Abstract
Aims: To describe the real-world economic burden of patients with anaplastic lymphoma kinase-positive (ALK+) non-small cell lung cancer (NSCLC) treated with post-crizotinib, second-line ALK inhibitor therapy.
Materials and methods: Retrospective analysis using data from US Optum: Clinformatics Data Mart administrative claims database. Adult patients with ALK + NSCLC treated with ceritinib or alectinib as second-line ALK inhibitors between 1 January 2011 and 30 September 2017 were included. Healthcare costs and resource utilization for up to 1 year of therapy were calculated on a per-patient-per-month (PPPM) basis and stratified by presence or absence of brain metastases (BM). Multivariate regression analysis was performed to identify factors associated with costs. Top ten cost drivers of non-inpatient procedure costs were recorded.
Results: One hundred and twelve patients received second-line ALK inhibitors. Total mean PPPM healthcare costs were $23,984 for all patients receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy. Total mean PPPM costs for patients with BM on or prior to post-crizotinib, second-line ALK inhibitor therapy were 1.37-times as high as those for patients without BM (p = 0.0406). Mean PPPM outpatient visits and inpatient hospitalization stays were higher for patients with BM versus no BM. The main cost drivers for non-inpatient procedures were radiation therapy, medications, and diagnostic radiology.
Limitations: Analyses did not include newer ALK-directed therapies. BM development after the index date (defined as the date of the first claim for a second-line ALK inhibitor) may have been misclassified as non-BM. Findings may not be generalizable to patients with no health insurance coverage.
Conclusions: Treatment of patients with ALK + NSCLC with ceritinib or alectinib as post-crizotinib, second-line ALK inhibitor therapy represents a high economic burden. Healthcare costs and resource utilization were significantly higher for patients with ALK + NSCLC with BM versus no BM.
Introduction
Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancer casesCitation1. Among these, ∼3–7% of NSCLC cases are anaplastic lymphoma kinase-positive (ALK+) and can benefit from targeted ALK inhibitor treatmentCitation2–4.
In 2011, crizotinib was approved as the first ALK inhibitor in the first-line setting for patients with ALK + NSCLC by the United States (US) Food and Drug Administration (FDA). Although crizotinib is associated with favorable treatment outcomes compared to cytotoxic chemotherapy in untreated and previously treated patients with ALK + NSCLCCitation5,Citation6, crizotinib-treated patients eventually acquire resistanceCitation7. Subsequent studies have shown superiority of next-generation ALK inhibitors with better brain penetrance compared to crizotinibCitation8–14.
Patients with ALK + NSCLC usually present with advanced disease, with the brain as one of the most common sites of metastasis. Brain metastases (BM) occur in 15–35% of patients with ALK + NSCLC at initial presentation, increasing to 60% over the course of first-line crizotinib therapy due to its modest intracranial activityCitation7,Citation15–18. BM are associated with high clinical burden, manifesting as physical and psychological dysfunctionCitation19. BM are a major cause of mortality, with 24-month overall survival rates of 14.7% reported in the Surveillance, Epidemiology, and End Results database for patients treated for NSCLC with BM between 2010 and 2013Citation20. Disease management for patients with ALK + NSCLC with BM requires radiotherapy (either whole-brain radiotherapy or stereotactic radiosurgery), surgical resection (for isolated metastases or cases with a good prognosis), and/or chemotherapy, as well as post-treatment rehabilitation due to deterioration of neurocognitive function and neurotoxicityCitation17,Citation18,Citation21. These treatments and associated imaging procedures and pharmacy utilization result in high healthcare costs that may be avoided or reduced by prevention or early treatment of central nervous system (CNS) progression among patients with ALK + NSCLCCitation22–24.
