Abstract
Background
Rising numbers of patients on the liver transplant waiting list has led to the utilization of organs from higher-risk donors that are more likely to be discarded and are prone to post-transplant complications. Storage and transportation of these livers at low temperatures can cause damage. OrganOx metra is a portable device intended to preserve and maintain the donated liver in normothermic conditions for up to 24 h prior to transplantation.
Objective
To evaluate the cost-utility of normothermic machine perfusion with OrganOx metra in liver transplantation compared to the current practice of static cold storage (SCS).
Methods
A de novo decision analytic model (a decision tree along with a Markov model), based on current treatment pathways, was developed to estimate the costs and outcomes. Results from a randomized clinical trial and national standard sources were used to inform the model. Costs were estimated from the National Health Service and Personal Social Services perspective. Deterministic and probabilistic sensitivity analyses (PSA) were conducted to explore uncertainty surrounding input parameters.
Results
Over a lifetime time horizon, liver transplantation with OrganOx metra was more costly and more effective than the current practice of static cold storage. The total costs per patient were £37,370 vs £46,711, and the total effectiveness per patient was 9.09 QALYs vs 10.27 QALYs for SCS and OrganOx metra groups, respectively. The estimated ICER was £7,876 per each QALY gained. Results from the PSA showed that use of OrganOx metra has 99% probability of being cost-effective at a £20,000 willingness-to-pay threshold. OrganOx metra led to the utilization of 54 additional livers with patients experiencing lower rates of early allograft dysfunction and adverse events.
Conclusions
Use of OrganOx metra for the perfusion and transportation of livers prior to transplantation is a cost-effective strategy.
Introduction of OrganOx metra into NHS could increase the utilisation of donated livers with patients experiencing lower rates of early allograft dysfunction and adverse events, compared with current practice.
Results of the economic analysis indicate that the OrganOx metra is highly likely to be cost-effective and result in improved patient outcomes.
KEY POINTS FOR DECISION MAKERS
Introduction
Liver transplantation is the only effective treatment in patients with end-stage liver disease and should be considered when there is a likelihood of extending patients’ life beyond the predicted natural history of the underlying liver diseaseCitation1. In the UK, the most common liver diseases requiring transplantation are alcohol-related liver disease (30%), non-alcoholic steatohepatitis (18%), primary sclerosing cholangitis (12%), hepatitis C virus infection (10%), and primary biliary cirrhosis (9%)Citation2.
Over the last decade in the UK, the number of organ donors and liver transplants has steadily increased. Despite this increased availability, there was also a 20% increase in the number of patients on the active transplant list in 2019 compared to 2018Citation3. As there are more patients on the active transplant list than there are currently available livers for transplantation, additional sources of donor livers are being explored. These additional sources include organs from living donors, donation after cardiac death (DCD) donors and other, less-than-optimal (‘higher risk’), deceased donors. Use of livers from these ‘higher risk’ or Extended Criteria Donors (ECD) is associated with an increased risk of the organ being discarded due to complications, such as primary non-function (PNF) and early allograft dysfunction (EAD)Citation4.
Despite the many advances in liver transplantation, the current standard method of organ preservation remains static cold storage (SCS). In SCS the liver is flushed and cooled with cold storage solution (e.g. UW solution) then stored in an icebox. This method has limitations; anaerobic activity takes place even at low temperatures, which further damages the liverCitation5. Alternative methods of preservation, such as normothermic machine perfusion (NMP), have gained popularity in recent years as there is evidence that maintaining the physiological temperature during preservation improves post-transplant survival as well as allowing for assessment of organ viability during the preservation periodCitation6.
Several alternative preservation devices exist, including the Organ Assist-Liver Assist (hypothermic and normothermic perfusion), the OrganOx metra (normothermic perfusion), and the Transmedics OCSFootnotei Liver Portable Perfusion System (normothermic perfusion)Citation7.
OrganOx metra is a portable device that is used during liver transplantation to preserve and maintain the donated liver for up to 24 h prior to transplantation. It preserves and maintains the donated liver in a functional state by creating an environment that mimics the physiology of the body by delivering oxygenated blood and nutrients to the liver at normal body temperature (37 °C)Citation8.
A recent systematic review and meta-analysis assessed transplant outcomes between NMP and SCS with the use of OrganOx metra and Liver Assist. The results showed that individuals who received a liver treated with NMP were less likely to develop EAD. Aspartate transaminase (AST) levels were also lower in the NMP group compared to SCS. However, no difference was found in serum bilirubin levels, PNF, and biliary strictures between the groupsCitation4.
