Abstract
Background: Evidence on the cost and risk of infection-related hospitalizations associated with targeted disease-modifying anti-rheumatic drugs (tDMARDs) in patients with RA previously treated with a tumor necrosis factor inhibitor (TNFi) is limited. This study compared the risk and cost of infection-related hospitalizations in commercially insured TNFi-experienced RA patients receiving abatacept, TNFi, or another non-TNFi.
Methods: A retrospective observational study was conducted using 2 large insurance claims databases (1 January 2009–30 June 2017). Adult TNFi-experienced RA patients initiating a subsequent tDMARD (initiation date of tDMARD = index date) with 12 months of continuous enrollment pre-index date, and who had ≥1 inpatient or ≥2 outpatient medical RA claims on 2 different dates were included. Abatacept was compared to TNFis (adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab) and other non-TNFis (tocilizumab, rituximab, and tofacitinib). Cox proportional hazards models estimated the adjusted risk for infection-related hospitalization; costs were calculated on a per-member-per-month (PMPM) and per-patient-per-month (PPPM) basis using generalized linear models.
Results: More patients in the abatacept cohort had an infection-related hospitalization at baseline (4.5%) vs TNFis (2.0%, p < .0001) and other non-TNFis (3.6%, p = .2619). However, during follow-up abatacept patients had fewer infection-related hospitalizations (abatacept: 2.8%, TNFi: 3.7% and other non-TNFis: 5.2%; p < .05). Regression results indicated that compared to patients on abatacept, patients receiving a TNFi [HR: 1.6 (95% CI: 1.1, 2.2)] and other non-TNFis [HR: 1.9 (95% CI: 1.3, 2.8)] had a significantly higher risk of infection-related hospitalization. Abatacept PMPM costs were lowest ($0.25 vs $0.39 and $0.43 for TNFi and other non-TNFi respectively). Mean PPPM (95% CI) cost in the follow-up was lower for abatacept compared to TNFi ($73 vs. $115; p = .042), and other non-TNFi ($73 vs. $125; p = .039).
Conclusions: There were significantly lower infection-related hospitalizations and associated costs in TNF-experienced RA patients treated with abatacept than TNFis and other non-TNFis.
Introduction
Rheumatoid arthritis (RA) is one of the most common autoimmune diseases, affecting nearly 1.3 million people in the U.SCitation1. RA is characterized by chronic inflammation of the joints that can ultimately lead to cartilage and bone destructionCitation2. Though not directly life-threatening, RA severely impacts patients’ quality of life and imparts a major economic burden on healthcare systems and societyCitation3. Mean annual healthcare costs in patients with RA have been noted to be more than double those of patients without RA ($7,197 vs. $20,919; p < .0001)Citation4. One US study estimated that the total annual RA costs for privately insured and Medicare populations in 2010 were $306 million and $600 million, respectively. Overall, RA contributes an estimated $19.3 billion in direct and indirect costs in the US annuallyCitation5.
The treatment of choice in RA is a group of medications known as disease-modifying antirheumatic drugs (DMARDs). Initial treatment of active RA is typically a conventional DMARD (cDMARD) such as methotrexate, sulfasalazine, or leflunamideCitation6,Citation7. Patients who are intolerant or show an inadequate respodnse to cDMARDs are often treated with biological original and biosimilar DMARDs (boDMARDs and bsDMARDs such as adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab, tocilizumab, abatacept and rituximab), or targeted synthetic DMARDs (tsDMARDs such as tofacitinib and baricitib). We collectively summarized the boDMARDs, bsDMARDs and tsDMARDs as targeted DMARDs (tDMARDs) throughout the manuscript. tDMARDs vary by the mechanisms of action such as tumor necrosis factor inhibitors (TNFis: adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab), anti-interleukin-6 agents (tocilizumab), anti-CD20 agents (rituximab), T-cell costimulation modulators (abatacept), and Janus kinase (JAK) inhibitors (tofacitinib)Citation8.
The immunomodulatory effects of RA, immunosuppressive treatments, and immunocompromising comorbidities all contribute to an increased risk of infection in patients with RACitation9. RA patients are twice as likely to have an increased risk for infection-related hospitalizationsCitation9,Citation10. Rates of infection, particularly for sepsis and pneumonia, have increased over the years, compared to patients without RACitation9. In addition, there is a significant economic burden associated with sepsis-and pneumonia-related hospitalizations (2013 USD: $23.6 billion and $9.5 billion, respectively) in the USCitation11.
