Abstract
Aims
Wild-type transthyretin amyloid cardiomyopathy (ATTRwt) is a fast progressing and fatal disease associated with substantial delays in diagnosis. Between the first symptoms and diagnosis, patients are frequently hospitalized, primarily with cardiac symptoms. After diagnosis, patients continue to experience frequent hospital admissions. The objective of this study was to estimate the Danish diagnostic and lifetime hospital costs associated with the treatment of patients with ATTRwt both before and after they are diagnosed.
Materials and Methods
We developed a cost model for Danish hospital costs associated with ATTRwt, including the costs of diagnosis, cardiac implants, and hospital admissions covering inpatient hospitalization and outpatient hospital care (ambulatory and emergency services). The number of diagnoses, cardiac implants, inpatient hospitalization and outpatient hospital care were estimated based on published data. Estimates of the unit costs were based on publicly available Danish reference costs. We calculated the total hospital costs covering the median lifespan of patients from onset of symptoms, which is 13 months prior to diagnosis, to 52 months after diagnosis which is the median survival time after diagnosis.
Results
The average cost of diagnosing ATTRwt was USD 3,424 per patient; the average costs of cardiac implants were USD 1,851 per patient. Hospital admissions costs totaled USD 3,345 pre-diagnosis and USD 59,449 post-diagnosis per patient, on average. The total diagnostic and lifetime (65 months) hospital costs associated with ATTRwt were USD 68,069.
Conclusions
Caring for patients with ATTRwt places a significant economic burden on the healthcare system. The study emphasizes the cost saving potential for medical interventions in this patient population.
Introduction
Wild-type transthyretin amyloid cardiomyopathy (ATTRwt) is a myocardial disease associated with advanced heart failure symptoms and increased morbidityCitation1–6. The epidemiology of ATTRwt is poorly characterized, however, new data suggest that the disease is substantially under- and misdiagnosedCitation5–8. Studies have reported delays in diagnosis from 13 to 78 monthsCitation9–11. Before the correct diagnosis is established, patients with ATTRwt experience multiple hospitalizations due to misdiagnosis and mistreatments, which has been reported to be as high as six hospital admissions annuallyCitation9–11.
Once patients are diagnosed, their hospitalization rates increase due to the expected natural disease progression of ATTRwt. A study from the United Kingdom found that patients required up to eight hospital visits per year following diagnosisCitation9. In addition to an increase in hospital visits, the disease progression is associated with substantial societal cost. In a recent registry study of 176,067 Danish patients, the total yearly net costs after an incidence of heart failure were USD 12,053Citation12.
The introduction and availability of new and improved treatments can reduce morbidity and all-cause mortality with a likely reduction of cardiovascular-related hospitalizations for patients with ATTRwtCitation13. To assess the cost-effectiveness of new and emerging therapies, it is important to estimate the diagnostic and hospital costs in terms of healthcare utilization by patients with ATTRwt.
As the first study of its kind, the present study aims to estimate the Danish diagnostic and lifetime hospital costs associated with the treatment of patients with ATTRwt both before and after they are diagnosed. Thereby we aim to establish reference costs for patients with ATTRwt which can be applied in the evaluation of potential forthcoming therapies.
Methods
Best-practice for cost-of-illness studies involves collecting real-world data by a retrospective registry analysis. Such analysis allows identification of all costs incurred by patients across healthcare sectors as well as of costs outside the healthcare sector, such as productivity loss, although we expect productivity loss to be minimal since the average age of Danish patients with ATTRwt is above 80. However, as ATTRwt patients currently are not assigned a separate diagnostic code in Denmark, it has not been possible to identify ATTRwt patients in the public Danish registries. Instead, this study applies a modelling-approach to estimate the lifetime hospital costs. Total hospital costs in Denmark are defined as publicly funded hospital service usage within the Danish healthcare system. The total hospital costs related to treating ATTRwt patients include the costs related to the diagnostic procedure, a cardiac implant when necessary and hospital admissions covering inpatient hospitalization and outpatient hospital care, such as ambulatory and emergency room services both prior to and after the date of diagnosis. Throughout this manuscript, we refer to ambulatory and emergency room services as outpatient hospital care. The cost of medication provided to the patient during the hospitalization is covered in the DRG rates. Costs of primary care and primary care medication are outside the scope of this study. The model was designed using an incidence-based cost-per-event design.
