Abstract
Aims: Cold agglutinin disease (CAD) is a rare subtype of autoimmune hemolytic anemia associated with increased thromboembolism risk and early mortality. Healthcare resource utilization (HRU) in CAD has not been reported. We aimed to compare HRU of patients with CAD with a matched non-CAD cohort in the United States.
Materials and methods: Patients with CAD were identified from 2006 to 2016 in the Optum-Humedica database using CAD terms in clinical notes and hematologist review. Patients were required to have Integrated Delivery Network records and ≥6 months’ follow-up before and after the first CAD mention date (index date). Patients with CAD were matched to a non-CAD cohort based on demographics. Multivariate analyses assessed inpatient hospitalizations, outpatient visits, emergency room visits, and transfusion use between cohorts 6 months before and 12 months after the index date.
Results: Of 814 patients with CAD, 410 met inclusion criteria and were matched to 3,390 patients without CAD. Mean age of patients with CAD was 68.0 years; approximately 62% were female. In the 12 months after the index date, mean inpatient hospitalizations (0.83 vs. 0.25), outpatient visits (17.26 vs. 6.77), emergency room visits (0.55 vs. 0.32), and transfusion days (1.05 vs. 0.05) were higher for patients with CAD than the matched non-CAD cohort (all p < .0001). Similarly, in the 6 months before the index date, patients with CAD had higher HRU than matched patients without CAD for all measures evaluated.
Limitations: Results of this study are based on patient information from the Optum-Humedica database, which is limited to commercially insured patients and may not represent the overall CAD population.
Conclusions: CAD places a substantial burden on patients and healthcare systems. In addition, the high HRU for patients with CAD observed in the 6 months before diagnosis indicates that disease awareness and better diagnostic practices may be needed.
Introduction
Cold agglutinin disease (CAD) is a rare chronic subtype of autoimmune hemolytic anemia with an estimated prevalence of 16 cases per 1 million individualsCitation1. CAD is mediated by complement-fixing autoantibodies, which bind to the I antigen on the surface of red blood cellsCitation2. Termed cold agglutinins, these monoclonal immunoglobulin M antibodies are most pathogenic when their thermal amplitude (the highest temperature at which they bind to red blood cell antigens) overlaps with vascular temperatures at the lower limit of normalCitation3. When bound to red blood cells, cold agglutinins activate complement component 1 (C1 complex) and trigger the classical complement pathway, resulting in extravascular hemolysis and, to a lesser extent, intravascular hemolysisCitation2,Citation4,Citation5. CAD typically affects middle-aged and elderly individuals and is slightly more frequent among females than malesCitation4. Clinical manifestations include anemia and debilitating fatigue as well as cold-induced circulatory symptoms such as Raynaud’s phenomenon and acrocyanosisCitation4,Citation6. CAD may be primary, in which it is considered a clonal lymphoproliferative disorder, or it may be secondary to an underlying disease (recently termed cold agglutinin syndrome in the literature), such as aggressive lymphoma, the Epstein–Barr virus infection, or Mycoplasma pneumoniae infectionCitation5,Citation6.
There are currently no treatments for CAD approved by the US Food and Drug Administration or the European Medicines Agency. Supportive treatment for CAD includes cold avoidance with transfusion support for acute hemolytic crisisCitation6. Corticosteroids have been used to treat CAD but are not currently recommended owing to low response rates and unacceptably high doses requiredCitation6. Off-label rituximab has been used as monotherapy with limited results. Combination therapy with rituximab and chemotherapy agents (i.e. fludarabine or bendamustine) has been used more recently with better results; however, this regimen is associated with higher toxicity including severe neutropeniaCitation4,Citation6.
CAD is associated with serious life-threatening consequences. Retrospective analyses revealed an increased risk of thromboembolism inpatients with CAD compared with a matched non-CAD cohortCitation7,Citation8, and a population-based study demonstrated an increased risk of early death in patients with CAD compared with a general population cohortCitation9.
Owing to the rarity of the condition and lack of specific diagnostic codes for CAD, treatment patterns and healthcare resource utilization (HRU) in patients with CAD have not been well characterized. One analysis of patients with CAD at Stanford University Medical Center reported that two-thirds of patients had a severe anemia event in the first 6 months after their initial therapy, and patients used a median of 3.5 therapies during the course of their diseaseCitation10. Two-thirds of patients with CAD had at least one hospitalization and 100% of patients used outpatient services within the first year after CAD onset with a median of 58.1 outpatient visits. No studies have compared HRU of patients with CAD with that of individuals without this condition. The objective of this study was to further delineate the burden of disease by comparing HRU of patients with CAD with that of a matched cohort of patients without CAD.