Selection of therapy may impact infection-related hospitalization risk. A recent analysis of claims data for a predominately commercially insured population found that patients who received abatacept had a lower risk for infection-related hospitalization compared to patients receiving a TNFi (HR 0.78, 95% CI 0.64–0.95)Citation12. An analysis conducted in Medicare patients found there were differences among the risk for hospitalized patients based on the biologic received after switching therapy (HR versus abatacept: etanercept 1.24, 95% CI 1.07–1.45; infliximab 1.39, 95% CI 1.21–1.60; rituximab 1.36, 95% CI 1.21–1.53)Citation13.
While the rates and risk of infections have been studied in the RA population, there is little information about the economic impact of infection-related hospitalizations. Understanding the differences in infection-related hospitalization risk and associated hospitalization costs across available tDMARD classes is helpful for informing treatment decisions. The purpose of this study was to compare the risk and cost of infection-related hospitalizations in TNFi-experienced RA patients subsequently receiving tDMARDs in the U.S.
Methods
Data sources and study design
A retrospective, observational study was conducted with 2 nationally representative insurance claims databases (MarketScan and PharMetrics; 1 January 2009–30 June 2017). Analyses were conducted in both datasets individually and in aggregate. De-identified analytical datasets were created by applying cohort inclusion/exclusion criteria on individual databases. These individual de-identified databases (MarketScan and PharMetrics) were then combined using only common variables to create a pooled analysis dataset.
The MarketScan database includes claims from employer-sponsored health insurance, with more than 42.6 million covered lives in the most recent year of data. The database is large enough to account for a nationally representative sample for the U.SCitation14. Similarly, the PharMetrics database offers claims information on 130 million de-identified lives from >100 commercial health plans in the U.SCitation15. Both databases provide claim level information on diagnoses, procedures, resource utilization (physician visits, ER visits, hospitalization, etc.), pharmacy claims, and associated costs and chargesCitation14,Citation15. Each of these databases included data from hundreds of health plans/self-insured employers, with very limited overlapping at health plan level. There is very low possibility for results to be driven by 1 or 2 plans with unique patient population or treatment pattern.
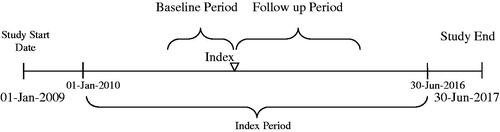
The index period was from 01 January 2010 through 30 June 2016. The date of first usage of a different tDMARD therapy after TNFi exposure in the baseline period was considered the index date. The tDMARD received on the index date was considered the index drug. The 12-months period (fixed) immediately preceding the index date was defined as the baseline period, and was used to identify RA diagnoses, assess prior history of infections, determine baseline characteristics, and assess tDMARD exposure. The follow-up period was at least 12 months ending at the earliest of one of the following endpoints: (1) end of patient insurance enrollment; (2) end of the overall study period; or (3) end of continuous index treatment date. A variable follow-up period was used to minimize the potential impact of patient disenrollment on study outcomes while maximizing the size of the study sample for data analysis ().
The discontinuation of the index drug treatment was defined as having a gap of 2 times of the days’ supply of previous tDMARD prescription. Gap was defined as the number of days between the end of last index drug claim and the next index drug prescription claim date, the end of patient insurance enrollment, or end of study period, whichever was the earliest. A gap of less than 30 days did not qualify as a discontinuation. For patients who discontinued their index drug treatment, the end of the continuous index treatment date was the end of last continuous prescription drug coverage (prescription claim date + drug days of supply). The days of supply for injectable drugs was not always well populated in the database. When the drug days of supply was missing in the database, it was imputed by the dosing frequency described in the corresponding drug Food and Drug Administration (FDA) package inserts. For patients who discontinued DMARD, an additional 90 days after the end of index drug usage was used to capture infectious events that might occur shortly after medication discontinuation (which might occur because of early symptoms of an infection).
