Abstract
Aims: The study evaluated the real-world cost of treatment in multiple sclerosis (MS) patients initiating infused disease-modifying-therapies (DMT) in the United States.
Materials and Methods: This retrospective cohort study using administrative claims data included adult patients with MS initiating index infusion DMT (ocrelizumab (OCR), natalizumab (NTZ) or alemtuzumab (ATZ)) from April 2017–September 2018 with 6-months pre/12-months post-index continuous enrollment. The primary cohort included patients who had prescribed annual dosing visits indicated by the approved product label (PL): 3 OCR, 5 ATZ, and 12–13 NTZ infusion visits within the first year of initiation. Annual treatment cost was the sum of all costs on index DMT infusion visit dates. Costs were summarized for a primary and secondary cohort of patients receiving additional doses than prescribed in PL (>3 OCR, >5 ATZ, and >13 NTZ infusion visits); and an overall cohort of patients who met minimum required annual dose (≥3 OCR, ≥5 ATZ, and ≥12 NTZ), further stratified by insurance type.
Results: For patients in the primary cohort (123 OCR, 18 ATZ, and 48 NTZ), mean (standard-deviation) annual cost of treatment with OCR, ATZ, and NTZ cohorts was $72,066 ($34,480), $121,053 ($51,097) and $93,777 ($38,815), respectively. Among patients initiating OCR and NTZ, 15 and 6% respectively, had additional infusion visits leading to greater costs. Mean annual costs of index infusion DMT treatment in the overall cohort (162 patients treated with OCR, 18 with ATZ, 56 with NTZ) were $80,582, $121,053, and $93,807, respectively. The mean costs for commercial enrollees were higher than those for MAPD enrollees.
Limitations: Small sample size, limited population generalizability, and cost-reduction for ATZ beyond the second year need to be accounted for.
Conclusions: Real-world infusion DMT treatment costs for commercially insured patients were higher than perceived expenditures based on wholesale acquisition cost and administration costs via a physician-fee schedule. Consideration of real-world costs in cost-effectiveness and treatment/coverage decisions is needed.
Introduction
Multiple sclerosis (MS) is a chronic autoimmune, inflammatory, demyelinating disease of the central nervous system characterized by neurological symptoms involving the motor, cognitive, sensory, visual, and autonomic systemsCitation1. In most patients, the disease course starts with relapsing-remitting (RRMS) progressing to secondary-progressive MS with a smaller proportion of patients experiencing a primary progressive MS (PPMS) disease course.
The estimated annual cost of MS in the United States is $28 billionCitation2 and spending on MS disease-modifying therapies (DMTs) alone was $19 billion in 2016Citation3. Currently, 18 DMTs are approved and marketed by the US Food and Drug Administration for treatment of relapsing forms of MS. MS DMT costs account for 63% of overall health care costs and 75% of MS-related healthcare expenditures in patients treated with DMTsCitation4,Citation5.
Wholesale acquisition costs (WAC) for DMTs in 2019 ranged from $25,420 per year for generic glatiramer acetate to $114,519 per year for alemtuzumabCitation6. Expenditures for some DMTs, especially those administered intravenously, include costs beyond drug acquisition, which may be attributed to administration, monitoring, co-administered medications such as corticosteroids, clinician visits, and management of adverse events during infusion. The additional costs for these and other ancillary services are often perceived as negligible and may be unaccounted for when overall treatment costs are determined. The costs of DMT administration are usually calculated by multiplying the estimated infusion frequency by costs from sources such as the physician-fee scheduleCitation7. Costs for infusion DMTs calculated from WAC plus cost of administration and monitoring calculated via physician fee schedules may underestimate the actual amounts paid by US payers and patients and may, in turn, affect the selection of DMTs based on cost-effectiveness.
The objective of the current study was to assess the real-world cost of infusion DMT treatment [ocrelizumab (OCR), alemtuzumab (ATZ) and natalizumab (NTZ)] in MS patients during their first year of treatment from a payer perspective.