Several more potent next-generation ALK inhibitors are available for use after crizotinib failure, including ceritinib, alectinib, and brigatinib, which were approved by the FDA in April 2014, December 2015, and April 2017, respectivelyCitation25. Still, clinical outcomes with second-line ceritinib and alectinib are suboptimal. Median systemic progression-free survival is <1 year, and therapy is not effective across all clinically relevant ALK mutationsCitation25,Citation26. Ceritinib and alectinib have improved intracranial activity compared to crizotinib; however, the intracranial duration of response was <1 year in patients with crizotinib-resistant ALK + NSCLC treated with ceritinib or alectinib, and BM remained the main site of progression among patients with BM at baselineCitation25,Citation26. Real-world evidence suggests that disease progression is the leading cause of discontinuation of second-line alectinib and ceritinib in patients who were previously treated with crizotinib. A retrospective chart review of patients with ALK + NSCLC who had progressed on crizotinib as their first ALK inhibitor revealed that 41.4% of alectinib discontinuations were due to disease progressionCitation27. In a French study of crizotinib-pretreated patients with ALK + NSCLC and temporary authorization for use of ceritinib, 58.1% of ceritinib discontinuations were secondary to disease progressionCitation28.
The increasing availability of structurally diverse next-generation ALK inhibitors has created the need to generate real-world data describing the healthcare costs associated with these therapies. These data will compliment clinical development programs in guiding treatments for patients with ALK + NSCLC, and will emphasize the cost-savings that can be provided by novel therapies, particularly those that delay development of BM in early lines of therapy. Although previous studies have described the substantial economic impact of post-crizotinib monotherapy and second-line ceritinib treatment for patients with stable and metastatic ALK + NSCLC, further research is needed to understand healthcare costs associated with progression on second-line ALK inhibitors, particularly in the setting of CNS metastasesCitation16,Citation23,Citation24. The objectives of the present study were to: (1) describe the real-world economic burden of patients with ALK + NSCLC treated with post-crizotinib, second-line ALK inhibitor therapy; (2) evaluate the economic impact of BM in these patients; and (3) identify predictors of cost and cost drivers.
Methods
Data source and study design
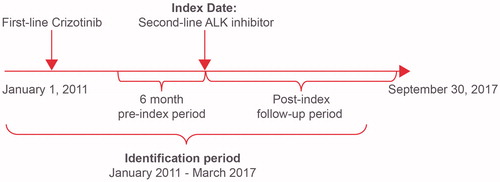
This retrospective observational study used the Optum: Clinformatics Data Mart, a US healthcare claims database that captures patient demographic characteristics, medical history, hospitalization utilization, laboratory payments, and pharmacy claims. Adult patients with NSCLC treated with post-crizotinib, second-line ALK inhibitor therapy (ceritinib or alectinib) between January 2011 and March 2017 were identified. The date of the first claim for a second-line ALK inhibitor was defined as the index date. Patients were observed for ≥6 months, and up to 12 months, of follow-up after the index date, until September 2017 ().
Inclusion criteria were: (1) ≥1 inpatient claim or ≥2 outpatient claims ≥30 days but ≤1 year apart with a lung cancer diagnosis code (International Classification of Diseases, Ninth Revision [ICD-9] codes: 162.2x, 162.3x, 162.4x, 162.5x, 162.8x, 162.9x; ICD-10 C34.0x, C34.1x, C34.2, C34.3x, C34.8x, C34.9x); (2) ≥2 claims for an ALK inhibitor; and (3) patients received crizotinib as first-line therapy and ceritinib or alectinib as post-crizotinib, second-line ALK inhibitors. Patients treated with first-line ceritinib or alectinib, patients treated with second-line crizotinib, other chemotherapy, immunotherapy, or radiation therapy, and patients with no observable second-line treatments were therefore excluded from the study.
Post-crizotinib, second-line ALK inhibitor was defined as a change from crizotinib to another ALK inhibitor (ceritinib or alectinib; second-line crizotinib was not included) between January 2011 and March 2017. BM were identified if the patient had a BM diagnosis (ICD-9: 198.3x, 198.4x; ICD-10: C79.31xx, C79.32xx, C79.4xxx; Note: codes for “other CNS” are included) on or prior to the index date.
Study measures
Patient baseline demographics and clinical characteristics were recorded, including age, gender, region, insurance type, Charlson Comorbidity Index (CCI), BM (on or prior to first- or second-line ALK inhibitor treatment), and death.
Study outcomes were healthcare costs and healthcare resource utilization. Total healthcare costs included pharmacy costs (ALK inhibitor costs and other pharmacy costs) and medical costs. Medical costs comprised inpatient admission, emergency care services, outpatient visits, office visits, professional services costs, and other costs (such as hospice care and nursing home care). Healthcare resource utilization included the number of office visits, outpatient visits, emergency department visits, professional service visits, inpatient hospitalization stays, and length of hospital stay.