Methods
Data source
Patients were retrospectively identified from January 2006 to June 2016 using the Optum-Humedica database, a commercial data repository containing de-identified information on medications, laboratory results, diagnoses, procedures, and clinical notes for more than 56 million privately insured people from all 50 states.
Study population
The methods used to identify patients with CAD in the Optum-Humedica database have been previously describedCitation8. The clinical notes of each patient were electronically searched for terms associated with CAD such as “cold agglutinin disease,” “cold agglutinin hemoglobinuria,” and “cold autoimmune hemolytic anemia.” To be considered for enrollment, patients were required to have a CAD-related term in their clinical notes on at least one occasion. Patients were considered to have a true case of CAD if they had CAD-related terms in their clinical notes on at least three separate dates. This requirement limited the unintended inclusion of “rule-out” diagnoses and ensured that only patients with physician-diagnosed CAD were identified. The validity of this search method was tested by gathering a random sample of 100 of the identified records and having two independent hematologists manually review physician notes to determine whether each patient had CAD. Agreement was 95%, and thus the three-mention criterion was deemed accurate. Clinical notes of patients with CAD terms mentioned on one or two occasions were reviewed by two hematologists and included in the analysis only after agreement by both physicians. The index date for each patient with CAD was defined as the first date of CAD mention in the patient’s clinical notes. Patients were required to be enrolled in Optum for at least 6 months before their CAD index date to ensure only incident cases were included in the cohort.
Patients with CAD were required to be in the Integrated Delivery Network to ensure complete records for HRU. Additionally, patients were required to have at least 6 months of follow-up in the Optum-Humedica database before and after their index date. A Charlson Comorbidity Index (CCI) score was calculated using the method defined in Quan et al.Citation11. Identified patients were then matched with up to 10 patients without CAD from 5% random samples of the general population available in Optum. Matching criteria included sex, age (±3 years), year of entry into the Optum healthcare plan, race (white vs. nonwhite), region of residence, CCI score category, and follow-up time (matched patients without CAD were required to have the same or longer follow-up time as patients with CAD, or at least 24 months of follow-up). For patients in the non-CAD cohort, the index date was the same as for the patient with CAD to whom they were matched.
Outcomes
Inpatient hospitalizations, outpatient visits, and emergency room visits were evaluated for patients with CAD and the matched non-CAD cohort and reported as the percentage of the cohort using the resource during two time periods (6 months before the index date and 12 months after and including the index date), mean number of visits, 75th percentile number of visits, and mean length of stay for inpatient hospitalizations. If patients with CAD had <12 months of follow-up after their index date, HRU was annualized by dividing the utilization by the entire follow-up time and multiplying by 12. Red blood cell transfusions were evaluated as transfusion days; multiple transfusion records in 1 day were de-duplicated.
Statistical analyses
Baseline demographic information, including age, sex, race, and region of residence, was summarized with descriptive statistics. Multivariate analyses adjusted for continuous CCI scores were used to evaluate HRU. Conditional regression models were used to calculate a p value for the differences in the percentage of patients with CAD and the matched non-CAD cohort using each HRU measure. The p value for the differences between cohorts in the mean number of HRU visits or transfusion days was calculated using mixed Poisson models. Mixed linear models were used to calculate the p value for the differences between patients with CAD and the matched non-CAD cohort in the mean length of stay for hospitalizations.
No codes were available to explicitly differentiate between patients with primary and secondary CAD. To evaluate HRU specifically in patients presumed to have primary CAD, a sensitivity analysis was conducted by excluding patients with CAD who also had coexisting malignant conditions (i.e. B-cell lymphomas and Waldenström macroglobulinemia), as well as specific infections (i.e. the Epstein–Barr virus, cytomegalovirus, and Mycoplasma pneumoniae). The International Classification of Diseases codes used to identify these patients are presented in Supplemental Table 1. Patients without CAD who were matched to any excluded patients with CAD were also removed from these analyses.