Methods
Study design and sample selection
This retrospective observational study used administrative claims data for commercial and Medicare Advantage with Part D (MAPD) enrollees in the US identified from the Optum Research Databasei from October 2016 through September 2018. Commercial enrollees are typically employees or their dependents who receive health insurance as a benefit of employment. MAPD enrollees are Medicare beneficiaries who receive publicly funded Medicare benefits from private insurers. Individuals may qualify for Medicare based on age (≥65-years-old) or disability status. The Optum Research Database is fully de-identified in compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA) and no identifiable or protected health information was extracted; consequently, institutional review board approval was not required for this study.
Initial patient selection was based on commercial and MAPD enrollees with ≥1 claim for an infusion DMT during the identification period of 01 April 2017 through 23 September 2017. The date of the first infusion DMT claim during the identification period was defined as the index date. The DMT on the index date (index infusion DMT) was used to determine the infusion DMT cohort. Adult patients (age ≥18-years-old) without missing demographic information, 6 months of continuous enrollment with medical and pharmacy benefits before the index date (baseline period) and 12 months of continuous enrollment beginning on the index date (follow-up period) were included. Additional selection criteria included ≥1 medical claim with an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10) diagnosis code for MS diagnosis (G35) during the baseline period and no claims for the index infusion DMT during the baseline period.
Patients were assigned to DMT cohorts based on their index infusion DMT: OCR, ATZ or NTZ. OCR was identified from medical claims with: (1) Healthcare Common Procedure Coding System (HCPCS) code C9494 or J2350; or (2) HCPCS code J3490, J3590, or J9999 or CPT code 96413, 96415, 96365, or 96366 and National Drug Code (NDC) 50242015001; or (3) pharmacy claims with NDC code 50242015001Citation8. ATZ was identified from medical claims with HCPCS code J0202. NTZ was identified from medical claims with HCPCS code J2323 or pharmacy claims with NDC 59075073015 or 64406000801. All medical claims for the infusion DMTs were required to have an ICD-10 diagnosis code for MS in the first or second position on the claim.
Based on the FDA product label, OCR is indicated to be administered as an infusion with a start dose of 300 milligrams (mg) followed two weeks later by a second 300 mg infusion, and then subsequent visits for 600 mg infusions every 6 months, that is patients are expected to have 3 infusion visits in the first year of treatmentCitation9. ATZ is administered with two treatment courses over 2 years: the first course is 12 mg/day on 5 consecutive days (i.e. patients are expected to have at least 5 infusion visits in the first year) followed 12 months later by a second course of 12 mg/day on 3 consecutive days. This may be followed by additional round of treatment in subsequent years with one infusion a day on 3 consecutive days as needed to be decided by healthcare providerCitation10. NTZ is administered as a 300 mg dose infused intravenously every 4 weeks, that is, patients are expected to have at least 12–13 infusion visits in the first yearCitation11.
Patient cohorts
In addition to the index infusion DMT cohorts, the patients were grouped into cohorts based on infusion visits:
The “primary cohort” comprised patients who had the number of infusion visits consistent with the prescribed annual dosing indicated by the approved product label for the first year of treatment. Each date with a claim for the index infusion DMT was defined as an infusion visit. Patients in the OCR, ATZ, and NTZ cohorts had 3 infusion visits, 5 infusion visits, and 12 or 13 infusion visits, respectively.
The “secondary cohort” included patients with more infusion visits than are prescribed in the label: ≥4 infusion visits for patients whose index infusion DMT was OCR; ≥6 for ATZ; and ≥14 for NTZ.
The “overall cohort” included patients who had at least prescribed annual dosing per the product label, that is, combining the primary and secondary cohorts.
Key study outcomes
Patient characteristics were measured in the primary cohort and captured age in the index year, gender, US Census region (Midwest, Northeast, South, West), insurance type (Commercial, MAPD), pre-index MS relapsesCitation12,Citation13, and comorbidities commonly observed in patients with MS and defined from diagnosis codesCitation14,Citation15. The main outcome was annual real-world costs of treatment with the index infusion DMT. Index infusion DMT costs were calculated for each infusion visit by summing all insurer and patient paid amounts on the infusion visit date, and included costs for drug acquisition, infusion administration, co-administered medication, other infusion-related services, and all other services that were billed on the infusion visit date.