Data analysis
Statistical analysis was conducted using SAS EG 7.11. Descriptive analyses of the demographic and clinical characteristics (age, gender, region, insurance, CCI, mortality, BM) of the included patients were performed; continuous measures are summarized as means and standard deviations (SD)/median and interquartile range, and categorical measures are summarized as counts and percentages.
Healthcare costs and healthcare resource utilization for patients receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy were calculated on a per-patient-per-month (PPPM) basis and stratified according to the presence or absence of BM. Healthcare costs represented reimbursed amounts from payers to healthcare providers and were adjusted to 2017 US Dollars using the medical component of the Consumer Price Index. Healthcare resource utilization was summarized as the number of visits.
Factors associated with PPPM costs in patients receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy were investigated in multivariate regression by fitting generalized linear models with gamma distribution and a log link adjusted for patient baseline characteristics, including region (Midwest vs. West, Northeast vs. West, South vs. West), gender (female vs. male), CCI (1 vs. 0, ≥2 vs. 0), BM on or prior to index date (Yes vs. No), insurance (commercial vs. Medicare), and age (continuous).
The total non-inpatient procedure costs for patients receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy were estimated and stratified according to the presence or absence of BM. The top ten cost drivers were then identified. Inpatient procedure codes were not listed, as the majority were missing.
Student t-tests were performed for continuous variables and chi-square tests for categorical variables; p <0.05 was considered statistically significant.
Results
Study population
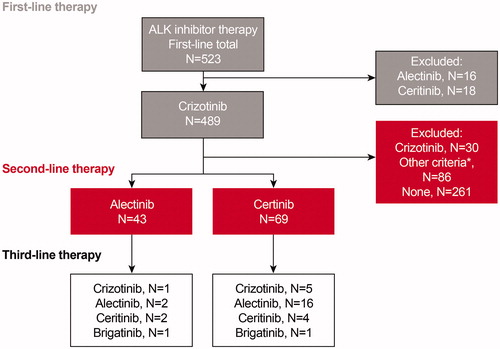
Of the 489 patients with ALK + NSCLC who received crizotinib as first-line therapy, 112 patients were treated with either alectinib (n = 43) or ceritinib (n = 69) as a second-line ALK inhibitor. Among patients receiving alectinib in the second-line setting, six (14%) patients were subsequently treated with crizotinib, alectinib, ceritinib, or brigatinib. Among patients receiving ceritinib as a second-line agent, 26 (38%) patients were subsequently treated with crizotinib, alectinib, ceritinib, or brigatinib ().
Figure 2. Attrition. If patient received ALK inhibitors and other National Comprehensive Cancer Network (NCCN)-recommended therapies post first-/second-line ALK inhibitor, then the ALK inhibitor was considered as second-/third-line therapy. *Other criteria were: (1) the period between the last date on ALK inhibitor treatment and the end of follow-up was <60 days; and (2) no other NCCN recommended therapies were used between the last date on ALK inhibitor treatment and data cut-off date. Abbreviation. ALK, anaplastic lymphoma kinase.
Demographic and clinical characteristics of all included patients are summarized in . Patients had a mean (±SD) age of 59.5 (±12.2) years, and 48.2% were male. Most patients lived in the South (36.6%), followed by the West (26.8%), Midwest (22.3%), and Northeast (14.3%). The majority of patients had a CCI ≤1 (75.9%); 23.2% of patients died during the study period.
Table 1. Baseline patient characteristics.
In total, 26.8% of patients had BM on or prior to first-line ALK inhibitor therapy (crizotinib), and 52.7% of patients had BM on or prior to second-line ALK inhibitor therapy (ceritinib or alectinib). Patients with BM had a mean (±SD) age of 56.0 (±11.2) years, and patients with no BM had a mean age of 63.3 (±12.3) years. Patients with BM were more likely to have commercial insurance than patients with no BM (78.0% vs. 52.8%).