Results
Of the nearly 56 million patients in the Optum-Humedica electronic medical records database, 814 patients with CAD were identified between 2006 and 2016, which equates to a prevalence of approximately 14.5 patients per 1 million individuals. Of these, 251 (31%) patients were excluded for not meeting the inclusion criterion of ≥6 months of follow-up time either before or after the index date. A further 153 (19%) patients were excluded for not having records in the Integrated Delivery Network. A final total of 410 patients with CAD were included in the HRU evaluation (). Patients with CAD were matched to 3,390 patients without CAD in the database at a mean ratio of 1:8.3 (median [range] number of matched patients per patient with CAD: 9 [1–10]). Demographics and baseline characteristics were similar between the CAD cohort and the matched non-CAD cohort (). The median age of patients in the CAD cohort was 71.5 years (range: 7–88 years) and approximately 62% were female. Most patients with CAD were white (87%) and nearly half were from the Midwest (46%). The mean time in the Optum-Humedica database was approximately 89 months for both cohorts. Mean follow-up times were similar between cohorts (). As part of the CCI assessment, we compared the incidences of 17 comorbid health disorders for patients with and without CAD. Of these disorders, 12 had similar incidences between cohorts (<3% difference in incidence). The remaining five disorders were as follows: cancer (CAD: 46.6% vs. non-CAD: 24.8%), diabetes without complications (24.9% vs. 37.9%), mild liver disease (16.8% vs. 12.2%), connective tissue disease–rheumatic disease (13.9% vs. 6.9%), and diabetes with complications (9.3% vs. 13.9%).
Figure 1. Patient selection diagram. Abbreviations. CAD, Cold agglutinin disease; EMR, Electronic medical record.
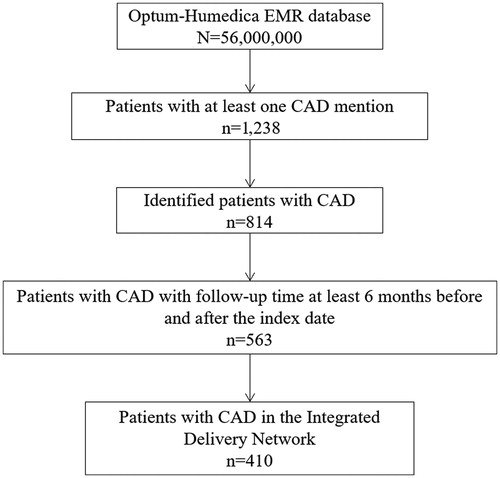
Table 1. Demographics and clinical characteristics of the CAD and matched non-CAD cohorts.
In the 12 months after the index date, the matched analysis showed that patients with CAD continued to have significantly higher HRU than the matched non-CAD cohort (), with more inpatient admissions (mean: 0.83 vs. 0.25; p < .0001), outpatient visits (mean: 17.26 vs. 6.77; p < .0001), and transfusions (mean number of transfusion days per patient: 1.05 vs. 0.05; p < .0001).
Table 2. Annualized healthcare resource utilization of CAD and matched non-CAD cohorts within 12 months after the index date.
In the 6 months before the index date, matched analyses revealed that patients with CAD had significantly higher HRU than the matched non-CAD cohort (), with more inpatient admissions (mean: 0.37 vs. 0.10; p < .0001), twice as many outpatient visits (mean: 6.40 vs. 2.92; p < .001), more frequent use of emergency room services (17% vs. 9%; p < .001), and more transfusions (mean number of transfusion days per patient: 0.35 vs. 0.01; p < .001).
Table 3. Healthcare resource utilization of the CAD and matched non-CAD cohorts within 6 months before the index date.
To attempt to evaluate HRU specifically among patients with primary CAD, sensitivity analyses were conducted excluding patients who had additional diagnostic codes known to be associated with cold agglutinin syndrome such as lymphoma (Supplemental Table 1). After excluding patients with these conditions, 272 patients with presumed primary CAD and 2,239 matched patients without CAD remained. HRU and transfusion patterns were similar between patients with primary CAD and the entire CAD cohort during the 6 months before and 12 months after the index date and were all statistically significantly higher than those of the matched non-CAD cohort (Supplemental Tables 2 and 3).
Discussion
In this study, we evaluated HRU in a large cohort of patients with CAD and a matched non-CAD cohort. We found that patients with CAD experienced significantly more HRU (including inpatient hospitalizations, outpatient visits, emergency room visits, and transfusion days) than those without CAD, both before and after their index date.