The largest randomized controlled trial (RCT) to date evaluating the effectiveness of OrganOx metra compared to SCS showed some very promising results; in 220 liver transplants, NMP was associated with a 50% lower rate of graft injury, a lower rate of organ discard, and a longer mean preservation time. Although no significant differences were observed on graft and patient survival, the findings have major implications for clinical practiceCitation5.
Despite the existence of several devices, only one economic evaluation has been conducted to examine the cost-utility of in-situ normothermic regional perfusion (NRP) compared to standard retrieval in DCD liversCitation9. That analysis showed that, compared to standard DCD retrieval, NRP was less costly and led to better outcomes, in terms of fewer post-transplant complications and better graft survival. However, no economic evaluations have examined the cost-effectiveness of ex-situ NMP compared to SCS. Given the potential benefits of these NMP devices, the aim of this study will be to evaluate whether NMP with OrganOx metra is a cost-effective option for the National Health Service (NHS) compared to the current practice of SCS.
Methods
Model overview
A de novo decision analytic model (a decision tree along with a Markov model) based on the current treatment pathway and available evidence, including the impact that EAD has on long-term mortality, was developed to estimate the costs and outcomes of each strategy over a lifetime time horizon. Outcomes in the model were the total cost of each strategy, number of EAD events, length of stay (LoS) in hospital/Intensive Care Unit (ICU), number of discarded organs, deaths, quality-adjusted life-years (QALYs), and incremental cost per QALY gained. Costs and outcomes beyond a 1-year time horizon were discounted at 3.5%, as recommended by the National Institute for Health and Care Excellence (NICE) reference caseCitation10.
The model was based on a hypothetical cohort of 432 patients with a mean age of 56 years old waiting for a liver transplantation. These values were based on NHS Blood and Transplant (NHSBT) data as of March 2019Citation3 and data from NHS Digital 2017–18. Each patient in the cohort received a liver which had been preserved and perfused by either (1) NMP with OrganOx metra (intervention) or (2) current standard method of SCS (comparator).
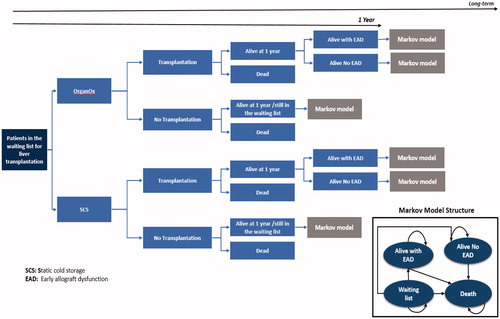
The decision-analytic model comprised of a decision tree followed by a Markov model with four health states (i.e. waiting list, alive, alive with complications, and dead) and yearly cycles. Patients entered the decision tree and were stratified by the preservation method (either NMP or SCS) of the liver intended for transplantation. Individuals had a chance of not receiving a liver based on the discard rates of each strategy; individuals who did not receive a transplant due to organ discard were assumed to either die within the same year or stay on the waiting list. Those who received a transplant had a chance of either dying within the first year after transplantation, staying alive with complications, or staying alive without complications. After the first year following transplantation, patients transitioned to one of the three states of the Markov model where long-term costs and consequences were calculated. The structure of the model is illustrated in .
Model inputs
The majority of clinical inputs were retrieved from a RCT which aimed to test the efficacy of machine perfusion against conventional cold storage in liver transplantation. From the initial 334 randomized livers, 170 were allocated to the NMP arm and 164 to the SCS arm. After application of the exclusion criteria, 137 and 133 livers were left at each arm, respectively. After organ retrieval and following the preservation period, 120 livers in the NMP arm and 100 livers in the SCS arm were available for transplantation; this gives a difference in the discard rate between arms of 12.4%Citation5. Furthermore, post-reperfusion syndrome (PRS) rates and EAD rates were higher in the SCS arm compared to the NMP arm. LoS in hospital/ICU and 1-year survival rates were not statistically different between the two groupsCitation5. The data for the model was supplemented with data from other sources and the Cochrane pyramid of evidence was considered where possible. Further information about the sources for the various model inputs are detailed in the following sections.
Clinical inputs
Clinical inputs for the model were: discard rates, EAD rates, PRS rates, renal replacement therapy (RRT) rates, adverse events (AE) rates, LoS in hospital and ICU postoperatively, and 5-year mortality rates ().
Table 1. Clinical input parameters.
