Abstract
Introduction
The aim of this study was to estimate the budget impact of lenalidomide and dexamethasone (RD) versus bortezomib, cyclophosphamide and dexamethasone (VCD) in newly diagnosed multiple myeloma (NDMM) and relapsed refractory (RR) MM patients, from the perspective of the Egyptian Ministry of health (MoH).
Methods
Two budget impact dynamic models were conducted to assess the budget impact of RD entry over a 3-year period. The clinical data for the modeled cohorts were based on published articles. Total annual medical costs associated with non-progression and progression disease states included the sum of estimated costs for adverse effects management, concomitant treatments, hospitalization and the follow up were measured. Deterministic sensitivity analyses were performed.
Results
The target population in a given year was estimated to include 245 patients with RRMM and 291 patients with NDMM receiving RD versus VCD. In RRMM, the annual budget savings of lenalidomide entry were estimated at EGP −1,103,969, −3,362,793 and −5,949,228 at year 1, year 2 and year 3, respectively. In NDMM, the annual budget savings of lenalidomide entry were estimated at EGP869,415, −1,779,776 and −2,139,311 at year 1, year 2 and year 3, respectively, to the payer after lenalidomide entry. The model results in RRMM were most sensitive to variations in patients eligible to transplantation in RRMM. In NDMM, the model results were most sensitive to the market share of VCD in the first year.
Conclusion
The results of our BI models suggest that not only does RD treatment have an effect on the budget, but also has major cost savings in other areas which are very important while considering the total costs of MM treatment. This study results provided evidence-based information to the MoH that will help in decision making of whether to implement RD as a treatment intervention or not.
1. Introduction
Multiple myeloma is a neoplasm of plasma cells that results in the overproduction of different chain monoclonal immunoglobulinCitation1. It is an incurable hematologic disease, accounting for 1% of all cancers and approximately 10% of all hematologic malignanciesCitation2. The incidence rate of multiple myeloma increases with age, particularly after 40 years, and is higher in men. The estimated number of multiple myeloma patients in Egypt is 552 (in 2015) but has continually increased in 2018 and 2019Citation3.
Patients can suffer from disease-related symptoms that significantly reduce the quality of life and survival. They can continue to be at a high risk of relapse and progressionCitation4. Clinicians must take into account several factors when making treatment decisions: patient preferences, access to treatment, age and comorbidities.
Myeloma management has changed over the years with an increase in available treatment options. The treatment algorithms are also changing because of the better outcomes obtained with newer agents. Maintenance therapies (MTs) after different induction regimens have been shown to improve response rate and progression-free survival (PFS). Lenalidomide 25 mg capsule is one of the therapies that was approved for use in the European Union (EU) in 2015 as first-line therapy for newly diagnosed multiple myeloma (NDMM), non-transplant eligible and for relapsed and refractory multiple myeloma (RRMM) patientsCitation5.
The increased survival benefit with lenalidomide was confirmed by Weisel et al., who conducted a network meta-analysis of survival in randomized controlled myeloma trials; lenalidomide and dexamethasone were associated with a significant progression-free and overall survival (OS) advantage versus other first-line treatments (bortezomib, melphalan, and prednisone [VMP] and melphalan, prednisone, and thalidomide [MPT])Citation6,Citation7.
Economic information including the budget impact and the cost-effectiveness in Egypt plays an important role in market access decision making for innovative medicines, particularly in an era of increasingly cost-conscious health systems. A budget impact analysis is needed to provide the decision-makers with evidence-based research to inform them about the impact of diffusion of new health care interventions from the health care system perspective for improving the outcomes of the population.
2. Research question
What is the budget impact of adopting lenalidomide (Immunomide, Hikma, Egypt) plus dexamethasone when compared with the current standard of care considering the health economic costs related to disease progression and adverse effects over a 3-year time horizon?
3. Objective
To estimate the budget impact of lenalidomide and dexamethasone (RD) versus bortezomib, cyclophosphamide and dexamethasone (VCD) in transplant-ineligible NDMM and transplant-eligible RRMM Egyptian patients, from the perspective of the Egyptian Ministry of health (MoH), over a 3-year time horizon.