HRU of patients with CAD in the current study was generally lower than HRU reported for patients with the same disease who received treatment at the Stanford University Medical CenterCitation10. Within the first year after diagnosis, the percentages of patients treated at Stanford with at least one inpatient hospitalization, outpatient visit, and emergency room visit were 67%, 100%, and 53%, respectivelyCitation10. Corresponding values for the Optum CAD cohort were 36%, 95%, and 26%, respectively. The differences in HRU between these studies may reflect variations in the healthcare systems from which patients were selected. The Optum-Humedica database represents commercially insured patients in an Integrated Delivery Network system covering more than 600 hospitals and 6,500 clinics nationwide, whereas the Stanford University Medical Center is an academic teaching hospital. Recent studies have shown that academic teaching hospitals have higher costs and HRU than community nonteaching centersCitation12–14.
High HRU has also been reported for patients with paroxysmal nocturnal hemoglobinuria (PNH), another rare complement-driven hemolytic anemia. Based on a prospective study of patients enrolled in the International PNH Registry, Schrezenmeier et al. reported that 23% of patients with this disease were hospitalized over a 6-month period (corresponding to an annualized rate of 45% of patients)Citation15. The authors considered this degree of HRU to be indicative of a poor quality of life. In the current study, the percentage of patients hospitalized before and after index date was similar to that reported for PNH. Using the International PNH Registry, Muus et al. later showed that HRU decreased for emergency room visits after approval of eculizumab, a complement inhibitor, for the treatment of PNHCitation16.
In the current study, the high HRU observed after the index date may reflect limitations associated with current CAD therapeutic approaches given the dearth of approved treatments. To verify that the increase in HRU was not caused by comorbid conditions such as diabetes or heart disease, patients with and without CAD were matched based on CCI scores. There was a higher incidence for cancer in patients with CAD than in the non-CAD cohort, which may reflect the contribution of specific types of cancer to secondary cold agglutinin syndrome. Accordingly, we conducted sensitivity analyses that excluded patients with underlying conditions that can be associated with CAD, such as specific infections and B-cell lymphoma. The persistence of increased HRU across all parameters (inpatient, outpatient, emergency room, transfusion days) in the CAD cohort after making these adjustments indicates that our findings cannot be solely owing to the presence of underlying comorbidities or other related disorders. Annualizing HRU for patients with less than 1 year of follow-up may introduce bias. To reduce the potential for this, we removed patients from the cohort who had less than 6 months of follow-up. In the current data set, 59 (14.4%) patients with CAD and 334 (9.9%) patients without CAD had follow-up times between 6 months and 1 year. The percentages of patients with annualized data were small and similar between cohorts.
Although commonly used to inform on disease burden for rare disorders (including other complement-driven hemolytic conditions such as PNH and atypical hemolytic uremic syndrome)Citation17–21, retrospective analyses have limitations. The current study may be subject to selection bias because it only included commercially insured patients from the Optum-Humedica database and may not accurately reflect the full spectrum of patients with CAD. Additionally, laboratory data were limited and inconsistent; therefore, we were not able to include it in our analyses. Despite these limitations, this large, real-world clinical-claims database provided sufficient power to conduct a comparison HRU study in a rare condition in which the ability to obtain prospective data with sufficient patient numbers is challenging.
Conclusions
The results of this study demonstrate that patients with CAD have high HRU before and after index date, placing a substantial burden on patients and healthcare systems, and suggest that improved disease awareness and diagnostic practices may be needed.
Transparency
Declaration of financial/other relationships
JS, JMA, and NJ are employees of Sanofi. LCB, XJ, and RJN were employees of EpidStrategies at the time of the analysis. EpidStrategies received funding from Sanofi for this research and from Amgen Inc., Merck, Genentech, Humacyte, Sanofi, and AstraZeneca for other research.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed that they have previously been a consultant/advisory board member for Sanofi related to atopic dermatitis. The reviewers have no other relevant financial relationships or otherwise to disclose.
Previous presentations
Su J, Bylsma LC, Jiang X, et al. Healthcare resource utilization among commercially insured cold agglutinin disease patients in the United States. Poster session presented at: International Society for Pharmacoeconomics and Outcomes Research Annual Meeting; 2019 May 18–22; New Orleans, LA, USA.
