Abstract
Background: Stress urinary incontinence (SUI) is a debilitating and highly prevalent condition in the UK. The condition is associated with a significant economic burden for affected patients and society. Current treatment options for SUI include minimally invasive therapies, medication and surgical intervention for the most serious cases. Electrical Muscle Stimulator with Multipath technology is a recently developed device for the treatment of SUI that relies on neuromuscular external electrical stimulation (NEES) technology. The clinical efficacy of the device has been proven in previous studies, but existing evidence surrounding its economic viability is limited.
Objectives: To assess the cost-utility of the Electrical Muscle Stimulator with Multipath technology Therapy device for the treatment of SUI amongst women in a UK setting.
Methods: An economic model was developed to consider the cost-utility (cost per quality-adjusted life-year [QALY] gained) of Electrical Muscle Stimulator with Multipath technology compared with current practice. A Markov model was developed, with costs and health effects estimated over the lifetime of the patient in the base-case analysis. The model was developed to reflect the treatment pathways typically followed by patients with SUI in the UK. Parameter uncertainty was explored in deterministic and probabilistic sensitivity analyses.
Results: Base-case results indicate that Electrical Muscle Stimulator with Multipath technology results in cost savings and QALY gains over the patient’s lifetime. In the “cure” analysis, the intervention is £250 less costly and leads to a 0.03 QALY gain per patient, while in the “improvement analysis”, the intervention is £327 less costly and leads to a 0.13 QALY gain per patient. Results from the probabilistic sensitivity analyses show that the likelihood of Electrical Muscle Stimulator with Multipath technology being cost-effective is greater than 74% across all willingness-to-pay thresholds in the two analyses presented.
Conclusions: Electrical Muscle Stimulator with Multipath technology is a potentially cost-effective treatment option for patients with SUI who have failed first-line treatment. It could reduce costs to the health care service and improve quality-of-life for selected patients over their lifetime.
1. Introduction
Urinary incontinence (UI) is a widespread condition affecting millions of women worldwideCitation1. In the UK alone, the National Health Service (NHS) estimates that between 3 and 6 million people have some degree of UICitation2. It is estimated that approximately half the female population will experience UI at some time in their lives, with women being five times more likely to develop UI than menCitation3. UI not only has a detrimental impact on the quality-of-life of the individual patient, but it also places a significant economic burden on society as a wholeCitation4. One published UK study estimated total expenditure for UI to be upwards of £1 billion (at today’s prices), while a 2004 UK study estimated a total health care cost of £117 million per yearCitation4.
There are various different types of UI, all of which are characterized by the involuntary leakage of urine. The most common forms are as follows: (1) Stress urinary incontinence (“SUI”) is the complaint of involuntary loss of urine on effort or physical exertion including sporting activities or on sneezing or coughing. (2) Urgency urinary incontinence (“UUI”) is involuntary urine leakage accompanied or immediately preceded by urgency (a sudden compelling desire to urinate that is difficult to defer)Citation5. (3) Functional incontinence, which exists when an individual is aware of the need to urinate, but for physical or mental reasons is unable to get to the bathroom. (4) Mixed urinary incontinence (MUI), which is the existence of both SUI and UUI together, and (5) Overflow incontinence is the complaint of UI in the symptomatic presence of an excessively (over-) full bladder. The prevalence of SUI alone steadily increases from 4.5% in 20–24 year olds to a peak prevalence of 18.2% at 45–49 years of age and then gradually falls to plateau at 10.9% from 60 years of age onwardCitation4, while approximately 40% of those patients that have UI are believed to be affected by SUICitation6.
Treatment options for SUI begin with simple measures including lifestyle changes (such as exercise like pilates and dietary adjustments) and pelvic floor muscle training (PFMT) including kegel exercises. At this stage, the patient may also be advised to use incontinence products, such as absorbent pads and handheld urinals. Patients may then try electrical stimulation therapy if first-line treatments are not successful. If this is unsuccessful for the management of symptoms, and the patient has a preference for pharmacological treatment over surgical treatment, or the patient is unsuitable for surgery, the patient may be prescribed a medication such as duloxetine. The last, and commonly employed, line of treatment for SUI is surgical management. However, medication is associated with side effects and is of questionable efficacy and there is considerable uncertainty surrounding the long-term efficacy of several surgical treatmentsCitation4. Data from Hospital episdoes statistics (HES), which show the frequency of different types of hospital admission in the UK, show that between April 2018 and April 2019, there were 18,492 total finished consultant episodes with HRG codes relating to surgical treatments for SUI. This involved 18,042 distinct patients who accrued a total length of stay in England hospitals of 18,490 days – an average of 1 day per episode (NHS digital 2018-19).