4. Methodology
4.1. Model overview
Two dynamic, budget impact models were conducted to assess the pharmacy and medical budget impact of lenalidomide on MoH health plan over a 3-year period in the treatment of RRMM and NDMM in Egypt ( and ). NDMM were ineligible for stem cell transplant due to older age (typically >65 years) or aged <65 years with other existing comorbidities or fit and aged ≤70 years. Transplant-eligible RRMM are defined as patients with a disease that has previously responded to therapy and subsequently progressed beyond 60 days of the last therapy or disease that is nonresponsive while on primary or salvage therapy, or progresses within 60 days of last therapy, eligible for first-time transplantation. The two models were constructed for a real Egyptian population based on local clinical practice and dose per each regimen (). The economic impact of lenalidomide entry was modeled considering the impact on drug costs, as well as changes in medical costs due to expected improvements in PFS. We measured the difference in total costs for the two strategies in the two models to produce the budget impact. Patients were assumed to have RD and VCD treatments for 6 cycles only and then a palliative treatment continued till death after those 6 cycles (each cycle consists of 21 days). Palliative Care includes pain control, prophylactic measures (antibiotic or antiviral), nutritional supplements and anemia treatment.
Figure 1. The Budget impact model in (a) RRMM and (b) NDMM. Abbreviations. RRMM, relapsed refractory multiple myeloma; NDMM, newly diagnosed multiple myeloma. Total and adult population were obtained from National data; the estimates of multiple myeloma, target and eligible patients were obtained from Delphi Panel. The number of patients of RRMM produced by multiplication of number of multiple myeloma patients by % of target population (9.6%); the number of eligible patients produced by multiplication of number of RRMM patients by % of eligible population (70%). The number of patients of NDMM produced by multiplication of number of multiple myeloma patients by % of target population (20%); the number of eligible patients produced by multiplication of number of NDMM patients by % of eligible population (40%).
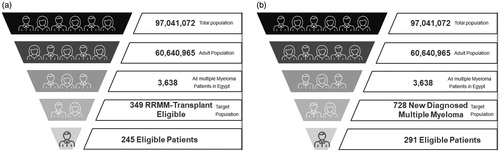
Figure 2. Transplant-ineligible NDMM patients flow chart (a) and transplant-eligible RRMM patients flow chart (b). Abbreviations. NDMM, newly diagnosed multiple myeloma; RRMM, relapsed refractory multiple myeloma.
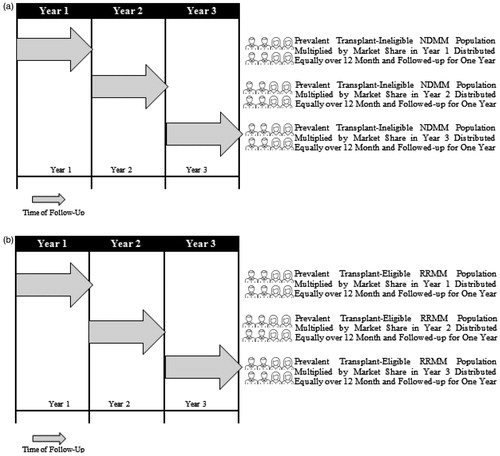
Table 1. Target populations, treatments and doses used in two cohorts.
The model was constructed for a hypothetical health plan population of 97,000,000 million lives in Egyptian health care settings. Model calculations, performed in Microsoft Excel 2010, included total annual incremental budget impact in EGP. All treatment lines, durations and doses used were as practiced within the Egyptian healthcare settings and as verified by our Delphi panel.
4.2. Model parameters and assumptions
The size of the target population in a hypothetical health plan was estimated using inputs derived from public databases, Delphi panel, and assumptions. Of the total population, 62.4% were assumed to be from 14 to 64-years-oldCitation12. Among these, 0.006% was prevalent with multiple myeloma, based on a Delphi panel (). Given the lack of accurate local epidemiological data on certain chronic diseases, this study consulted a Delphi panel of 5 clinicians specialized in hematology through a well-structured questionnaire as mentioned in .
Table 2. Delphi panel questions.
Table 3. The resources applied to each side effect.