Statistical analyses
All analyses were performed using SAS version 9.4 (SAS Institute, Inc.). Categorical variables are reported as frequencies (n) and percentages. Continuous variables are reported as mean, median, quartiles and standard deviation (SD). Patients’ baseline characteristics and key outcomes were summarized by index infusion DMT and by type of insurance. The annual costs of treatment with the index infusion DMTs were analyzed for the primary, secondary, and overall cohorts and stratified by index infusion DMT and insurance type. In addition, we conducted a sensitivity analysis of patients who met the selection criteria for the primary cohort, except for 12-month post-index continuous enrollment; this provided a larger sample of patients who were treated with their index infusion DMTs while retaining the required dosing per product label.
Results
Patient selection and cohorts
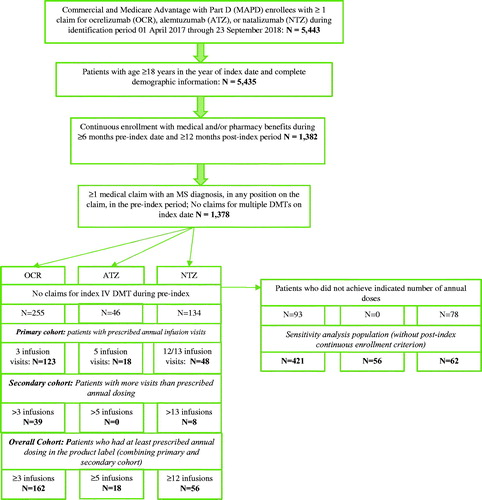
Between 01 April 2017 and 23 September 2017, 5,443 commercial and MAPD enrollees had ≥1 claim for OCR, ATZ, or NTZ. After applying additional inclusion criteria for minimum age, non-missing demographic information, pre-index MS diagnosis, and continuous enrollment, 1,378 patients were identified who then were further divided into initial index infusion DMT cohorts: OCR (n = 255), ATZ (n = 73), and NTZ (n = 1,050). Then, we applied the criterion for initiation of the index infusion DMT on the index date based on no pre-index claims for the index infusion DMT; this reduced the cohorts to 255 OCR, 46 ATZ and 134 NTZ patients (). Additionally, 36.5% (n = 93), 0% (n = 0), and 58.2% (n = 78) of patients in the OCR, ATZ and NTZ cohorts, respectively, did not achieve indicated number of annual doses (i.e., had fewer than 3 infusion visits for OCR, <5 infusions for ATZ and <12–13 for NTZ in a year) and were excluded from the overall cohort.
The primary cohort comprised 123 patients in the OCR cohort, 18 patients in the ATZ cohort, and 48 patients in the NTZ cohort. The secondary cohort included 39 OCR patients (15.3% of 255 initiators), no ATZ patients, and 8 NTZ patients (6.0% of 134 initiators) who had more infusion visits than the prescribed annual dose in the label. Thus, the overall cohort, combining the primary and secondary cohorts, was 162 OCR patients, 18 ATZ patients, and 56 NTZ patients. The sensitivity analysis population based on excluding the post-index continuous enrollment criterion in the primary cohort included 421 OCR, 56 ATZ, and 62 NTZ patients.
Baseline characteristics in the primary cohort
Primary cohort
The mean age for patients in the primary cohort starting OCR, ATZ, and NTZ was 53.1, 49.9 and 42.5 years, respectively. Overall, there were more female patients than males in each of the index infusion DMT cohorts. Most patients in OCR and NTZ cohorts had commercial insurance coverage while 61% of the ATZ cohort were MAPD enrollees. The average number of relapses for patients across 3 primary infusion DMT cohorts during the baseline period was [mean (SD)]: OCR = 1.4 (0.6); ATZ =2.5 (1.0) and NTZ = 1.1 (0.4). shows the demographics and clinical characteristics of the primary cohort by index infusion DMT.