INNOVO is a recently developed non-invasive pelvic floor exerciser that treats SUI, rather than managing the symptoms. The device relies on neuromuscular external electrical stimulation (NEES) technology and has been shown to be effective in previous clinical studiesCitation7,Citation8. It provides an alternative option for patients suffering from SUI, particulary for those who have not had success with lifestyle changes and pelvic floor exercises, and who are reluctant to undergo pharmacological or surgical treatment. The purpose of this study is to assess the cost-effectiveness of INNOVO, by developing an economic model to understand the economic viability of introducing this therapy device for the treatment of patients suffering from SUI in the UK.
2. Methods
A de novo economic model was developed in Microsoft Excel to reflect the clinical pathways typically followed by patients with SUI who have failed first-line treatment, i.e. PFMT, kegel exercises etc. The clinical effectiveness of the intervention, INNOVO NEES technology for the treatment of SUI, compared with current pathways of care, was considered in the model. The impact of alternative treatment strategies on the probability of being dry/cured, the likelihood of experiencing treatment-related adverse events and death, and associated NHS resource use was captured in the model. Therefore, costs and outcomes were calculated based on the clinical events experienced by patients across the model pathways.
The model was based on a hypothetical cohort of patients with SUI who have not had success with their first line of treatment, and are seeking further treatment. NICE guidelines indicate that the time horizon of the model should be long enough to fully capture the costs and benefits of the intervention and relevant comparators, and in the base-case analysis a lifetime time horizon was appliedCitation9. The recommended discount rate in the UK (3.5% per annum) was applied for both costs and benefitsCitation9, with discounting applied after the first year of the model. All costs included in the model were considered from the UK NHS and personal social services perspective.
2.1. Model overview
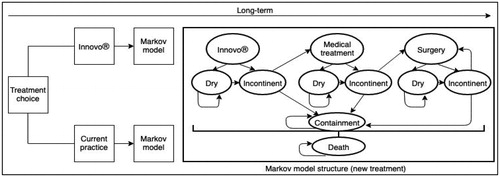
A simplified version of the Markov cohort model that was developed is shown in . Progression over the model time horizon is represented by various health states and clinical probabilities of progressing from one health state to the next. All SUI patients in the model begin at a point where they have failed first-line treatment, are incontinent and have chosen to undergo subsequent treatment, i.e. existing electrical stimulation methods with current practice or INNOVO in the intervention pathway.
In the intervention pathway, for patients treated with INNOVO, the intervention can either be successful or unsuccessful. If successful (i.e. cured or improved), patients progress to a “dry” health state and if unsuccessful, patients progress to an “incontinent” health state. Patients who have had a successful treatment may remain in the “dry” health state in subsequent cycles or they may have a recurrence of the condition, rendering them “incontinent”. Patients who are incontinent following treatment with INNOVO may move on to the next line of treatment, i.e. medication, or they may choose to use containment products such as incontinence pads in a “containment” health state, rather than undergo further treatment. For patients progressing to medication, similar pathways are followed; treatment can again be successful or unsuccessful, with unsuccessful patients either progressing to surgical intervention or choosing to use containment products rather than undergo further treatment. New “dry” and “incontinent” health states are used to follow the patients experiencing success or failure of treatment with medication. Patients in the “dry” health state following treatment with medication may also experience recurrence, moving them to the “incontinent” health state following medication.