Data sources
The input to the model is based on real-world data from published international peer-reviewed journals, treatment guidelines and dialogue with a clinical expert in ATTRwt from a Danish university hospital. Estimates of resource use associated with diagnosis are based on current Danish treatment guidelines and clinical practice with treatment and monitoring of patients with ATTRwt. Frequencies of cardiac implants and hospitals admissions prior to diagnosis are estimated from a Danish study by Ladefoged et al.Citation10. Ladefoged et al. (2020) includes 50 contemporary ATTRwt patients diagnosed between 2017 and 2019 and present the only available Danish data on ATTRwt. The average age of the included patients is 81.1. The study includes frequency of misdiagnoses as well as inappropriate investigations and treatments of other suspected diseases prior to diagnosis. In the cost analysis, we use the average frequency of investigations and treatments from this study.
Because of a lack of specific Danish data, healthcare utilization in the period after diagnosis is based on historical data from the UK by Lane et al.Citation9 covering 711 ATTRwt patients from 2000 to 2017.
Unit costs
Estimates of the costs of diagnosis, implants, and hospitalizations are based on relevant publicly available Danish reference costs from 2020 (DRG rates)Citation14.
DRG rates are national average unit costs for inpatient hospitalizations, ambulatory and emergency services based on hospital data gathered in a national costs databaseCitation15. All results are presented in USD and at the 2020 price level. Cost estimates in DKK are converted to USD using an exchange rate of 0.145 (as of February 18, 2020).
Time horizon
Three studies report the median diagnostic delay measured as the time from the first cardiological examination to the date of the diagnosis by endomyocardial biopsy or DPD scintigraphy. Ladefoged et al.Citation10 reports median delays of 13 (2–47) months, Bishop et al.Citation11 reports median delays of 34 (15–72) months, and Lane et al.Citation9 reports median delays of 39 (8–78) months in diagnosis. We use the Danish estimate by Ladefoged et al. (2020) with a median diagnostic delay of 13 months in our base case. However, Ladefoged et al. (2020) presents data from a center of excellence, where diagnostic delay is most likely shorter than the national average. Therefore, we present the results from a sensitivity analysis that assumes a diagnostic delay of 39 months as reported in Lane et al.Citation9.
There are currently no Danish data on median life expectancy for patients diagnosed with ATTRwt. The average weighted median life expectancy from published studies is 52 months (26–68 months)Citation2,Citation4,Citation5,Citation9,Citation16,Citation17. It is reported that life expectancy depends on disease stage. Lane reports that median survival for patients with ATTRwt classified by the United Kingdom National Amyloid Centre suggested stage system (referred to as NAC stage) is 68 months in stage 1, 48 months in stage 2, and 26 months in stage 3Citation9. Lane reports a median life expectancy of 57 months. In the Danish study by Ladefoged et al.Citation10, 42% of patients were in stage 1, 40% in stage 2, and 18% in stage 3. Using the UK NAC-specific survival data and the Danish distribution of patients according to the NAC distribution, the average weighted median life expectancy is 52 months. We use this for the base case analysis, but apply a sensitivity analysis using the median life expectancy of 26 and 68 months as reported in the literature. To assess the total lifetime cost per patient with ATTRwt, we therefore consider the cost of ATTRwt from 13 months prior to diagnosis through 52 months after diagnosis.
To investigate the impact of key assumptions on the estimate of the diagnostic and lifetime hospitalization costs associated with ATTRwt and to address potential uncertainties regarding each of the cost components included in the analysis, we have carried out several sensitivity analyses.