Inclusion and exclusion criteria
Patients were required to meet all of the following criteria to be selected for study inclusion:
Patients initiating any of the index drug therapies of interest during the index period were eligible. All patients were required to have evidence of previous TNFi exposure during the baseline period. This TNFi was required to be different from the index therapy
≥1 inpatient claim or ≥2 outpatient medical claims on 2 different dates with an ICD-9-CM/ICD-10-CM diagnosis code for RA during the baseline period prior to the index date
18 years or older at index date
Continuous enrollment in the database for at least 12 months prior to the index date
At least 12 months of continuous enrollment after the index date
Patients with any of the following criteria were excluded from the study population:
Medical or pharmacy claim for more than 1 type of tDMARD on the index date
Diagnosis of other conditions including Crohn’s disease, ulcerative colitis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, chronic lymphocytic leukemia, juvenile idiopathic arthritis, Wegener’s granulomatosis, non-Hodgkin’s lymphoma, or polyarteritis nodosa, at any time during the baseline period
Patients with any cancer diagnosis at any time during the baseline or follow-up periods, since these patients have unusual infection rates and profiles
Prescription data validity issues (e.g, invalid or missing strength, quantity, daily dose, or days’ supply that could not be easily imputed from available information) from pharmacy claims
Based on the study inclusion and exclusion criteria specified, patients who qualified for the study were grouped into the following treatment cohorts: abatacept, any TNFi (adalimumab, certolizumab pegol, etanercept, golimumab, and infliximab), or other non-TNFi drugs (tocilizumab, rituximab, and tofacitinib). Although a granular comparison at the mechanism of action (MOA) level would have been ideal for abatacept, JAK inhibitor, IL-6 inhibitor, IL-1 inhibitor, and B-cell depleting agents, we were unable to do a MOA level comparison due to sample size constraints. It is important to note that considerable variation exists in the literature on the nomenclature used for tDMARDs.
Baseline measures
Patient demographics and characteristics were captured during the baseline period. Variables included age, gender, U.S. geographic region, race, health care plan type, baseline Charlson Comorbidity Index (CCI) score, baseline comorbid conditions (diabetes, renal disease, COPD, neutropenia, infection, hypertension, congestive heart failure, cancer, and liver disease), infection-related hospitalizations (by type as based on a primary or non-primary diagnosis code, including genitourinary, joint, pneumonia, respiratory, sepsis, and skin), and glucocorticoid use.
Outcome measures
The percentage of patients with an infection-related hospitalization at baseline and in the follow-up period was captured, as was the type of infection. Risk of infection-related hospitalization was also calculated. Length of stay for infection-related hospitalizations was also assessed. Direct medical costs for infection-related hospitalizations in the follow-up period were calculated on a PMPM and PPPM basis from a payer perspective. Costs were inflated to 2016 USD using the medical care component of the Consumer Price Index.
Statistical analysis
Outcomes were compared among the patient cohorts by using both descriptive statistics (unadjusted) as well as multivariable regression analyses (adjusted). A Cox proportional hazards model was used to estimate the hazard ratio for the risk of infection-related hospitalization. A two-part zero-inflated generalized linear model (GLM) with gamma distribution and log link function was used to estimate PPPM costs. 95% Confidence intervals for PPPM costs were estimated by bootstrapping with replacement. Key variables used to estimate the adjusted outcomes include the Charlson Comorbidity Index (CCI), payer type, and demographic measurements including age, gender and region, glucocorticoids usage in baseline, comorbidities, baseline infections, and a dummy variable for the dataset (MarketScan vs. PharMetrics). All key variables were controlled for to avoid model misspecification.
Per month cost in all TNFi-experienced patients (PPPM × number of TNFi − experienced RA patients) was divided by the total plan membership to estimate the PMPM. The number of TNFi-experienced RA patients in a hypothetical plan of 1,000,000 members was estimated in several steps. First, the prevalence of RA by age groups and gender was identified via a targeted literature review. Second, this prevalence was age-and gender-adjusted to the 2014 US Census populationCitation16. Next, the prevalence was applied to the 1,000,000 membership to estimate the number of RA cases. Last, of these RA cases, we estimated those who were TNFi-experienced and eligible for subsequent tDMARD treatment from the published literatureCitation17.
Results
Patient selection and baseline characteristics
Risk of infection-related hospitalizations
In the pooled analysis, a higher percentage (4.5%) of patients in the abatacept cohort had an infection-related hospitalization in the baseline period compared to TNFis (2.0%, p < .0001) and other non-TNFis (3.6%, p = .2619), but this trend reversed in the follow-up period (2.8% for abatacept vs 3.7% for TNFi and 5.2% for other non-TNFi; p < .05) ( and ).
Table 1. Baseline Characteristics (Pooled Data).