Economic burden in patients with ALK + NSCLC treated with second-line ALK inhibitors
Total mean PPPM healthcare costs were $23,984 for all patients receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy, $26,415 for patients with BM, and $21,278 for patients with no BM. Mean PPPM inpatient costs ($8,953 vs. $3,644; p = 0.05), pharmacy costs ($10,531 vs. $8,896; p = 0.046), and outpatient costs ($4,861 vs. $2,103; p = 0.038) were significantly higher for patients with BM compared to no BM. Approximately 60% of healthcare expenditures for patients with or without BM were medical costs ().
Figure 3. Economic burden associated with use of post-crizotinib, second-line ALK inhibitor therapy. (a) PPPM healthcare costs (mean, 2016 USD). Patients with BM vs. no BM: inpatient costs p = 0.05; pharmacy p = 0.046; outpatient costs p = 0.038; *Other costs include all not listed miscellaneous services, such as hospice, nursing home, etc. (b) PPPM healthcare utilization (mean). Patients with BM vs. no BM: inpatient hospital days p = 0.05; inpatient hospital admission times p = 0.019. Abbreviations. BM, brain metastases; PPPM, per-patient-per-month; USD, United States Dollars.
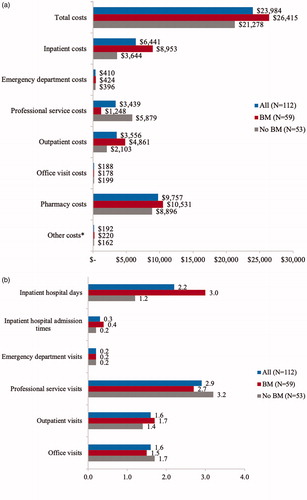
Mean PPPM office visits and outpatient visits were each 1.6, professional service visits were 2.9, emergency room department visits were 0.2, and inpatient hospital admission times were 0.3 for all patients receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy. Mean PPPM outpatient visits (1.7 vs. 1.4) and inpatient hospital admission times (0.4 vs. 0.2) were higher for patients with BM compared to no BM, while office visits (1.5 vs. 1.7) and professional service visits (2.7 vs. 3.2) were higher for patients with no BM, possibly because patients with no BM were older. Mean PPPM inpatient hospitalization days were 2.2 for all patients receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy, and 3.0 and 1.2 for patients with and without BM, respectively ().
In multivariate analysis controlling for patient characteristics, including age, gender, CCI, BM on or prior to index date, region, and insurance, total mean PPPM costs for patients with CCI ≥2 were 59.0% higher than for those with CCI = 0 (p = 0.0175), and total mean PPPM costs for patients with BM on or prior to post-crizotinib, second-line ALK inhibitor therapy were 1.37-times as high as those for patients without BM (p = 0.0406) (). Sensitivity analyses conducted to assess inclusion of death, development of BM in the follow-up period, and second-line therapy showed these covariates did not impact the PPPM cost analysis.
Table 2. Adjusted PPPM cost analysis.
The total non-inpatient procedure costs for patients receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy were $2,34,485 per patient, $2,57,445 for patients with BM, and $2,08,925 for patients with no BM. The main cost drivers were radiation therapy (12.1%), medications (11.6%), and radiographic procedure (10.2%). For patients with BM, the main cost drivers for non-inpatient procedures were radiation therapy (16.9%), medications (11.2%), and radiographic procedure (12.5%). In patients with no BM, the main cost drivers for non-inpatient procedures were medications (11.8%), diagnostic radiology (7.1%), and radiation therapy (6.0%). Notably, these data are underestimations, as 27.4% of non-inpatient procedure costs had missing procedure information ().
Table 3. Top ten non-inpatient procedure cost drivers in patients receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy.
Among the non-inpatient procedure costs, radiation therapy costs were 3.5-times higher in patients with BM compared to no BM. These therapies include radium/radioisotope therapy, radiation treatment aids, stereotactic radiosurgery (multisource), and stereotactic body radiation therapy. In addition, the cost of radiographic procedures was 2.2-times higher in the patients with BM compared to no BM. The costs for medication procedures, including chemotherapy treatments such as pemetrexed and anti-programmed death-ligand 1, accounted for a similar percentage of total non-inpatient procedure costs for patients with and without BM.