Results
Cost of diagnostic procedure
The diagnosis of ATTRwt is obtained primarily by either endomyocardial heart biopsy or radionuclide bone scintigraphy with technetium-labeled bisphosphonate. Echocardiography and cardiac magnetic resonance imaging are also used to raise the suspicion of ATTRwt amyloidosis among patients with heart failureCitation18.
lists the diagnostic tests included in the Danish diagnostic procedure for ATTRwt along with the percentage of patients receiving the tests and the frequency of each diagnostic test per patient.
Table 1. Diagnostic tests and costs per patient of diagnostic procedure in patients with ATTRwt in Denmark.
presents the costs associated with each test (DRG rates) and the total cost of diagnostic procedures per patient. The estimate of 25% myocardial biopsy is based on the fact that some patients will only be grade 1 on the bone scintigraphy. They will require supplementary diagnostics such as a biopsy. In addition to this, a substantial part of elderly patients suspected to have ATTRwt will have elevated serum levels of kappa and lambda light chains and a subsequent increased kappa and lambda light chain ratio above 1.65 which will require a myocardial biopsy to rule out amyloid light chain amyloidosis.
Cardiac implants
For patients with ATTRwt, a pacemaker is a standard treatment for symptom management of particular arrhythmias. According to Ladefoged et al.Citation10, 30% of Danish ATTRwt patients have received a pacemakerCitation10. Another recent study from Spain found that up to 44% of patients with ATTRwt carry a pacemakerCitation19. We use 30% in our base case and assume that 99% of the patients who had a pacemaker implanted experienced no device associated complications.
presents the costs per patient of pacemaker implantation in Denmark and the assumptions regarding the prevalence and type of cardiac implants in Danish patients with ATTRwt, as well as the associated costs of different types of placement.
Table 2. Prevalence and Costs per Patient of Cardiac Implants in Patients with ATTRwt in Denmark.
Our calculations are based on implementations of pacemakers only, since implanted cardiac devices (ICDs) are only seldomly implanted in patients with ATTRwt. Moreover, we limit the analysis to only include the cost of the first cardiac implant since the time until renewal is estimated to be seven years which is longer than the median survival of 52 months for patients with ATTRwt. A population-based cohort study of all Danish patients who underwent a cardiac implantable electronic device (CIED) procedure from May 2010 to April 2011 by Kirkfeldt et al.Citation20 reports that only few patients experienced complications with their pacemaker implantation. Therefore, we assume that 1% of patients will experience complications with a pacemaker implant.
Hospitalizations prior to diagnosis
Ladefoged et al.Citation10 presents the number of investigations and treatments the population had during a median period of 13 months. We use this data to estimate the number of inpatient hospitalizations and outpatient hospital care visits patients have per year prior to diagnosis. We assume that one investigation requires one outpatient hospital care visit and one treatment require one inpatient visit.
Ladefoged et al.Citation10 shows that the 50 patients in the study were investigated a total of 104 times and that each patient, on average, had 2.08 investigations. We assume that each patient has 2.08 investigations and hence 2.08 outpatient hospital care visits in 13 months. Based on DRG rates, we calculate an average cost of USD 276 for each outpatient hospital care visit. Similarly, these 50 patients received a total of 49 treatments prior to their diagnosis. Therefore, we apply an average of 0.98 inpatient visits in 13 months per patient in the analysis. We calculate the unit cost per inpatient visit as a weighted average of treatment costs (DRG rates) resulting in a unit cost of USD 2,828 per inpatient visit.
presents the share of patients investigated and treated for a number of specific diseases prior to diagnosis and the costs associated with these investigations and treatments.
Table 3. Hospital Contacts per year prior to diagnosis of ATTRwt and Costs per Hospital Contact of Patients with ATTRwt in Denmark.
