1359–1367.
- McBride A, Krendyukov A, Mathieson N, et al. Febrile neutropenia hospitalization due to pegfilgrastim on-body injector failure compared to single-injection pegfilgrastim and daily injections with reference and biosimilar filgrastim: US cost simulation for lung cancer and non-Hodgkin lymphoma. J Med Econ. 2020;23(1):28–36.
- ASP Drug Pricing Files April 2019. Update. Center for Medicare and Medicaid Services; [cited 2019 Apr 14]. Available from: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2019ASPFiles.html.
- Pegfilgrastim and pegfilgrastim-bmez. Micromedex Solutions. Truven Health Analytics, Inc. Ann Arbor, MI; [cited 2019 Dec 22]. Available from: http://www.micromedexsolutions.com.
- Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy- induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32.
- Gascón P, Aapro M, Ludwig H, et al. Treatment patterns and outcomes in the prophylaxis of chemotherapy-induced (febrile) neutropenia prophylaxis with biosimilar filgrastim (the MONITOR-GCSF study). Support Care Cancer. 2016;24(2):911–925.
- Aapro M, Ludwig H, Bokemeyer C, et al. Predictive modeling of the outcomes of chemotherapy-induced (febrile) neutropenia prophylaxis with biosimilar filgrastim (MONITOR-GCSF study). Ann Oncol. 2016;27(11):2039–2045.