Discussion
This study described the real-world economic burden of patients with ALK + NSCLC receiving up to 1 year of ceritinib or alectinib as post-crizotinib, second-line ALK inhibitor therapy, and evaluated the economic impact of BM in these patients. Findings showed that treatment of patients with ALK + NSCLC with ceritinib or alectinib as post-crizotinib, second-line ALK inhibitor therapy represents a high economic burden. Total PPPM costs were associated with CCI scores of ≥2 and the presence of BM on or prior to post-crizotinib, second-line ALK inhibitor therapy. In patients with BM, total PPPM costs were 1.37-times higher compared to patients with no BM, representing an increase of ∼40%. In all patients and those stratified according to the presence or absence of BM, over half of the healthcare expenditures were due to medical costs. Medical costs associated with inpatient and outpatient services accounted for ∼60% of healthcare expenditures. Radiotherapy, radiographic procedures, and medication were the main non-inpatient procedure cost drivers among patients with BM. The total PPPM drug co-pay amount for all patients included in the study was $221; there were no significant differences in drug co-pay for patients with BM compared to those with no BM ($197 vs. $248). Taken together, data from this study suggest that clinical benefit in patients with ALK + NSCLC may be derived from novel treatments that delay disease progression in early lines of therapy, and agents that prevent or impede the development of BM may be particularly advantageous.
These findings are consistent with previous real-world studies. A retrospective analysis that used two large US commercial claims databases (January 2006–December 2015) to investigate healthcare resource utilization and costs among 164 patients with ALK + NSCLC receiving ceritinib in second or later line of therapy reported a mean total healthcare cost per patient per 6 months of $111,468. Medical costs accounted for 44.3% of the total healthcare costs, which were driven by outpatient costs (mainly office visit costs) and inpatient costsCitation23. A retrospective study of three large US claims databases (Source Healthcare Analytics’ Source Lx, IMS LifeLink Health Plan Claims, and Truven Health Analytics MarketScan; August 2011–June 2013) that investigated costs of BM in patients with ALK + NSCLC without consideration for line of treatment, showed monthly costs per patient were significantly higher after a diagnosis of BM ($22,645 vs. $5,983), compared to pre-diagnosisCitation16. Costs were driven by pharmacy (42.0%), inpatient (29.6%), and outpatient expenditures (26.0%)Citation16. In a retrospective database analysis using two large US claims databases (PharMetrics Plus and MarketScan; January 2008–March 2016) to estimate the real-world economic burden of BM in newly diagnosed patients with ALK + NSCLC who received alectinib or crizotinib as first-line treatment, the mean total unadjusted PPPM cost of patients with BM was $29,497 vs. $22,791 without BM. Similar to the present study, in which the main cost drivers for non-inpatient procedures in patients with BM were radiation therapy, medications, and other diagnostic radiology, the 29.4% higher total unadjusted PPPM costs in patients with BM were driven by inpatient and outpatient costs, especially costs for radiation therapy and radiology imagingCitation22.
Real-world evidence and data from clinical trials show that patients with ALK + NSCLC discontinue ceritinib or alectinib use in later lines of therapy because of disease progression and/or toxicity, potentially resulting in further healthcare resource utilization and costs. One retrospective study that used two large US commercial claims databases of patients with ALK + NSCLC, most of whom were treated with crizotinib before initiation of ceritinib, showed that 37.2% of patients discontinued ceritinib over a mean observation period of 7.4 monthsCitation23. In the French temporary authorization for use program, among 193 patients with ALK + NSCLC receiving crizotinib as the last regimen before ceritinib, the median duration of ceritinib treatment was 3.9 monthsCitation28. In the present study, among patients with ALK + NSCLC receiving ceritinib or alectinib as a post-crizotinib, second-line ALK inhibitor therapy, median time to discontinuation of ceritinib or alectinib was 5.7 months. As time to discontinuation may serve as a proxy measure for treatment effectiveness, the treatment duration implies these therapies are suboptimal, while adding considerable economic burdenCitation29,Citation30.