Hospitalizations after diagnosis
There are very limited data on hospitalization of ATTRwt patients after diagnosis. The only published reference we have been able to identify concludes that 94% of all hospitalizations after diagnosis are cardiac relatedCitation21.
presents the applied input regarding unit costs of inpatient hospitalizations based on a case mix of diagnoses and procedure groups experienced by ATTRwt patients in Denmark after diagnosis, as assumed by the authors. The average weighted cost of an inpatient hospitalization for a patient diagnosed with ATTRwt is USD 5,483.
Table 4. Case Mix of Inpatient Stays Following Diagnosis of ATTRwt, and Average Weighted Costs of Patients with ATTRwt in Denmark.
We rely on estimates from Lane et al.Citation9 to estimate the frequency of hospitalizations after diagnosis. Based on a subset of 364 patients with ATTR-CM (including both wild type ATTR-CM, ATTRwt, and hereditary ATTR-CM, ATTRm), the study indicates that the median number of inpatient hospital admissions per patient was 2 in the first 2 years after diagnosis and 3 in the third year. We therefore assume that patients on average have 2.33 inpatient hospitalizations per year following their diagnosis. The study also finds that in the first year after diagnosis the median number of outpatient and emergency department visits per patient was 8 and 1 respectively. We find that the estimates of 8 ambulatory visits of patients from Lane et al.Citation9 are too high to be applied directly in a Danish setting. Danish patients are partly monitored at the general practitioner, which would crowd out some of the outpatient stays reported for the population of patients in the UK. Expert opinion from the author with extensive clinical experience in ATTRwt (Professor Steen Hvitfeldt Poulsen) suggests that an average of 4.5 ambulatory visits and one emergency room visit per year is closer to the average for Danish patients.
The cost per ambulatory visit and emergency room visit is USD 168 for both types of contacts, according to the DRG rates from 2020.
presents the yearly frequency and costs of inpatient hospitalization, ambulatory and emergency visits in patients with ATTRwt in Denmark.
Table 5. Hospital Contacts per year after diagnosis of ATTRwt and Costs per Hospital Contact in Patients with ATTRwt in Denmark.
Total diagnostic and lifetime hospital costs per patient with ATTRwt
presents the results of total diagnostic and lifetime hospital costs associated with a patient with ATTRwt from 13 months prior to diagnosis to 52 months after diagnosis. The table shows that the costs total USD 68,069 per ATTRwt patient. Of these, we estimate that the diagnostic procedure and cardiac implant account for USD 3,424 and USD 1,851 respectively.
Table 6. Total diagnostic and lifetime hospital costs of patients with ATTRwt in Denmark.
In the 13 months prior to diagnosis, the total hospitalization costs associated with ATTRwt are equal to USD 3,345 per patient. The single largest cost driver is hospitalizations after diagnosis, accounting for USD 59,449 per patient (87% of total costs).
Sensitivity analyses
provides the results from the sensitivity analyses. One previous study found that 44% of patients have a cardiac implant. To address this uncertainty, we varied the share of cardiac implants by +/-50%, that is, to 15% and 45%, respectively. Both sensitivity analyses have only modest impact on the overall results.
Table 7. Deterministic sensitivity analyses.
Lane et al.Citation9 finds that ATTR-CM patients, on average, had one inpatient visit per year and 4.7 outpatient visits per year prior to diagnosis, which collectively is higher than the numbers we use in the base case (1.92 and 0.90, respectively). There are no published data on frequency of hospitalizations after diagnosis, nor are there any data on unit cost per event. Therefore, we have based the analysis on the best available knowledge and estimates from Professor Steen Hvitfeldt Poulsen. However, we assume that the unit cost per event is a significant underestimation of the true cost as the DRG rate represents only the first hospitalization and omits costs due to any readmissions as well as costs borne by the rest of the healthcare system and social care costs. Recognizing that the uncertainties regarding these parameters are considerable, we have added sensitivity analyses increasing/decreasing the total cost of hospitalization by +/- 50% both prior to and after diagnosis. This range covers the variation in both the frequency and the unit cost of hospitalizations. The sensitivity analyses show that the variation in the cost of hospitalizations in the years following diagnosis has the largest effect on the total cost estimates.