For patients progressing to surgery, the surgical procedure is assumed to be retropubic mid-urethral sling (RMUS). This was based on the fact that in previous clinical and cost-effectiveness work, RMUS has been applied as the reference surgery against which other surgical treatments have been comparedCitation4. Surgery may be successful, in which case the patient will progress to a new “dry” health state following surgery or it may be unsuccessful, in which case the patient will progress to a new “incontinent” health state following surgery. Patients who have had a successful surgery may either remain dry or they may have a recurrence, meaning that they move to the “incontinent” health state following surgery. Patients who are incontinent following surgery may choose to use maintenance products in the “containment” health state or they may progress to a second, and third, surgical procedure (RMUS each time), with the same pathways as described previously being followed. New “dry” and “incontinent” health states are used to capture success and failure of treatment with second, and third, surgeries. Patients who are still incontinent following a third surgery will remain in the “containment” health state for the duration of the model. The same pathways as described above are used to represent treatment in current practice, however INNOVO is not offered as a treatment option prior to medication. Rather, patients will use existing electrical stimulation treatment initially, with a possibility of subsequently progressing to medication and surgical treatment. Patients may also experience death in each cycle of the model. The mortality rate associated with the age of patients in the model with this condition is applied, but a death rate associated with treatment is not considered.
2.2. Model inputs
All model inputs are presented in the following section. Point estimate values for the clinical effectiveness, utility, and cost parameters are presented in . Additionally, uncertainty around the point estimate, as well as a distribution of uncertainty, are presented where appropriate, and available, in order to allow for a probabilistic analysis to be undertaken.
Table 1. Parameters included in economic model.
2.2.1. Clinical effectiveness parameters
The probability of being dry (cured) at 3 and 6 months following treatment with INNOVO was derived from a previous randomized controlled non-inferiority trial comparing external electrical stimulation with intravaginal electrical stimulation for the treatment of SUI in womenCitation10. The probability of being dry at 1 year and in the long term with INNOVO was estimated using a parametric survival model studyCitation10. The probabilities of cure at 3 months, and 1 year, from existing electrical stimulation treatment were derived from a study looking at the effectiveness and cost-effectiveness of non-surgical treatments for women with SUICitation11. The probabilities of cure at 3 months, and 1 year, from medication were derived from the same studyCitation11. The probabilities of cure at 3 months, and 1 year, with surgery were derived from a previous systematic review and economic evaluation in this clinical areaCitation4. Probabilities of progressing to the next line of treatment following failure of the current line of treatment were all based on assumptions informed by expert clinical input. The relative succuss rate of repeat surgeries was derived from the previous economic evaluation in this areaCitation4.
In a separate analysis, success of each treatment based on improvement rate (rather than cure), was also conducted. Data from Dmochowski et al.Citation10 were used to estimate the improvement rate associated with INNOVO at 6 months, and 1 year, based on the Patient Global Impression of Improvement measure. The probabilities of improvement (based on a combination of measures) at 3 months, and 1 year, from existing electrical stimulation treatment were derived from previous clinical studiesCitation11,Citation12. Short-term and longer-term probabilities of improvement associated with medication and surgery were derived from Imamura et al.Citation11 and Brazelli et al.Citation4, respectively. In these studies, improvement was based on a combination of measures.
The clinical probabilities of experiencing multiple adverse events were modelled. For INNOVO, the probabilities of experiencing infection, pain, muscoskeletal and connective tissue disorders, renal disorders and skin and subcutaneous tissue disorders were considered. All of these probabilities were derived from the study which included a randomized trial of the deviceCitation10. For existing electrical stimulation treatment, the probabilities of experiencing infection and pain were considered. Appropriate values were derived from Imamura et al.Citation11. For medication, the probabilities of experiencing nausea, headache and constipation were considered. These probabilities were derived from a previous trial comparing duloxetine with placeboCitation13. Finally, for surgery the probabilities of experiencing infection, pain, tape erosion, urethral perforation, and voiding difficulties were considered. The probabilities of these events occurring were derived from the previous economic evaluationCitation4.