Table 2. Percentage of patients with infections-related hospitalization by treatment type.
Pneumonia was the most common type of infection in the pooled analysis in the baseline and follow-up period, followed by sepsis. Pneumonia was highest in the abatacept group in the baseline period (2.8% vs. 1.1% and 2.4% for TNFi and other TNFi, respectively), but was lowest in the abatacept group in the follow-up period (1.3% vs 1.6% and 2.7% for TNFi and other TNFi, respectively) (Supplemental Figure 1).
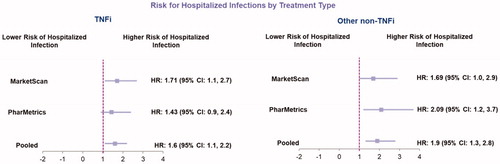
Regression results varied in significance by database and cohorts, but pooled database results indicated a significantly higher risk for infection-related hospitalizations for patients who switched to another TNFi [HR: 1.6 (95% CI: 1.1, 2.2)] or other non-TNFi [HR: 1.9 (95%: 1.3, 2.8)] vs abatacept ().
Hospitalization length of stay
Per-Patient length of stay
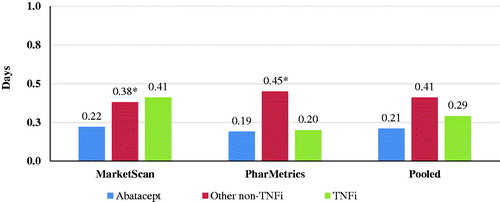
There were 54 hospitalizations in the abatacept cohort, 247 in the TNFi cohort, and 114 in the other non-TNFi cohort in the follow-up period. Average length of stay was numerically lower for abatacept vs TNFi cohorts (0.21 days vs 0.29 days, respectively) and statistically significantly lower for abatacept compared to other non-TNFis (0.21 vs 0.41 days, respectively; p = .0016) ().
Economic outcomes
Per-Patient per month health care costs
Unadjusted baseline inpatient PPPM costs in the pooled analysis were higher for abatacept compared to the TNFi cohort ($87 vs $27, p < .0001). Similarly, the baseline PPPM cost in the abatacept group was numerically higher but not statistically significant versus other non-TNFi ($87 vs $61, p = .2394). Unadjusted inpatient PPPM costs in the follow-up period in the pooled analysis were not significantly different for abatacept vs TNFi ($51 vs $49, p = .1599), but were significantly lower for abatacept vs other non-TNFis ($52 vs $88, p = .0017).
Adjusted mean PPPM (95% CI) inpatient costs in the follow-up period were $73 ($17–$158) for abatacept, $115 ($27–$224) for TNFi, and $125 ($29–$264) for other non-TNFi. Follow-up PPPM inpatient costs was significantly lower for abatacept (difference of $42 versus TNFi and $52 versus other non-TNFi, p < .05) ().
Table 3. Pooled difference in follow-up inpatient costs.
Per member per month health care costs
The age-and gender-adjusted prevalence of rheumatoid arthritis in the US was estimated to be 0.53%Citation16,Citation18. An estimated 2,529 RA patients were TNFi-experienced and eligible to receive a subsequent tDMARD treatment in a plan of 1,000,000 members. Overall, patients who switched to abatacept had lower PMPM costs ($0.18) compared to patients who switched to another TNFi ($0.29) or other non-TNFi ($0.32).
Discussion
Results from this analysis showed that in a commercially insured population of RA patients with prior TNFi treatment, abatacept may lower the risk and cost of infection-related hospitalizations compared to TNFi and other non-TNFi. The abatacept cohort had the lowest frequency of hospitalizations in the follow-up period, as well as a shorter average length of stay per hospitalization. Differences in the risk of hospitalization was driven by the lower incidence of pneumonia, sepsis and genitourinary infections in the abatacept group. Of note, a reduction in the risk of pneumonia-related hospitalization was observed post-initiation of abatacept, whereas the risk increased post-initiation for both TNFi and other non-TNFi groups. The increase in the risk of sepsis and genitourinary infection-related hospitalization post-initiation of tDMARD was lowest in the abatacept group.