Even though this paper focuses on published real-world data, we have included a sensitivity analysis where we reduce the number of hospitalizations per year to 0.7 as found in the ATTR-ACT trial.
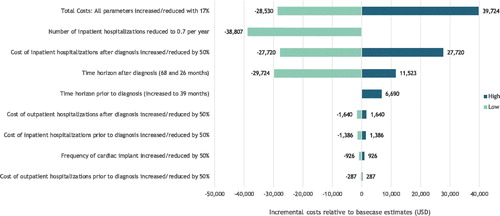
In addition to this, we add three sensitivity analyses in which we change the time horizon. In the first, we use the information from Lane et al. (2019) who found a median diagnostic delay of 39 months instead of the baseline of 13 months. In the second and third, we set the time horizon to the minimum and maximum median survival for this patient population from published studiesCitation2,Citation4,Citation5,Citation9,Citation16,Citation17. As seen in , changing the assumptions on the time horizon has a modest effect on the overall cost estimates.
To establish the joint uncertainty of the total lifetime hospital costs, we provide a sensitivity analysis where we increase and reduce all 16 model parameters with 17%. In the case where it is assumed that each variable is independently uniformly distributed with mean of the point estimate from the base-case analyses and a minimum of 0, the estimates will provide the 5th and the 95th percentile of the total lifetime hospital costs.
All estimates from the sensitivity analyses are graphically presented in .
Discussion
To our knowledge, this is the first study to provide an estimate of the total diagnostic and lifetime hospital costs for a patient with ATTRwt. We found that the cost estimates were robust when key assumptions were changed in the sensitivity analyses except for the cost of hospitalizations after diagnosis. Cost of hospitalizations after diagnosis is an aggregated measure of frequency of hospitalizations and the unit costs (DRG rate), and when changed +/-50%, the total cost of diagnostic and lifetime hospital visits changed significantly.
There are limited data on frequencies of hospitalizations and the source used in this study represents the best available evidence. Adding to the uncertainty, is the fact that that the frequency of hospitalizations increases with disease progression. Results from a recent randomized controlled trial suggest that the risk of cardiovascular (CVD)-related hospitalizations is elevated by 116%, for patients in the more severe New York Heart Association (NYHA) class III relative to patients in NYHA class ICitation13. The proportion of NYHA class III patients is likely higher in Denmark than in the study by Ladefoged et al.Citation10. This study included mainly patients in an expert clinical setting where patients are diagnosed relatively early. This could potentially cause Danish patients to have more frequent hospitalizations than what was found in Lane et al.Citation9 and thereby the yearly hospitalization costs to increase. At the same time, patients with a more advanced disease are expected to have shorter median survival. It is difficult to say how these two effects combined will affect the lifetime hospital costs.
The DRG rates include only the cost of the first hospitalization and omit costs due to any readmissions as well as costs carried by the rest of the healthcare system and social care costs. A broader healthcare perspective is typically included in real-world registry studies; as an example, the Danish study by Bundgaard et al.Citation12 finds that cost per event of heart failure is USD 12,053 compared with the DRG rate for heart failure of USD 4,972 used in the present study. In addition to heart failure, atrial fibrillation is a common comorbidity of ATTRwt. In a Danish registry-based study, the average three-year societal cost per patient attributable to atrial fibrillation was estimated at USD 22,039–28,672Citation22. Furthermore, the cost of ischemic stroke in patients with atrial fibrillation has been estimated at USD 30,295 per patient per 3 years in DenmarkCitation23. A common non-CVD related co-morbidity of ATTRwt is pneumonia, which has been estimated to cost USD 24,155 per patient in DenmarkCitation24. These dollar amounts are substantially higher than the average weighted cost of inpatient hospitalization after diagnosis of USD 5,483 reported in this study. This suggests overall that the cost per inpatient hospitalizations is considerably underestimated.