2.2.2. Utilities
Utility values, and utility decrements, were applied to relevant health states and adverse events in the model. SUI pre-treatment, re-treatment, cured and incontinent health state utility values were derived from a previous cost-utility analysisCitation14 and a quality-of-life study amongst women with UICitation15. Utility decrements associated with adverse events related to INNOVO, electrical stimulation treatment and surgery, and duration of effect, were derived from a previous decision analysis comparing retropubic and transobturator midurethral slingsCitation16, a study looking at the impact of adverse events on health utility and health-related quality-of-life amongst patients with metastatic breast cancerCitation17, and from a systematic review of health state utility values in metastatic non-small cell lung cancerCitation18. Utility decrements associated with adverse events related to medication were derived from prior studies exploring patient preferences and quality-of-life in various patient populationsCitation19–21.
2.2.3. Costs
The cost of treatment with INNOVO was estimated based on information on resource use provided by the device manufacturersCitation22. The cost of existing electrical stimulation treatment was derived from Imamura et al.Citation11. The 3-monthly cost of medication was derived from the British National Formulary, 2018Citation23, while the cost of the surgical intervention was derived from the UK NHS Reference Costs 2017/18Citation24. All additional costs associated with delivery of medication and surgery were derived from previous literature and routine sources, and are presented in below. The costs associated with treating adverse events were derived from the British National Formulary, 2018Citation23, and a previous economic evaluation in the area of SUICitation4. Finaly, the cost of remaining dry was based on an assumption, and the cost of containment products was estimated based on information from the NICE clinical guidelines for the management of UI in womenCitation26.
2.3. Analysis
The model was run to obtain the expected costs and health outcomes associated with each strategy, with results presented in terms of cost per quality-adjusted life-year (QALY) gained. A Monte Carlo simulation, with 10,000 iterations, was conducted in order to obtain probabilistic results, and results of these analyses are presented in the form of cost-effectiveness planes and cost-effectiveness acceptability curves (CEACs). Deterministic sensitivity analyses were also carried out in order to explore the impact of parameter variation on the model results, with results presented in the form of tornado diagrams. Cost-utility analyses were carried out firstly with “cure” applied as the measure of success for all treatments, and then with “improvement” applied as the measure of success.
3. Results
This section presents the results of the economic analysis; results of the “cure” analysis are presented first followed by results of the “improvement” analysis.
3.1. Cure analysis
3.1.1. Base-case analysis
Results of the base-case analysis presented in indicate that the treatment strategy including INNOVO leads to cost savings of £250 per patient over a lifetime time horizon, and an increase in QALYs gained over the patient’s lifetime (+0.03). Therefore, INNOVO is dominant, i.e. less costly and more effective than current patient management.
Table 2. Base-case probabilistic results: cure analysis.
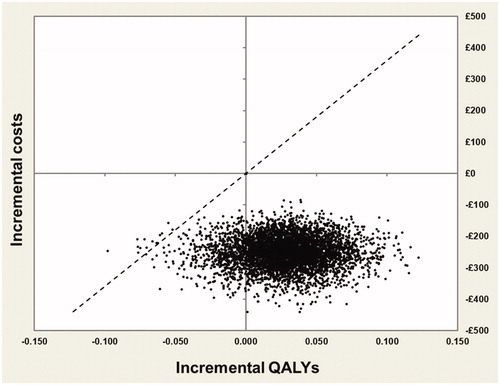
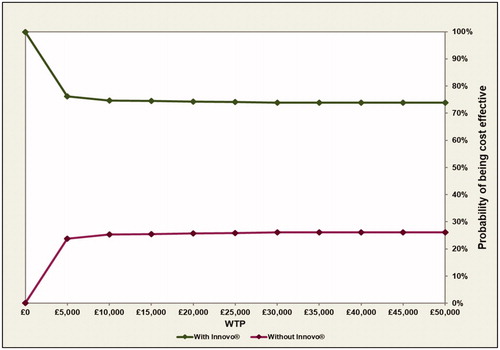
The cost-effectiveness plane produced from the probabilistic analysis () shows that most points are in the South-East quadrant, indicating that the intervention is less costly and more effective than the comparator. This plot also shows that although INNOVO is not always more effective than current practice, it is almost always less costly, based on the results of this probabilistic analysis. The CEAC shown in indicates that INNOVO has a high probability of being cost-effective (>74%) across all willingness-to-pay (WTP) thresholds presented. Finally, the probability that the intervention is solely cost saving was also estimated and results shown in indicate that there is a >99% probability that INNOVO will lead to a cost reduction.