Table 1. Baseline characteristics: primary cohort.
Primary cohort by commercial and MAPD
MAPD patients in general were older and sicker than were commercial patients. The mean age and percentage of patients at least 65-years-old among MAPD patients was higher. MAPD and commercial patients were similarly distributed across gender and majority of patients were female. Patients with MAPD coverage had higher percentages of co-morbidities compared with commercial enrollees, with hypertension the most common comorbid condition ().
Annual cost of treatment with the index infusion DMT
The real-world mean annual cost of treatment in the primary cohort OCR, ATZ and NTZ cohorts was $72,066, $1,21,053, and $93,777 respectively. The sensitivity analysis for patients who met all criteria for inclusion in the primary cohort except continuous post-index enrollment criteria resulted in slightly higher mean annual real-world treatment costs of $78,422 for OCR (n = 421), $126,470 for ATZ (n = 56), and $96,076 for NTZ (n = 62).
The mean annual costs of treatment in the secondary cohort were $107,439 among those treated with OCR, and $93,988 for those who initiated NTZ. No patients who initiated ATZ were in the secondary cohort; all patients had 5 infusion visits.
The mean annual costs of infusion DMT treatment for the combined overall cohort were $80,582 for OCR, $121,053 for ATZ, and $93,807 for NTZ. Mean annual treatment costs for all cohorts are presented in and Supplementary Figure 1. Subgroup analysis of costs across patient cohorts according to insurance type observed that mean annual costs appeared to be higher in commercially insured patients compared with MAPD patients irrespective of the DMT.
Table 2. Mean annual costs of index infusion DMTs.
Exploration of site of infusion visits
Additionally, as a part of exploratory evaluation, we found that 7.3% of OCR infusion visits and 10.9% of NTZ infusion visits were home-based; no ATZ patients were found to have home infusions. Overall, ≥13% of patients on OCR and NTZ switched infusion sites during the course of first year of treatment (data not presented in a table).
Discussion
To our knowledge, this is the first study to evaluate real-world costs of treatment with infusion DMTs (OCR, ATZ and NTZ) during the first year of treatment. Our study showed that the real-world costs of treatment of these infusion DMTs (OCR = $80,582, ATZ = $121,053, NTZ = $93,807) for the for the patients who had at least prescribed annual dosing per the product label were greater than the treatment cost usually perceived and documented in literature which is most commonly calculated based on wholesale acquisition cost via redbook and other ancillary costs for administration and monitoring via physician fee scheduleCitation16–18. For example, published treatment costs for OCR based on wholesale acquisition cost ($65,000) and administration and monitoring costs per fee schedules ($313.85) in 2017 was $65,313Citation19. In another study (2018), Beckerman et al. estimated 4-year cost of administration ($1,582) and monitoring for OCR ($121) via 2018 physician fee schedule as $1,704, for an annual cost of $426; this amount for administration and monitoring coupled with the 2018 WAC for OCR would yield an annual treatment cost of $65,426Citation6,Citation17. Similarly, the average annual cost of NTZ in 2018 can be approximated as $81,797: drug cost of $80,561 per the WAC plus annual administration and monitoring costs of $1,236 from Beckerman et al.’s 4-year estimate of $4,943Citation6,Citation17. Moreover, treatment cost was calculated for infused DMT by using WAC based on redbook and administration/monitoring cost via physician fee schedule by Institute of Clinical and Economic Review (ICER) for evaluation of cost-effectiveness of DMTs (2017) approved for treatment of MSCitation18. All of these estimates of treatment costs were lower than the real-world treatment costs we observed using claims data in our study over a similar timeframe (2017–2018).
Our study also highlighted that difference between the perceived and real-world cost was evident among commercial population. Along with ancillary costs, we also acknowledge that rebates, discounts and increased mark-up on the DMT may also drive the incremental difference between estimated costs as described above and overall real-world cost.