The main limitation of this study is the lack of national registry data for this specific patient population. As there has been no systematic national collection of data on patients with ATTRwt, there are no national registry data available on which to perform a real-world cost-of-illness analysis. Because of this limitation, we have had to estimate real-world costs by applying published sources of incidence of hospitalizations along with published Danish reference costs (DRG rates). Based on previously published studies, we assume that the exclusion of primary sector cost and social care cost has led to considerable underestimation of the real-world life-time costs of ATTRwt. The availability of registry data would have made it possible to estimate the cost directly attributable to ATTRwt. This could for example have been carried out by estimating the cost of ATTRwt patients in a retrospective registry analysis and comparing these to the healthcare costs of a matched control group.
However, since ATTRwt patients are not currently assigned a separate diagnostic code in Denmark, it has not been possible to identify ATTRwt patients in the public Danish registries.
Since it is not possible to estimate whether a hospitalization or an outpatient stay is directly caused by ATTRwt, we have chosen to present the full lifetime hospital costs.
Thereby, the results presented in this section will in the best way possible establish a counterfactual for the cost of patients initiated on potential forthcoming therapies for treatment of ATTRwt.
It should be noted that the costs presented in this paper are based on estimates on frequencies and unit costs in Denmark and this should be considered if the results are to be extrapolated to other settings where utilization or unit costs may differ.
The estimated costs for patients with ATTRwt can be modified by earlier diagnosis obtained through greater awareness of the disease among healthcare professionals, proper heart failure education of the patients and the introduction of new pharmacological treatments. These interventions are expected to reduce the morbidity for these patients and will thereby reduce the yearly hospital costs. These interventions are also expected to improve survival among these patients. For that reason, it is not possible to estimate how these interventions will affect the total lifetime hospital costs for this patient population.
Conclusion
Caring for patients with ATTRwt places a significant economic burden on the healthcare system. The study emphasizes the cost saving potential for medical interventions in this patient population. Due to the limited data on healthcare utilization for this population, uncertainty persists regarding the exact costs of ATTRwt. This study presents the total lifetime hospital costs estimated using unit prices for hospitalizations which omit costs due to any readmissions as well as costs borne by the rest of the healthcare system and social care costs. For that reason, the estimates of the total diagnostic and lifetime hospital costs of ATTRwt, presented in this paper, are conservative and actual costs are most likely higher.
Transparency
Declaration of funding
This work was supported by Pfizer Denmark.
Declaration of financial/other interests
This study was supported by Pfizer Denmark. Trine Pilgaard is an employee at Pfizer Denmark. Mikkel Hasse Pedersen is an employee at Incentive, which is a paid vendor of Pfizer Denmark. Steen Hvitfeldt Poulsen did not receive any funding from Pfizer to conduct this study, and he paid for his own work in this project.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
Trine Pilgaard and Mikkel Hasse Pedersen contributed to the study design, development of the economic model, the interpretation of the results and the drafting of the manuscript. Steen Hvitfeldt Poulsen contributed to the study design, the interpretation of the results and revision of the manuscript and gave clinical expert input. All authors have approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Previous presentations
An earlier version of this paper was accepted for a poster presentation at the annual ISPOR (The Professional Society for Health Economics and Outcomes Research Company) conference in 2020. In connection with that conference, an earlier version of the abstract is forthcoming in the abstract supplement of the May issue of Value in Health, 2020.
Acknowledgements
The authors acknowledge Daniel Sloth Hauberg, Mette Strand and Peter Bo Poulsen from Pfizer Denmark and Jens Olsen from Incentive for their valuable comments and suggestions throughout the research and writing of this paper.
References
- Ruberg F, Berk J. Transthyretin (TTR) Cardiac Amyloidosis. Circulation. 2012;126(10):1286–1300.
- Grogan M, Scott C, Kyle R, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–1020.