3.1.2. Sensitivity analysis
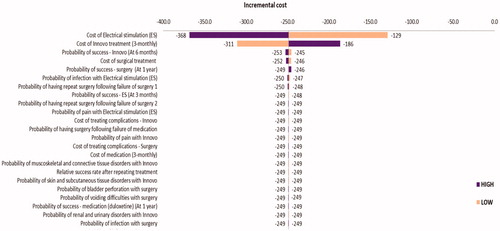
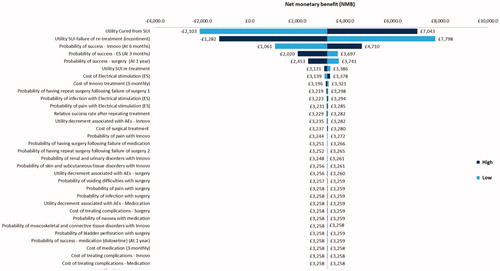
A number of deterministic sensitivity analyses were also carried out in order to explore the impact that specific parameter variation had on the model results. In , the impact that varying each model parameter by 25% had on the incremental cost of the intervention (INNOVO) is presented. The most impactful parameter variations are displayed at the top of the Figure while those which had the least impact are displayed towards the end. In the base-case analysis, the incremental cost of the intervention was −£250, meaning that it was cost saving. Parameter variations which had the strongest impact on the base-case results were the cost of electrical stimulation treatment, the cost of INNOVO treatment, and the probabilities of successful INNOVO treatment, electrical stimulation treatment and surgical treatment.
Figure 3. Cost-effectiveness acceptability curve at various WTP thresholds (£0–£50,000): cure analysis.
In a separate analysis (), the impact that parameter variation had on the net monetary benefit (NMB) of the intervention was explored. NMB is calculated as: (Incremental benefit x WTP threshold) – Incremental cost, with a positive NMB indicating superiority from an economic standpoint. In the base-case analysis, the NMB of the intervention was £821. The utility of patients who have been cured from SUI was a strong driver of results, as well as the probabilities of having a successful INNOVO, electrical stimulation and surgical treatment. The utility of patients who have SUI following failure of re-treatment was also a strong driver of results, with an increase in this value by 25% reducing the NMB of the intervention (£131), and a decrease in this value increasing the NMB of the intervention by £1510.
3.2. Improvement analysis
3.2.1. Base-case analysis
In this analysis, “improvement” was applied as the measure of success of treamtment rather than “cure”. Results of the base-case analysis presented in indicate that the treatment strategy including INNOVO leads to cost savings of £327 per patient over a lifetime time horizon, and an increase in QALYs gained over the patient’s lifetime (+0.13). As in the previous analysis, INNOVO is absolutely dominant, i.e. less costly and more effective than current patient management.
Figure 5. Impact of changing the input parameters by ±25% on the estimated Net Monetary Benefit (NMB): cure analysis.
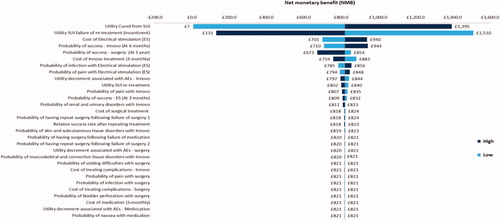
Table 3. Base-case probabilistic results: improvement analysis.