Taken together, data from this study highlight the unmet need for more effective early lines of treatment for patients with ALK + NSCLC. Therapies that have better tolerability and progression profiles should result in lower discontinuation rates, as patients are more likely to persist with their medication, resulting in improved patient outcomesCitation31. Our study and others showed that up to 30% of ALK + NSCLC patients already have a diagnosis of BM on or before the start of ALK inhibitor therapyCitation16. Early treatment of symptomatic and asymptomatic BM using targeted agents with CNS activity may prolong the period before radiotherapeutic interventions or impairment of cerebral symptoms ensues due to the development of BM. Considering the costs and resource use involved in radiotherapy treatment, as well as the availability of efficacious and tolerable ALK inhibitors with intracranial activity, use of ALK inhibitor therapy in the first-line may delay or prevent CNS progression. Improved management of BM in patients with ALK + NSCLC may offer patients a longer duration of relatively greater health-related quality-of-life and reduce the economic burden of BM treatmentCitation19.
Limitations
This study was associated with several limitations. First, there were constraints inherent to any retrospective study and claims-based analysis, such as the possibility of inaccurate recording of patients’ diagnoses and limited outcomes data. Second, due to the study period, the study population included only patients treated with crizotinib as first-line therapy and ceritinib or alectinib as second-line therapies according to the prevailing treatment guidelines for ALK + NSCLC. During the study period, treatment paradigms changed as a result of clinical development, approval of next-generation ALK inhibitors, and the need to optimize sequence of treatments, which may be dependent on specific resistance mechanisms and the presence of BMCitation32–36. Third, patients who developed BM after the index date may have been misclassified to the non-BM group; this misclassification may attenuate the cost burden of BM. Fourth, the study is limited by the 1-year follow-up period; therefore, costs across the full trajectory of disease could not be ascertained. In particular, costs for patients who develop BM beyond 1 year of initiating second-line therapy may be postponed. Fifth, investigation of treatment side-effects for patients with and without BM was not performed due to limitations associated with incomplete inpatient claims data. Last, the study population consisted of a relatively large and racially diverse population of patients with commercial healthcare coverage, such that the findings may not be generalizable to patients with other or no health insurance coverage. Economic analyses of prospective randomized trials, such as J-ALEX, ALEX and ALTA-1L, with a focus on patients who developed BM versus those who did not, could provide a better estimate of costs associated with treatment of BM among patients with ALK + NSCLCCitation9–11.
Conclusions
In this real-word study, healthcare costs were high for patients with ALK + NSCLC receiving ceritinib or alectinib as post-crizotinib, second-line ALK inhibitors. Total PPPM costs for patients with BM receiving up to 1 year of post-crizotinib, second-line ALK inhibitor therapy were 1.37-times as high as those for patients without BM. The presence of BM increases overall treatment costs, particularly those for radiation therapy in patients receiving second-line ALK-inhibitors.
Transparency
Declaration of funding
This study was funded by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Declaration of financial/other interests
HML, YW, HH, and KR are employees of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and may own stock. XP and PH were employees of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, at time of study design, completion, and manuscript development, and may own stock. MJ has received consulting and research funding from Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, Roche/Genentech, Novartis, and Pfizer.
A peer reviewer on this manuscript has disclosed that they have previously received funding from Millennium Pharmaceutical, though not in this disease area. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose
Previous presentations
Huamao M Lin, Xiaoyun Pan, Peijie Hou, Hui Huang, Avinash Desai, and Mohammad Jahanzeb. Economic Burden of Brain Metastases in Patients with Anaplastic Lymphoma Kinase Positive (ALK+) Non-Small Cell Lung Cancer (NSCLC) Receiving Second-Line ALK Inhibitors. Presented at ASCO 2018; 28–29 September, Phoenix, AZ.
Acknowledgements
Medical writing support by SNELL Medical Communication Inc., with funding provided by Millennium Pharmaceuticals, Inc.
References
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res. 2016;5(3):288–300.
- Gainor JF, Varghese AM, Ou SHI, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non–small cell lung cancer. Clin Cancer Res. 2013; 19(15):4273–4282.
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115(8):1723–1733.
- Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–4283.
- Shaw AT, Kim D-W, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394.
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177.
- Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27(Suppl 3):iii42–iii50.
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. JCO. 2017;35(22):2490–2498.
- Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell Lung cancer. N Engl J Med. 2018;379(21):2027–2039.
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838.