These incremental costs found in the real-world are a noteworthy percentage of the overall treatment costs for infused DMT costs documented in the literature. Such costs can accumulate to substantial amounts with large patient volume, resulting in significant financial implications for the payers, MS patients and, perhaps, for providers if adequate reimbursement cannot be obtained. The costs reported in our study are direct costs calculated via paid amounts by the payer and patient. We were unable to compute indirect costs including patient and caregiver travel time, and lost work productivity and these costs can also represent a significant cost to patients and society. Therefore, the costs from our analysis should be considered a conservative (lower bound) treatment cost of infusion DMTs to payers and patients. Future research should examine and include these additional societal costs and burden.
Real-world costs were higher in the secondary cohort of patients with more visits than prescribed annual dosing in OCR and NTZ cohort. It was not clear from the data why OCR and NTZ patients in the secondary cohort had more than 3 or 13 infusion visits, respectively. Some patients in the OCR cohort may have needed more frequent dosing. Based on preliminary exploration of our data, 8.3% of 255 OCR patients had fewer than <20 weeks between the second loading and subsequent dose found on further evaluation. Additional infusion visits may also have been a function of scheduling logistics or physician acquisition of the drug for later administration rather than a visit during which the DMT was infused. Nevertheless, the additional dates in the secondary cohort did result in higher real-world costs.
Among MS patients who met the study inclusion criteria until the required dosing for the primary cohort, 36.5% of patients who initiated OCR and 58.2% of patients who initiated NTZ were did not achieve indicated number of infusion visits for a year as prescribed in the product label. This might be attributed to some OCR patients with more advanced MS given the older mean age in the OCR cohort or with primary progressive MS who might have higher discontinuation rates or due to tolerability issues (e.g., infusion site reaction/adverse events)Citation20. It is also possible that patients prescribed NTZ were not adherent to a regular 4-week dosing schedule, or that their physicians administered NTZ less frequently in extended off-label dosing intervals.
The cost of infusion treatment and its components (with micro-costing) has been well studied and quantified in other therapeutic areas including oncology and in patients with rheumatoid arthritis (RA). In oncology, one published review of the costs of cancer suggests that costs other than cancer drug costs, such as IV administration procedures, other oral and IV drugs, evaluation and management, laboratory services, and radiology, account for 41–43% of total costs, whereas a study in lung cancer reports these costs to be >40%Citation21,Citation22. For patients who received an IV biologic agent to treat RA, IV administration costs and other services accounted for 9% of the total cost of the treatmentCitation23. Similarly, another study by Schmier et al. in 2017 showed that personnel, supplies, and overhead costs also contributed substantially to overall costs (8–16%) of biologic therapies for RACitation24.
Similar to other therapeutic areas, we found that drug, administration, and related services that make up total infusion DMT treatment costs result in substantial burden to the payer and patient in MS. Furthermore, a recent complementary analysis in MS patients using IBM Marketscanii commercial data has corroborated our findings for commercial enrolleesCitation25.
Currently, there are 18 approved MS DMTs, which include injectable, oral pills and infused therapies. Therefore, a true understanding of the real-world comprehensive average costs for infusion DMTs, including drug, infusion administration, and related services, is critically important to assess cost-effectiveness of these DMTs. Adherence, patient preference, and formulary placement play vital roles in selection of DMTs as newer DMT options are available with different routes of administration and dosing schedules. In addition to selecting an MS DMT based on efficacy, safety and life-style considerations, quantifying the real-world cost of DMTs in patients with MS provides clinicians and payers with additional information helpful to assess value and guide treatment and formulary decision.
Limitations
Claims data have inherent limitations, including potential misclassification of diagnoses and procedures. Another key limitation of claims data is that they lack clinical measures of disease progression and severity (e.g., Expanded Disability Status Scale score, magnetic resonance imaging findings, time from initial MS diagnosis). And, as noted above, we cannot measure indirect costs, such a productivity loss, from claims data, although indirect costs are not borne by payers. Specific to this study, we were unable to consistently separate the treatment costs on infusion visit dates into components of drug, administration, and related services because costs are often bundled into one or a few aggregate cost amounts on medical claims.