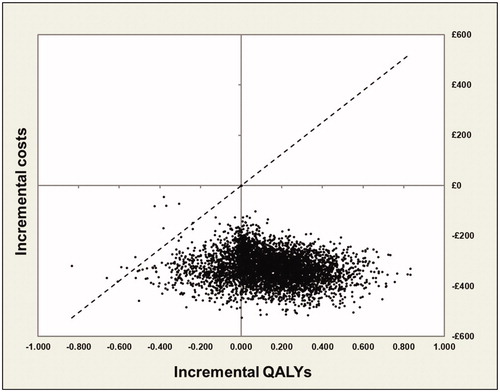
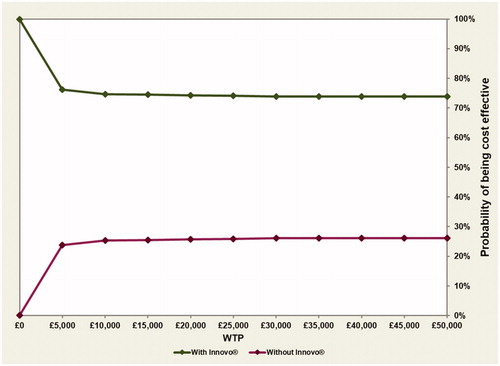
Probabilitic results are also similar to the results presented in the “cure” analysis, with the majority of points presented in the cost-effectiveness plane () lying in the South-East quadrant (indicating that the intervention is less costly and more effective than the comparator). Similarly, the CEAC shown in indicates that once again, INNOVO has a high probability (>74%) of being cost-effective across all WTP thresholds presented. The probability that the intervention is solely cost saving was also estimated and results shown in indicate that, as previously, there is a >99% probability that INNOVO will lead to a cost reduction.
3.2.2. Sensitivity analysis
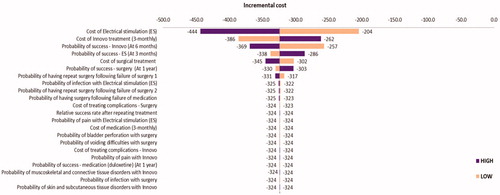
Parameters were again increased and decreased by 25% in a deterministic sensitivity analysis to explore the impact of this variation on the model results. In the base-case “improvement” analysis, the incremental cost of the intervention was −£327, meaning that it was cost saving. Parameter variations which had the strongest impact on the base-case results were the cost of electrical stimulation treatment, the cost of INNOVO, and the probabilities of successful INNOVO treatment, electrical stimulation treatment and surgical treatment (). All of these parameter variations changed the incremental cost of the intervention. Results also showed that when cost of containment products were included (i.e. societal perspective) the cost saving per women increased to £792.
Figure 8. Tornado diagram showing the impact of changing the input parameters by ±25% on the estimated incremental cost: improvement analysis.
shows the impact of the same parameter variations on the NMB of the intervention. In the base-case analysis, the NMB of the intervention was £3,258. The utility of patients who have been cured from SUI was a strong driver, as well as the probabilities of having a successful INNOVO, electrical stimulation and surgical treatment. The utility of patients who have SUI following failure of re-treatment was also a strong driver of results, with an increase in this value by 25% reducing the NMB of the intervention (−£1,282), and a decrease in this value increasing the NMB of the intervention by £7,798.
4. Discussion
This study has aimed to assess the cost-effectiveness of a new therapy device for the treatment of SUI (INNOVO) in the UK, compared with current treatments. Due to the substantial impact that UI has on public expenditureCitation4, it is important that evidence-based decisions are made on which interventions should be funded so as to ensure that resources are allocated in the most effieicent way. Not only this, but a robust cost-effectivness analysis is required in order to consider the optimal treatment strategy for a condition which has such a detrimental impact on the quality-of-life of individual patientsCitation4.
Results of the analysis show the high likelihood that the INNOVO therapy device is cost-effective over the lifetime of the patient (>74% probability at a WTP threshold of £20,000), in both “cure” and “improvement” analyses. Indeed, results of the probabilistic analyses show that the probability of the device being cost-effective is greater than 74% across all WTP thresholds in both of the analyses presented. Additionally, the probability of the intervention being cost saving is greater than 99% in both “cure” and “improvement” analyses. Deterministic sensitivity analyses were also conducted in order to explore the impact that 25% parameter variations had on the model results. Amongst the most important drivers of results was the probability of having a successful INNOVO treatment. This is hardly surpising, given that improving the probability of success at an earlier stage in the treatment pathway will reduce costs for the health care service and improve patient outcomes.