Our findings indicate that the choice of tDMARD may not only have clinical consequences for patients but may also reduce the economic burden for payers. A recent 2018 Magellan Rx Medical Pharmacy Trend Report showed a 9% increase in the PMPM healthcare cost from 2016 to 2017 ($1.11 vs $1.21) for patients with RACitation19. Our analysis indicates a PMPM saving of $14–$18 from the use of abatacept due to infection-related hospitalizations alone, which may help payers control the increase in economic burden of RA among plan members. Moreover, this study assumes significance given the projected increase in prevalence of RA from 1.28 million in 2014 to 1.39 million in 2020 due to an aging population in the USCitation18.
To the best of our knowledge, this is the first study to examine the economic consequences associated with infections in patients with RA. Risk estimates from previous real-world studies were consistent with our findings. Chen et al. retrospectively evaluated the risk of infection-related hospitalization in patients with RA who initiated therapy with either abatacept or a TNFi using the Truven MarketScan database (2006–2015)Citation12. Patients initiating abatacept could have used non-biologic DMARDs or a TNFi during the baseline period; TNFi initiators could have used non-biologic DMARDs or abatacept during the baseline. The risk of infection-related hospitalization for patients receiving abatacept was lower compared to patients receiving a TNFi [HR (95% CI): 078 (0.64, 0.95)]Citation12. Yun et al. evaluated Medicare patients (2006–2011) for risk of infection-related hospitalizations among the various agents in patients who had received a previous biologic agentCitation13. Cox regression analysis showed that patients receiving etanercept [HR (95% CI): 1.24, (1.07, 1.45)], infliximab [HR (95% CI): 1.39, (1.21, 1.60)], and rituximab [HR (95% CI): 1.36, (1.21, 1.53)] had significantly higher risk for infection-related hospitalization when compared to abataceptCitation20. Results from both analyses may help to provide support to show that patients who receive abatacept after a previous biologic agent may experience fewer infection-related hospitalizations, regardless of age.
Our study evaluated patients initiating abatacept after receiving prior therapy with a TNFi. Montastruc et al. recently conducted a retrospective study in patients with RA newly-treated with a biologic using the Truven MarketScan and Supplemental US Medicare data from 2007–2014. Results of the study showed that the use of abatacept was not associated with an increased incidence of serious infections overallCitation21. Of note, the study included patients with cancer and other autoimmune diseases, and prevalence of comorbidities was higher in the abatacept group.
Limitations
This study has several limitations that warrant mentioning. As with most real-world studies, unobserved heterogeneity may exist between patients and claims databases. This analysis was conducted only with respect to the 2 therapeutic groups described (TNFis and other non-TNFis). Consequently, the results of this analysis do not permit any conclusions to be drawn between abatacept and any specific products within those groups. The goal our study was to compare the risk and cost of infection-related hospitalizations at the mechanism of action (MOA) level. While we were able to compare abatacept to TNFis at the MOA level, we were unable to compare abatacept to JAK inhibitor, IL-6 inhibitor, IL-1 inhibitor and B-cell depleting agents due to sample size constraints. Therefore, we broadly categorized the latter into the ‘Other non-TNFi’ arm. Differences may persist between various MOAs within the ‘Other non-TNFi’ group. The results of the analysis are only generalizable to RA patients who are covered by commercial insurance or Medicare, with data primarily from large employers. It was not feasible to identify whether the infection-related hospitalization event occurring shortly after the initiation of index tDMARD was related to the prior TNFi or the index treatment using only claims data. However, of note, all patients included in this study were using a TNFi prior to the index date. We would expect that the distribution of such events should be similar between the three tDMARD arms, and therefore, not impact the findings of our study. It should be noted that claims data are secondary data and are not collected for the purposes of research. Furthermore, clinical data on disease severity, progression, or functional status were not available. The presence of a claim for a tDMARD therapy does not indicate that the tDMARD was actually used.
Conclusions
There were significantly lower infection-related hospitalizations and associated costs for TNFi-experienced RA patients who were switched to abatacept compared to patients switched to other tDMARDs. Future research is recommended for Medicare patients given the high prevalence of RA and comorbidities in the elderly population, and understanding physician preferences and prescribing behavior for patients who are at high risk for infections.
Transparency
Declaration of funding
Funding for this study was provided by Bristol-Myers Squibb, Inc.
Declaration of financial/other relationships
Damemarie Paul, Dhaval Patil, Laura McDonald, Vardhaman Patel and Francis Lobo were employees of Bristol-Myers Squibb, Inc. at the time of this study.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Damemarie Paul designed the study, guided the analysis, interpreted the findings and co-developed the manuscript. Dhaval Patil conducted the statistical analysis, and co-developed the manuscript. Laura McDonald and Francis Lobo provided inputs for the study design. Francis Lobo interpreted the findings and co-developed the manuscript. Vardhaman Patel contributed towards the interpretation of results and co-developed the manuscript.