We captured infusion DMT treatment costs incurred on the dates of infusion visits, which might lead to underestimation as some of the costs (e.g., pre-testing or extended monitoring, etc.) might be incurred few days prior or following the infusion visit date. Conversely, patients may have received services on the dates of MS infusion visits that were unrelated to the index infusion DMT and these services would result in overestimates of costs.
The sample identified from the Optum Research Database was small for the primary cohort. To mitigate the impact of the small sample size in the primary sample, we performed sensitivity analysis with more relaxed criteria for continuous enrolment, which showed us similar results. Furthermore, the results from the current analysis may not be generalizable to patients who are not commercially insured or MAPD enrollees, for example, individuals enrolled in Medicaid, fee-for-service Medicare, Veterans Affairs, or who are uninsured. And, these results cannot be generalized outside of the United States.
Our study objective was to capture real-world costs during the first year of infusion DMT treatment. We would expect these mean annual costs to recur in the OCR and NTZ cohorts as long as patients continued treatment. ATZ, however, is expected to be administered for three consecutive days at the beginning of the second year of treatment, after which treatment should be completed, although treatment in subsequent years may be needed for some patients. The cumulative annual cost of treatment for patients who do not need more than two courses of ATZ infusion might decrease over time.
Conclusions
Despite limitations, this study provides an account of true real-world costs of treatment for MS patients who initiated infusion DMTs. Real-world costs of treatment with infusion therapies, including, drug, administration, and related services, should be consistently and preferably used to help evaluate cost-effectiveness and guide treatment and coverage decisions.
Transparency
Declaration of funding
This study was supported by Novartis Pharmaceutical Corporation.
Declaration of financial/other interests
JN works at the OhioHealth Multiple Sclerosis Center, in Columbus, Ohio and is a consultant for Novartis Pharmaceuticals Corporation, Alexion, Biogen Idec, Genzyme, Genentech, Celgene and the Multiple Sclerosis Association of America. JN has research grants from Novartis Pharmaceuticals Corporation, Biogen Idec, and Genzyme. RH, JP, and ML are employees of Optum Life Sciences, which was contracted by Novartis to conduct the analysis documented in this manuscript. MZ and CD are employees of Novartis Pharmaceuticals Corporation.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed that they have previously directed research on the impact of multiple sclerosis for EMD Serono. The reviewers have no other relevant financial relationships or otherwise to disclose.
Author contributions
CD and RH were responsible for conceptualization and overall development of the study. JN and MZ were key advisors closely involved in the development of protocol and contextualization of the results and findings. RH, JP, and ML were responsible for execution and analysis of the study.
Supplemental Material Figure 1
Download MS Power Point (615.8 KB)Acknowledgements
The authors acknowledge Sirish Cholasamudram (Novartis Healthcare Private Limited) for providing support for writing the manuscript.
Notes
Notes
i Eden Prairie, MN, USA.
ii NY, USA.
References
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517.
- National Multiple Sclerosis Society [Internet]. New York (NY): NMSS. Heath policy fact sheet #2: financial burdens for people with MS, their families, and society; 2019 [cited 2019 October 10]. Available from: https://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Documents/Health-Policy-Fact-Sheet-2-Costs.pdf
- IQVIA Institute for Human Data Science [Internet]. Medicines use and spending in the U.S. A review of 2016 and outlook to 2021; 2017 [cited 2019 October 10]. Available from: https://www.iqvia.com/institute/reports/medicines-use-and-spending-in-the-us-areview-of-2016
- Milliman [Internet]. Report commissioned by Biogen. Multiple sclerosis: new perspectives on the patient journey; 2016 [cited 2019 October 10]. Available from: http://us.milliman.com/uploadedFiles/insight/2016/multiple-sclerosis newperspectives.pdf.
- Optum Rx [Internet]. Six cost drivers of multiple sclerosis; 2019 [cited 2019 October 20]. Available from: https://www.optum.com/resources/library/ms-cost-drivers.html.