There is limited information available in the literature to directly compare the results presented in our own study with previous work. Stewart et al.Citation27 carried out a systematic review of non-invasive electrical stimulation therapy for the treatment of SUI in women, in order to understand the relative clinical effectiveness and value for money of available treatments. However, of the 56 trials identified comparing electrical stimulation to no treatment or any other available treatment, none included estimates of cost-effectiveness. While there is not much evidence of cost-effectiveness with which we can compare our results, evidence from the literature re-iterates our finding that use of an electrical stimulation device can improve quality-of-life amongst patients with SUI. Correia et al.Citation28 conducted a randomized controlled trial to explore the effects of surface and intravaginal electrical stimulation in the treatment of women with SUI. The authors found that after treatment in the intervention groups, there was a significant reduction in scores (improvement in quality-of-life) in domains including; limitations of daily activities, incontinence impact, and physical limitation. These results are in line with the findings of our own study, which indicate that INNOVO can improve the quality-of-life of patients when compared to current patient management.
There are limitations to the analysis presented here. Firstly, evidence on the long-term effectiveness of INNOVO is lacking, with only short-term evidence of success reported in Dmochowski et al.Citation4. Indeed, evidence on the effectiveness of the device in general is sparse, with the randomized trial presented in this study being the only robust source of dataCitation4. For this reason, the input of clinical experts was required to inform the longer-term success outcomes associated with INNOVO (based on cure and improvement rates). In addition, longer term outcomes from other electrical stimulation studies with similar early results were used as a guide. Secondly, results of our analysis are presented separately depending on whether cure or improvement is applied as the measure of success in the model. While studies in this clinical area tend to universally define “cure” as dryness, “improvement” is measured in a variety of ways across studies, which made it difficult to ensure consistency in our “improvement” analysis. While Dmochowski et al.Citation10 use the Patient Global Impression of Improvement scale to measure improvement amongst patients using the INNOVO device, Imamura et al.Citation11 and Brazelli et al.Citation4 utilize data from meta-analyses which combine data from studies defining improvement in a variety of ways. Therefore, for the treatments that we are modelling (INNOVO, electrical stimulation therapy, medication and surgery), success based on improvement is defined in various ways, which introduces inconsistency in the analysis. Finally, due to data limitations, there was uncertainty surrounding the probability of progressing to the next line of treatment following failure of current treatment, which meant that assumptions had to be made for these parameter values.
Depite these limitations, a robust economic model has been developed to consider the current treatment pathways for SUI in the UK, and the potential impact that the introduction of INNOVO to this pathway could have on health care costs and patient outcomes.
5. Conclusion
INNOVO is a potentially cost-effective treatment option for women with SUI in the UK. Initiated at a point following the failure of minimally invasive first-line treatment, this therapy device could result in reduced costs for the health care service and improved quality-of-life for selected patients.
Transparency
Declaration of funding
This report is independent research funded by Atlantic Therapeutics. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the Department of Health, or the company.
Declaration of financial/other relationships
AM has no conflicts of interest that are directly relevant to the content of this article. Device Access (MJ, AM, MBH and JA) received funds from Atlantic Therapeutics during the conduct of the study. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Mehdi Javanbakht was responsible for developing and populating the economic model and drafting the final version of the paper. All authors provided inputs for the model, read, and approved the final draft of the manuscript.
Acknowledgements
None reported.
Data availability statement
The authors declare that all of the data supporting the findings of this study are available within the article.
References
- Irwin DE, Milsom I, Hunskaar S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50:1306–1314.
- Irwin DE, Milsom I, Kopp Z, et al. Impact of overactive bladder symptoms on employment, social interactions and emotional well-being in six European countries. BJU Int. 2006;97:96–100.
- Aoki Y, Brown HW, Brubaker L, et al. Urinary incontinence in women. Nat Rev Dis Primers. 2017;3:17042. DOI:10.1038/nrdp.2017.42
- Brazzelli M, Javanbakht M, Imamura M, et al. Surgical treatments for women with stress urinary incontinence: the ESTER systematic review and economic evaluation. Health Technol Assess. 2019; 23:1–306.
- National Institute for Health and Care Excellence. Urinary and pelvic organ prolapse in women: management. London: National Institute for Health and Care Excellence; 2019.
- Hunskar S, Lose G, Sykes D, et al. The prevalence of urinary incontinence in women in four European countries. BJU Int. 2014;93:324–330.