Supplemental Material
Download MS Word (45.4 KB)Acknowledgements
The authors thank Dr. Jon Fryzek for his critical review of the manuscript and Dr. Jon Fryzek and Dr. Adam Rosenthal for their assistance in the development of the CAD patient algorithm for the Optum-Humedica database. The authors also thank all the participants in the Optum-Humedica database. Editorial assistance for the development of this paper was provided by JK Associates Inc., a member of the Fishawack Group of Companies, and was funded by Sanofi.
Data availability
Qualified researchers may request access to patient level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient level data will be anonymized, and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/
Additional information
Funding
References
- Berentsen S, Ulvestad E, Langholm R, et al. Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91(4):460–466.
- Berentsen S. Complement activation and inhibition in autoimmune hemolytic anemia: focus on cold agglutinin disease. Semin Hematol. 2018;55(3):141–149.
- Rosse WF, Adams JP. The variability of hemolysis in the cold agglutinin syndrome. Blood. 1980;56(3):409–416.
- Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood. 2013;122(7):1114–1121.
- Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):226–231.
- Berentsen S. How I manage patients with cold agglutinin disease. Br J Haematol. 2018;181(3):320–330.
- Kamesaki T, Nishimura J, Wada H, et al. Clinical characteristics, treatment patterns, and thromboembolic risk of patients with cold agglutinin disease (CAD) in Japan. HemaSphere. 2019;3(Suppl. 1):169.
- Broome CM, Cunningham JM, Mullins M, et al. Increased risk of thrombotic events in cold agglutinin disease: a 10-year retrospective analysis. Res Pract Thromb Haemost. 2020. DOI:10.1002/rth2.12333
- Bylsma LC, Ording AG, Frøslev T, et al. The occurrence and survival of cold agglutinin disease in Denmark. HemaSphere. 2018;2(Suppl. 1):513.
- Mullins M, Jiang X, Bylsma LC, et al. Cold agglutinin disease burden: a longitudinal analysis of anemia, medications, transfusions, and health care utilization. Blood Adv. 2017;1(13):839–848.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139.
- Caveney AF, Silbergleit R, Frederiksen S, et al. Resource utilization and outcome at a university versus a community teaching hospital in tPA treated stroke patients: a retrospective cohort study. BMC Health Serv Res. 2010;10(1):44.
- Reed SD, Blough DK, Meyer K, et al. Inpatient costs, length of stay, and mortality for cerebrovascular events in community hospitals. Neurology. 2001;57(2):305–314.
- Burke L, Khullar D, Orav EJ, et al. Do academic medical centers disproportionately benefit the sickest patients? Health Aff (Millwood). 2018;37(6):864–872.
- Schrezenmeier H, Muus P, Socié G, et al. Baseline characteristics and disease burden in patients in the International Paroxysmal Nocturnal Hemoglobinuria Registry. Haematologica. 2014;99(5):922–929.
- Muus P, Langemeijer S, Hochsmann B, et al. Patient-reported outcomes and healthcare resource utilization before and during treatment with eculizumab: results from the International Paroxysmal Nocturnal Hemoglobinuria Registry. Poster session presented at: European Hematology Association 22nd Annual Congress; 2017 Jun 22–25; Madrid, Spain.
- Peffault De Latour R, Huynh L, Ivanova JI, et al. A retrospective chart review to assess burden of illness among patients with severe aplastic anemia with insufficient response to immunosuppressive therapy. Blood. 2017;130(Suppl. 1):678.
- Thayer S, Bell C, McDonald CM. The direct cost of managing a rare disease: assessing medical and pharmacy costs associated with Duchenne muscular dystrophy in the United States. J Manag Care Spec Pharm. 2017;23(6):633–641.
- Placzek H, Xu Y, Mu Y, et al. Clinical and economic burden of commercially insured patients with acromegaly in the United States: a retrospective analysis. J Manag Care Spec Pharm. 2015;21(12):1106–1112.
- Cohen B, Balcells C, Hotchkiss B, et al. A retrospective analysis of health care utilization for patients with mitochondrial disease in the United States: 2008–2015. Orphanet J Rare Dis. 2018;13(1):210.
- Belk KW, Craver CW. Hospital-based utilization in patients with atypical hemolytic uremic syndrome. Value Health. 2014;17(3):A61.