The clinical data and adverse events for the modeled cohorts ( and ) were based on published articlesCitation8–11. Two partitioned survival models were performed using Weibull distribution on PFS and OS Kaplan–Myer curves to capture the proportion of patients that existed in each health state (). PFS and OS for lenalidomide in patients with RRMM were estimated from a pooled update of two large, multicenter MM-009 and MM-010 placebo-controlled randomized phase III trials that included 704 patients and assessed lenalidomide plus dexamethasone versus dexamethasone plus placeboCitation8, while PFS and OS for bortezomib were obtained from randomized (1:1), open-label, phase 3 study on 669 patients with RRMM comparing intravenous bolus of bortezomib versus high-dose oral dexamethasone from 93 centers in the United States, Canada, Europe, and IsraelCitation9. Both studiesCitation8,Citation9 have the same comparator and homogenous population. In NDMM, PFS and OS for lenalidomide were estimated from a randomized open-label trial comparing lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone on 445 patientsCitation11, while PFS and OS for bortezomib were obtained from open-label phase III study conducted on 482 patients with NDMM at 89 sites in France, Belgium, and Switzerland comparing vincristine plus doxorubicin plus dexamethasone (VAD), VAD plus dexamethasone, cyclophosphamide, etoposide, and cisplatin (DCEP) consolidation, bortezomib plus dexamethasone, or bortezomib plus dexamethasone plus DCEPCitation10. Adverse event incidence rates were converted to reflect the incidence rates per month. Only the most common adverse events in Egyptian clinical practice were selected.
Table 4: Model Input Parameters.
Separate market share distributions across the treatment options were used for patients with RRMM and NDMM as shown in . These assumptions are based on the patient population and the expected penetration of the treatment options for RRMM and NDMM.
Table 5. Market penetration for treatment options.
Pharmacy costs for treating RRMM and NDMM (MoH perspective) were estimated for 3 years in the current and future scenarios. The health care resource consumption in both arms has been reported in and . Pharmacy costs for the management of each adverse event are based on Egyptian drug-treatment algorithms. The pharmacy and the medical costs per year for each treatment option were calculated based on the reimbursed treatment cost, treatment duration and the follow-up. The total pharmacy cost for each regimen was calculated by multiplying the pharmacy cost by the number of patients expected to receive each treatment based on the market share. The unit costs were obtained from the MoH hospitals. If multiple unit costs were available, the average of these costs was used. Compliance was assumed to be 100% for all medications while patients were on medication. Total annual pharmacy costs included the sum of estimated pharmacy costs for all available treatments. The pharmacy budget impact of lenalidomide entry was estimated as the difference in pharmacy costs between the pre- vs post-lenalidomide entry scenarios.
Total annual medical costs associated with non-progression and progression disease states included the sum of estimated costs for adverse effects management, concomitant treatments, hospitalization and the follow-up. For each treatment option, the average survival time of a patient during the year was computed using survival analysis by assigning a Weibull distribution as reported by NICE and mean follow-up time. Similar to pharmacy costs, the medical budget impact of lenalidomide was estimated as the difference in medical costs during the 1 year before and after lenalidomide entry. All costs beyond year one were discounted at 3.5% annually, as recommended by CHEERS and the Egyptian Pharmacoeconomic GuidelinesCitation13,Citation14.
We have incorporated some assumptions in the two models. First, we assumed that the size of the target population would remain constant before and after the entry of lenalidomide. Second, the cost of dexamethasone and cyclophosphamide was not incorporated in the calculations as they are very low costs relative to the cost of lenalidomide and bortezomib. Third, we used the mean OS predicted from the published trials instead of median because the calculated values were a ratio of means (not medians) and to ensure methodological consistency in adjusting survival calculationsCitation15.
4.3. Sensitivity analyses
One-way sensitivity analyses were conducted for all treatment options to assess the model’s robustness. The parameters considered in the sensitivity analyses were included the progression rates, all costs of treatments and laboratory tests, population data, and market share. They were varied within plausible ranges based on base case values. The discount rate was varied between 2 and 6%.
5. Results
In a hypothetical health plan with 97 million members, the target population in a given year was estimated to include 245 patients with RRMM and 291 patients with NDMM receiving RD versus VCD. summarizes the expected budgetary impact of lenalidomide entry in terms of total annual pharmacy and medical costs in RRMM and NDMM.
Table 6. The base case results in RRMM and NDMM over 3 years (costs in EGP).