- Soeder S, Tunn R. Neuromuscular electrical stimulation (NMES) of the pelvic floor muscles using a non-invasive surface device in the treatment of stress urinary incontinence (SUI); a pilot study. Proceedings of the 38th Annual Meeting of the International Urogynecological Association (IUGA), Dublin. 2013.
- Maher RM, Caulfield B. A novel externally applied neuromuscular stimulator for the treatment of stress urinary incontinence in women – a pilot study. Neuromodulation. 2013;16:590–594.
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. 2013. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781
- Dmochowski R, Lynch CM, Efros M, et al. External electrical stimulation compared with intravaginal electrical stimulation for the treatment of stress urinary incontinence in women: A randomized controlled noninferiority trial. Neurol Uerodyn. 2019;38:1834–1843.
- Imamura M, Abrams P, Bain C, et al. Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence. Health Technol Assess. 2010;14:1.
- Eriksen BC, Bergmann S, Mjolnerod OK. Effect of anal electricostimulation with the ‘Incontan’ device in women with urinary incontinence. BJOG. 1987;94:147–156.
- Millard RJ, Moore K, Rencken R, et al.; for the Duloxetine UI Study Group. Duloxetine vs placebo in the treatment of stress urinary incontinence: a four-continent randomized clinical trial. BJU Int. 2004;93:311–318.
- Manca A, Sculpher MJ, Ward K, et al. A cost-utility analysis of tension-free vaginal tape versus colposuspension for primary urodynamic stress incontinence. BJOG . 2003;110:255–262.
- Haywood KL, Garratt AM, Lall R, et al. EuroQol EQ-5D and condition-specific measures of health outcome in women with urinary incontinence: reliability, validity and responsiveness. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. Qual Life Res. 2008;17:475–483.
- Shepherd JP, Lowder JL, Jones KA, et al. Retropubic and transobturator midurethral slings: a decision analysis to compare outcomes including efficacy and complications. Int Urogynecol J. 2010;21:787–793.
- Hagiwara Y, Shiroiwa T, Shimozuma K, et al. Impact of adverse events on health utility and health-related quality-of-life in patients receiving first-line chemotherapy for metastatic breast cancer: results from the SELECT BC study. Pharmacoeconomics. 2018;36:215–223.
- Paracha N, Abdulla A, MacGilchrist KS. Systematic review of health state utility values in metastatic non-small cell lung cancer with a focus on previously treated patients. Health Qual Life Outcomes. 2018;16:179.
- Beusterien KM, Davies J, Leach M, et al. Population preference values for treatment outcomes in chronic lymphocytic leukaemia: a cross-sectional utility study. Health Qual Life Outcomes. 2010;8:50.
- Stafford MR, Hareendran A, Ng-Mak DS, et al. EQ-5D-derived utility values for different levels of migraine severity from a UK sample of migraineurs. Health Qual Life Outcomes. 2012;10:65.
- National Institute for Health and Care Excellence. Palliative care for adults: strong opioids for pain relief. 2012. Available from: https://www.nice.org.uk/guidance/cg140
- Atlantic Therapeutics. Available from: https://atlantictherapeutics.com/; https://www.myinnovo.com/uk/about-us/
- British National Formulary. BNF Online. 2019 Jul 15. Available from: https://www.medicinescomplete.com/mc/?utm_source=bnforg&utm_medium=homepage&utm_campaign=medicinescomplete
- Department of Health. Reference Costs 2017–18. 2018.
- Curtis L, Burns A. Unit costs of health & social care 2018. Canterbury: The University of Kent; 2018.
- National Institute for Health and Care Excellence. Urinary incontinence in women: the management of urinary incontinence in women. 2011. Available from: https://www.nice.org.uk/guidance/cg171/documents/urinary-incontinence-update-draft-scope2
- Stewart F, Berghmans B, Bo K, et al. Electrical stimulation with non-implanted devices for stress urinary incontinence in women. Cochrane Database Syst Rev. 2017; 22:12.
- Correia GN, Pereira VS, Hirakawa HS, et al. Effects of surface and intravaginal electrical stimulation in the treatment of women with stress urinary incontinence: randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2014;173:113–118.