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29–39.
- Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global phase III ALEX study. J Thorac Oncol. 2019;14(7):1233–1243.
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. JCO. 2016;34(7):661–668.
- Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in adult patients with ALK-rearranged non-small cell lung cancer, previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017;18(7):874–886.
- Guérin A, Sasane M, Wakelee H, et al. Treatment, overall survival, and costs in patients with ALK-positive non-small-cell lung cancer after crizotinib monotherapy. Curr Med Res Opin. 2015a;31(8):1587–1597.
- Guérin A, Sasane M, Zhang H, et al. Brain metastases in patients with ALK + non-small cell lung cancer: clinical symptoms, treatment patterns and economic burden. J Med Econ. 2015b;18(4):312–322.
- Preusser M, Winkler F, Valiente M, et al. Recent advances in the biology and treatment of brain metastases of non-small cell lung cancer: summary of a multidisciplinary roundtable discussion. ESMO Open. 2018;3(1):e000262.
- Owen S, Souhami L. The management of brain metastases in non-small cell lung cancer. Front Oncol. 2014;4:248.
- Peters S, Bexelius C, Munk V, et al. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev. 2016;45:139–162.
- Bates JE, Milano MT. Prognostic significance of sites of extrathoracic metastasis in patients with non-small cell lung cancer. J Thorac Dis. 2017;9(7):1903–1910.
- Saito H, Nakano T, Aoyama H. Clues to improve the cost-effectiveness of radiotherapy for brain metastases from non-small cell lung cancer: cost reduction, patient selection, and better understanding of neurocognitive deterioration. Ann Palliat Med. 2019;8(2):199–202.
- Burudpakdee C, Wong W, Seetasith A, et al. Economic impact of preventing brain metastases with alectinib in ALK-positive non-small cell lung cancer. Lung Cancer. 2018;119:103–111.
- Dalal AA, Guerin A, Mutebi A, et al. Treatment patterns, clinical and economic outcomes of patients with anaplastic lymphoma kinase-positive non-small cell lung cancer receiving ceritinib: a retrospective observational claims analysis. J Drug Assess. 2018;7(1):21–27.
- Girard N, Cozzone D, de Leotoing L, et al. Extra cost of brain metastases (BM) in patients with non-squamous non-small cell lung cancer (NSCLC): a French national hospital database analysis. ESMO Open. 2018;3(6):e000414.
- Thai AA, Solomon BJ. Treatment of ALK-positive nonsmall cell lung cancer: recent advances. Curr Opin Oncol. 2018;30(2):84–91.
- Remon J, Besse B. Brain metastases in oncogene-addicted non-small cell lung cancer patients: incidence and treatment. Front Oncol. 2018;11(8):88.
- DiBonaventura MD, Wong W, Shah-Manek B, et al. Real-world usage and clinical outcomes of alectinib among post-crizotinib progression anaplastic lymphoma kinase positive non-small-cell lung cancer patients in the USA. OTT. 2017;11:75–82.
- Cadranel J, Cortot AB, Lena H, et al. Real-life experience of ceritinib in crizotinib-pretreated ALK + advanced non-small cell lung cancer patients. ERJ Open Res. 2018;4(1):00058–2017.
- Lai EC-C, Chang C-H, Yang Y-HK, et al. Effectiveness of sulpiride in adult patients with schizophrenia. Schizophr Bull. 2013;39(3):673–683.
- Lieberman JA, Stroup S, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223.
- Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59(1):56–66.
- ESMO Guidelines Committee. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Updated version published September 18, 2019.
- NCCN Clinical Practice Guidelines in Oncology. Non-small cell lung cancer. Version 7 August 30, 2019.
- McCusker MG, Russo A, Scilla KA, et al. How I treat ALK-positive non-small cell lung cancer. ESMO Open. 2019;4(Suppl 2):e000524.
- Pirker R, Filipits M. From crizotinib to lorlatinib: continuous improvement in precision treatment of ALK-positive non-small cell lung cancer. ESMO Open. 2019;4(5):e000548.
- Ricciuti B, De Giglio A, Mecca C, et al. Precision medicine against ALK-positive non-small cell lung cancer: beyond crizotinib. Med Oncol. 2018;35(5):72.