The target population in a given year was estimated to include 245 patients with RRMM and 291 patients with NDMM receiving RD versus VCD. In RRMM, the annual budget savings of lenalidomide entry were estimated at EGP −1,103,969, −3,362,793 and −5,949,228 at year 1, year 2 and year 3, respectively. In NDMM, the annual budget savings of lenalidomide entry were estimated at EGP869,415, −1,779,776 and −2,139,311 at year 1, year 2 and year 3, respectively, to the payer after lenalidomide entry.
5.1. Sensitivity analyses
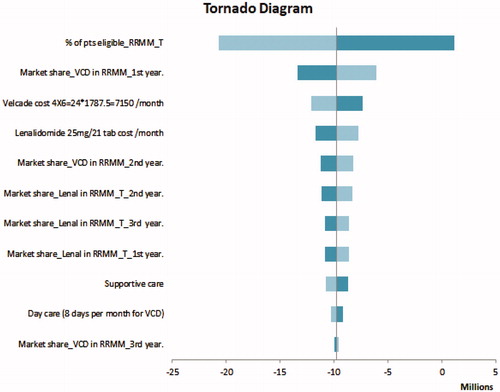
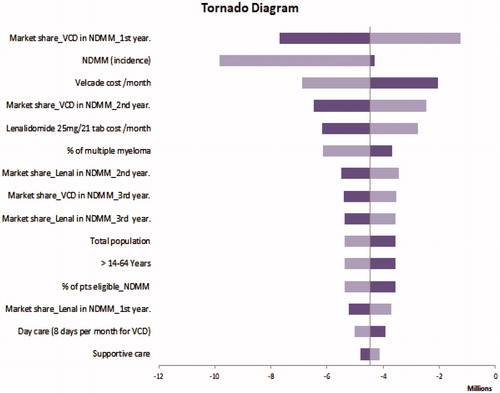
Given the uncertainty of all the parameters for the two regimens, a sensitivity analysis was conducted using plausible ranges in . One-way sensitivity analyses showed that model results were robust to changes in key input parameters. The model results in RRMM ( Tornado Diagram) were most sensitive to variations in patients eligible to transplantation in RRMM. In NDMM, the model results ( Tornado Diagram) were most sensitive to the market share of VCD in the first year.
6. Discussion
This study is the first to report the estimated budget impact of introducing lenalidomide as a treatment option for patients with NDMM and RRMM in Egypt. Budget impact analysis is increasingly important to the comprehensive economic evaluation of new pharmaceutical products. The estimated impact of a new drug on annual pharmacy and medical spending is crucial not only for financial planning but also for anticipating its effect on service provision within the healthcare system.
The total costs of multiple myeloma are important because many factors contribute to the overall costs including healthcare-related costs, management of complications, and hospital admissions, in addition to the cost of therapy. The results of the budget impact analysis suggest that lenalidomide entry is associated with lower costs versus the VCD combination among RRMM and NDMM patients.
A strength of this model is its analysis of the adverse event profiles of both drug combinations. The results highlight a favorable toxicity profile for RD versus VCD. This finding was confirmed in a prior study on the adverse event profile of patients with RRMM with different therapiesCitation16.
The bortezomib related costs of monitoring of adverse events grade 3 and 4 add on to the total cost of treatment such as additional therapy for IV hydration and acyclovir for the management of herpes zoster virus. Our model suggests that RD would not require the same costs associated with VCD complications, specifically those associated with bortezomib. Durie noted that the bortezomib medical and adverse event profile was higher than RD in RRMM by $40 per day which contributed to an annual excess cost of $17,000Citation17.
An additional strength of our study is the use of PFS, OS, and adverse effects data from available randomized clinical trials that may be more relevant than any other source. This is the first study to evaluate the budget impact of lenalidomide in transplant-ineligible NDMM/transplant-eligible RRMS patients within the Egyptian healthcare settings. We also conducted a Delphi panel to fill the important gaps in data needed for the budget impact analysis.
Our results confirm that initiating therapy with lenalidomide in NDMM would result in budget savings. A prior study also demonstrated how lenalidomide-based treatments compared to bortezomib-based treatments had a lower cumulative cost of approximately $120,000Citation5. Lenalidomide also delays the progression of the disease by approximately 5.1 months in NDMMCitation5, which reduces overall cost as demonstrated in our model. That is, newly diagnosed patients initiated on lenalidomide are less likely to receive subsequent therapies within the three year time horizon of the model given that lenalidomide delays the time to progression and thus reducing the overall cost of treatment.