- Analysource [Internet]. AnalySource online: premier access to the first Databank drug pricing database. 2019 [cited 2019 January 15]. Available from: https://www.analysource.com
- Centers for Medicare and Medicaid Services [Internet]. Woodlawn (MD): CMS. Physician fee schedule; 2020 [cited 2020 April 06]. Available from: https://www.cms.gov/apps/physician-fee-schedule/
- Ocrevus sample coding [Internet]. Multiple sclerosis; 2019 [cited 2019 October 20]. Available from: https://www.genentechaccess.com/content/dam/gene/accesssolutions/pdfs/coding/OCERVUS-Billing-Coding-for-ALL.pdf
- Ocrevus® highlights of prescribing information [Internet]. 2019 [cited 2019 October 20]. Available from: https://www.gene.com/download/pdf/ocrevus_prescribing.pdf
- Lemtrada® prescribing information, including boxed warning. [cited 2019. October 20]. Available from: https://www.lemtradahcp.com/dosing
- Tysabri® highlights of prescribing information [Internet]. 2019 [cited 2019 October 2019]. Available from: https://www.tysabrihcp.com/content/dam/commercial/tysabri/hcp/en_us/pdf/tysabri_prescribing_information.pdf
- Ollendorf DA, Jilinskaia E, Oleen-Burkey M. Clinical and economic impact of glatiramer acetate versus beta interferon therapy among patients with multiple sclerosis in a managed care population. JMCP. 2002;8(6):469–476.
- Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ. 2010;13(4):618–625.
- Marrie RA, Patten SB, Tremlett H, et al. Sex differences in comorbidity at diagnosis of multiple sclerosis. A population-based study. Neurology. 2016;86(14):1279–1286.
- Edwards NC, Munsell M, Menzin J, et al. Comorbidity in US patients with multiple sclerosis. PROM. 2018;9:97–102.
- O’Day K, Meyer K, Miller RM, et al. Cost-effectiveness of natalizumab versus fingolimod for the treatment of relapsing multiple sclerosis. J Med Econ. 2011;14(5):617–627.
- Beckerman R, Aragão F, Duff S, et al. Four-year resource use and costs associated with administration and monitoring of disease modifying drugs in patients with relapsing multiple sclerosis. Paper presented at: International Society for Pharmocoeconomics and Outcomes Research Europe (ISPOR EU); 2018 November 10–14; Barcelona, Spain.
- Institute for Clinical and Economic Review [Internet]. Boston (MA): ICER. Disease-modifying therapies for relapsing-remitting and primary progressive multiple sclerosis: effectiveness and value. Final Evidence Report; 2017 [cited 2019 October 20]. Available from: https://icer-review.org/wpcontent/uploads/2016/08/CTAF_MS_Final_Report_030617.pdf
- Yang H, Duchesneau E, Foster R, et al. Cost-effectiveness analysis of ocrelizumab versus subcutaneous interferon beta-1a for the treatment of relapsing multiple sclerosis. J Med Econ. 2017;20(10):1056–1065.
- Alimohamadi S, Vollmer B, Nair K, et al. Ocrelizumab safety and effectiveness in the one year treatment of multiple sclerosis compared to other disease modifying therapies. Neurology. 2019;92(15):P3.2.
- Kruse GB, Amonkar MM, Smith G, et al. Analysis of costs associated with administration of intravenous single-drug therapies in metastatic breast cancer in a U.S. population. JMCP. 2008;14(9):844–857.
- Skarin AT, Duh MS, Weiner JR, et al. Costs associated with intravenous (IV) chemotherapy administration in patients with lung cancer. JCO. 2007;25(18):18092–18092.
- Wong BJ, Cifaldi MA, Roy S, et al. Analysis of drug and administrative costs allowed by U.S. private and public third-party payers for 3 intravenous biologic agents for rheumatoid arthritis. JMCP. 2011;17(4):313–320.
- Schmier J, Ogden K, Nickman N, et al. Costs of providing infusion therapy for rheumatoid arthritis in a hospital-based infusion center setting. Clin Ther. 2017;39(8):1600–1617.
- Dieguez G, Engel T, Jacobson N. Site of service and cost dispersion of infused drugs. Seattle (WA): Milliman; 2019. (Milliman white paper).