Acknowledgements
Meg Franklin, PharmD, PhD assisted with the writing and editorial support for this manuscript.
Previous presentations
Portions of this analysis were presented at the European Congress of Rheumatology (EULAR) in Madrid, Spain, 12–15, June 2019.
JME-2019-0229-RT_Supplemental_Materials.docx
Download MS Word (54.6 KB)References
- Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25.
- Pruijn GJ, Wiik A, van Venrooij WJ. The use of citrullinated peptides and proteins for the diagnosis of rheumatoid arthritis. Arthritis Res Ther. 2010;12(1):203.
- Brooks PM. The burden of musculoskeletal disease-a global perspective. Clin Rheumatol. 2006;25(6):778–781.
- Chen C-I, Wang L, Wei W, et al. Burden of rheumatoid arthritis among US Medicare population: co-morbidities, health-care resource utilization and costs. Rheumatology Advances in Practice. 2018;2(1):rky005.
- Birnbaum H, Pike C, Kaufman R, et al. Societal cost of rheumatoid arthritis patients in the US. Curr Med Res Opin. 2010;26(1):77–90.
- Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26.
- Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis. 2017;76(6):960–977.
- Stevenson M, Archer R, Tosh J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016;20(35):1–610.
- Jinno S, Lu N, Jafarzadeh SR, et al. Trends in Hospitalizations for Serious Infections in Patients With Rheumatoid Arthritis in the US Between 1993 and 2013. Arthritis Care Res. 2018;70(4):652–658.
- Smitten AL, Choi HK, Hochberg MC, et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol. 2008;35(3):387–393.
- Torio C, (AHRQ), Moore B. (Truven Health Analytics). National Inpatient Hospital Costs: The Most Expensive Conditions by Payer. 2013. HCUP Statistical Brief #204. May 2016. Agency for Healthcare Research and Quality, Rockville, MD; [cited 2020 May 21]. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb204-Most-Expensive-Hospital-Conditions.pdf.
- Chen SK, Liao KP, Liu J, et al. Risk of Hospitalized Infection and Initiation of Abatacept versus TNF Inhibitors among Patients with Rheumatoid Arthritis: a Propensity Score-Matched Cohort Study. Arthritis Care Res. 2018;72(1):9–17.
- Yun H, Xie F, Delzell E, et al. Comparative risk of hospitalized infection associated with biologic agents in rheumatoid arthritis patients enrolled in medicare. Arthritis Rheumatol. 2016;68(1):56–66.
- Truven MarketScan Database. [cited 2017 May 27]. Available from: https://truvenhealth.com/Portals/0/Assets/2017-MarketScan-Databases-Life-Sciences-Researchers-WP.pdf.
- IQVIA Real-world Data Adjucicated Claims (PharMetrics Plus); [cited 2019 Jun 13]. Available from: https://www.iqvia.com/institute/research-support.
- U.S. Census Bureau, Population Division. Annual Estimates of the Resident Population for Selected Age Groups by Sex for the United States, States, Counties, and Puerto Rico Commonwealth and Municipios: April 1, 2010 to July 1, 2014.
- Pawar A, Desai RJ, Solomon DH, et al. Risk of serious infections in tocilizumab versus other biologic drugs in patients with rheumatoid arthritis: a multidatabase cohort study. Ann Rheum Dis. 2019;78(4):456–464.
- Hunter TM, Boytsov NN, Zhang X, et al. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37(9):1551–1557.
- Magellan Rx Management Medical Pharmacy Trend Report. 2018. 9th Edition; [cited 2019 Jun 13]. Available from:https://www1.magellanrx.com/documents/2019/03/medical-pharmacy-trend-report_2018.pdf/.
- Yun H, Xie F, Delzell E, et al. Risk of hospitalised infection in rheumatoid arthritis patients receiving biologics following a previous infection while on treatment with anti-TNF therapy. Ann Rheum Dis. 2015;74(6):1065–1071.
- Montastruc F, Renoux C, Hudson M, et al. Abatacept initiation in rheumatoid arthritis and the risk of serious infection: a population-based cohort study. Semin Arthritis Rheu. 2019;48(6):1053–1058.