Our results also confirm a significant reduction in medical costs associated with RD treatment. Lenalidomide entry reduces the cost of hospitalization and complications compared to bortezomib. In a prior study by Schey, the cost of hospitalization related to first-line lenalidomide was €867 per patient compared to €1173 per patient for bortezomibCitation5.
Comparable budget savings were presented in another studyCitation18 that evaluated administration charges among RRMM patients on RD or VCD. The study reported budget savings of $10/day/patient for RD versus bortezomib.
Our study is limited by the estimate for the size of the targeted population obtained from a Delphi panel due to a lack of reliable data in Egypt. Another limitation was the use of separate clinical trial data given the lack of head-to-head comparisons. Although the selected trials had similar eligibility criteria, heterogeneity across the trial populations will still exist, which may affect the PFS estimates associated with different treatments.
7. Conclusion
RD treatment is budget-saving over the 3-year time horizon of our budget impact analysis when compared with VCD treatment among RRMM and NDMM patients in Egypt. This study considered all the major cost drivers of treatment in multiple myeloma such as medication cost, hospitalization, and adverse event management. These results provide the necessary evidence for the Egyptian MoH to facilitate decision making in providing lenalidomide as a treatment option.
Transparency
Declaration of Funding
This work was financially supported by Hikma Pharmaceuticals.
Declaration of financial/other relationships
The views expressed in this article are those of the authors only. No other author conflict of interest to declare.
Author contributions
GE collected the data, performed the analysis, and wrote the manuscript. All other authors interpreted the data, revised and edited the manuscript.
Acknowledgements
None reported.
References
- El Husseiny NM, Kasem N, El Azeeim HA, et al. Multiple myeloma: a descriptive study of 217 Egyptian patients. Ann Hematol. 2014;93(1):141–145.
- Moreau P, San Miguel J, Ludwig H, et al. Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(6):vi133–vi137.
- Ibrahim AS, Khaled HM, Mikhail NN, et al. Cancer incidence in Egypt: results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:437971.
- Shustik J, Tay J, Hollmann S, et al. A Canadian cost impact analysis comparing maintenance therapy with bortezomib versus lenalidomide in multiple myeloma patients ineligible for stem cell transplant. Value Health. 2014;17:A1–A295.
- Schey S, Montero LFC, Stengel-Tosetti C, et al. The cost impact of lenalidomide for newly diagnosed multiple myeloma in the EU5. Oncol Ther. 2017;5(1):31–40.
- Weisel K, Doyen C, Dimopoulos M, et al. A systematic literature review and network meta-analysis of treatments for patients with untreated multiple myeloma not eligible for stem cell transplantation. Leuk Lymphoma. 2017;58(1):153–161.
- Kumar M, Panigrahi A, Dolai TK, et al. VTD in newly diagnosed myeloma: an institutional experience. Egypt J Haematol. 2015;40(4):175–176.
- Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147–2152.
- Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498.
- Harousseau J-L, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010;28(30):4621–4629.
- Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma:an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37.
- Central Intelligence Agency. The world fact book. 2018. Fairfax (VA): CIA.
- Elsisi G, Kalo Z, Eldessouki R, et al. Recommendations for reporting pharmacoeconomic evaluations in Egypt. Value Health Reg Issues. 2013;2(2):319–327.
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)-explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250.
- NICE technology appraisal Committee. Lenalidomide for the treatment of multiple myeloma in people who have received at least one prior therapy. NICE technology appraisal guidance; 2009. (TA171).
- Roy A, Kish JK, Bloudek L, et al. Estimating the costs of therapy in patients with relapsed and/or refractory multiple myeloma: a model framework. Am Health Drug Benefits. 2015;8(4):204–215.
- Durie B, Binder G, Pashos C, et al. Total cost comparison in relapsed/refractory multiple myeloma. J Med Econ. 2013;16(5):614–622.
- Durie BG, Moreau P, Sonneveld P, et al. Regional differences in the treatment approaches for relapsed multiple myeloma: an IMF study. J Clin Oncol. 2012;30(15):8095.