- Damy T, Kristen A, Suhr O, et al. Transthyretin cardiac amyloidosis in continental Western Europe: an insight through the Transthyretin Amyloidosis Outcomes Survey (THAOS). Eur Heart J. 2019. DOI:10.1093/eurheartj/ehz173
- Connors L, Doros G, Sam F, et al. Clinical features and survival in senile systemic amyloidosis: comparison to familial transthyretin cardiomyopathy. Amyloid. 2011;18(sup1):157–159.
- González-López E, Gagliardi C, Dominguez F, et al. Clinical characteristics of wild-type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J. 2017;38(24):1895–1904.
- Bhogal S, Ladia V, Sitwala P, et al. Cardiac amyloidosis: an updated review with emphasis on diagnosis and future directions. Curr Probl Cardiol. 2018;43(1):10–34.
- Mohamed-Salem L, Santos-Mateo J, Sanchez-Serna J, et al. Prevalence of wild type ATTR assessed as myocardial uptake in bone scan in the elderly population. Int J Cardiol. 2018;270:192–196.
- Tanskanen M, Peuralinna T, Polvikoski T, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40(3):232–239.
- Lane T, Fontana M, Martinez-Naharro A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26.
- Ladefoged B, Dybro A, Povlsen J, et al. Diagnostic delay in wild type transthyretin cardiac amyloidosis – A clinical challenge. Int J Cardiol. 2020;304:138–143.
- Bishop E, Brown E, Fajardo J, et al. Seven factors predict a delayed diagnosis of cardiac amyloidosis. Amyloid. 2018;25(3):174–179.
- Bundgaard J, Mogensen U, Christensen S, et al. The economic burden of heart failure in Denmark from 1998 to 2016. Eur J Heart Fail. 2019;21(12):1526–1531.
- Maurer M, Schwartz J, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–1016.
- Sundhedsdatastyrelsen. Interaktiv DRG 2020. http://drgservice.ssi.dk/grouper/Modules/Home/. (accessed May 5, 2020).
- Ankjaer-Jensen A, Rosling P, Bilde L. Variable prospective financing in the Danish hospital sector and the development of a Danish case-mix system. Health Care Manag Sci. 2006;9(3):259–268.
- Givens RC, Russo C, Green P, et al. Comparison of cardiac amyloidosis due to wild-type and V122I transthyretin in older adults referred to an academic medical center. Aging Health. 2013;9(2):229–235.
- Ruberg F, Mathew SM, Judge D, et al. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: The Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J. 2012;164(2):222–228.
- Gillmore J, Maurer M, Falk R, et al. Nonbiopsy diagnosis of cardiac transthyretin Amyloidosis. Heart Fail. 2016;133(24):2404–2412.
- Gonzálo-López E, Gallego-Delgado M, Guzzo-Merello G, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–2594.
- Kirkfeldt R, Johansen J, Nohr E, et al. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35(18):1186–1194.
- Nativi-Nicolau J, Vieira M, Chen Y, et al. Hospitalizations and Mortality Among Medicare Beneficiaries With Diagnosis of Wild-Type Amyloidosis Cardiomyopathy, Poster presented at AMCP Nexus 2019, National Harbor, Maryland, USA: 2019.
- Johnsen S, Dalby L, Täckström T, et al. Cost of illness of atrial fibrillation: a nationwide study of societal impact. BMC Health Serv Res. 2017;17(1):714
- Jakobsen M, Kolodziejczyk C, Fredslund E, et al. Societal costs of first-incident ischemic stroke in patients with atrial Fibrillation-A Danish Nationwide Registry Study. Value Health. 2016;19(4):413–418.
- Brogaard S, Nielsen M, Nielsen L, et al. Health care and social care costs of pneumonia in Denmark: a register-based study of all citizens and patients with COPD in three municipalities. Int J Chron Obstruct Pulmon Dis. 2015;10:2303–2309.
- Danske Regioner. Danske Regioner - Økonomisk Vejledning 2020 n.d. https://www.regioner.dk/aftaler-og-oekonomi/oekonomisk-vejledning/oekonomisk-vejledning-2020. (accessed Jun 7, 2020).