Due to the fact that differences in survival rates within the trial period were not statistically significant, external sources were used to extrapolate survival to the long-term time horizon. NHS BT survival rate for 1- (92%), 2- (89%), and 5-years (79%) post-transplantation were obtained and were assumed to be similar for the NMP and SCS groupsCitation3. Individuals with EAD were assumed to enter the model in the “alive with complications” health state of the Markov model. Various studies have demonstrated that patients who experience EAD post-transplantation have higher mortality rates compared to those without complicationsCitation11–13. Lee et al.Citation11, in a study with 1950 liver transplants, showed that the 1-, 3-, and 5-year survival rates for those who did not and those who did develop EAD were 94.7% vs 85.1%; 87.4% vs 74.8%; and 78.8% vs 65.2%, respectivelyCitation11. The survival rates from the EAD arm were used in our model for those individuals in the “alive with complications” health sate.
Health utility values
In order to estimate the QALYs gained in each strategy, it is necessary to adjust the period of time the average patient is alive in the model by using an appropriate health utility weight. The utilized utility weights were based on a study conducted by Ratcliffe et al.Citation14; this study estimated pre- and post-liver transplantation quality-of-life in patients selected from six liver transplantation centers in England. In total, 455 individuals responded and provided information on their health-related quality-of-life over a period of 24 months; health-related quality-of-life was captured with the EQ-5D and SF-36 tools. In our model, the EQ-5D values of the study were used; utilities beyond the 24 months following transplantation were assumed to be equal to the utility value at 24 months ().
Costs
Costs were estimated from the NHS and Personal Social Services (PSS) perspective. The NHS perspective includes costs of transplantation, as well as follow-up costs including costs of general practitioner (GP) or transplant surgeon visits, costs of medicines, and costs of managing AEs. The following costs were included in the model: cost of the initial intervention (i.e. OrganOx metra), cost of an icebox, cost of stay in the ICU, cost of treating end-stage liver disease, cost of additional days in hospital, cost of transplantation, cost of RRT, cost of medicines for the management of PRS, as well as costs of post-transplantation immunosuppressants. As data from NHS reference costs for 2019 were not available, costs were presented in UK pounds sterling for the 2018 price year and were obtained from the NHS reference costsCitation15, NHS Digital 2017–2018 and the British National FormularyCitation16 ().
Costs of the OrganOx metra device included both the cost of disposables and solutions (£6,000) as well as staff costs (£500). An additional device lease fee of £30,000 per year per each device was also included. The cost of other solutions and post-transplant medications are also listed in . Management of PRS involves administration of vasopressors such as vasopressinCitation5. In the trial by Nasralla et al.Citation5, 33.9% in the NMP and 59.4% in SCS received a vasopressor bolus, while 53.7% of patients in the NMP and 79.2% in the SCS arm received vasopressor infusion. For the management of PRS we assumed that individuals received Argipressin; those requiring bolus received 3 U of Argipressin, while those requiring infusion received 3 U/h for 20 min.
Furthermore, 22.3% of individuals in the NMP arm and 20.8% in the SCS arm required RRT. For those requiring RRT, thrice weekly hemodialysis is necessaryCitation17. For the purposes of this model, we assumed that individuals receive RRT only for the first 6 months after transplantation.
AEs were experienced by a number of individuals in the trialCitation5; 55.4% of patients in the NMP arm and 57.4% in the SCS arm experienced some type of AE which were categorized based on the Clavien-Dindo grading system. In order to incorporate the costs associated with the management of AEs in the model, we used the approach used by Ramsay et al.Citation18 that considered that the costs of each Clavien-Dindo grade were equivalent to the costs of an extra day in the hospital. Thus, those who experienced Clavien-Dindo I AEs experienced one extra day in the hospital, for Clavien-Dindo II AEs two extra days, etc. Due to the fact that the differences in AE rates for some grades were not statistically significant, we only considered those AE grades where the incidence was 1.5-times greater in one grade compared to the other.
Follow-up care post-transplantation is significant since patients require visits to a clinician to check the status of the transplanted graft. We assumed that for the first 2 months after transplantation patients visit a transplant surgeon weekly and for the remaining 10 months of the first year patients have monthly visits. After the first year post-transplantation, the risk of complications is low so we assumed that individuals visit their GP once annually for review. Transplant recipients are at high risk of graft rejection so must take immunosuppressant medications for the rest of their lives. Although various immunosuppressants can be used, we assumed that individuals receive tacrolimus, a calcineurin inhibitor. Guidelines recommend the use of 0.1–0.15 mg/kg per dayCitation19; for the model we assumed that individuals’ weight is approximately 60 kg.
Individuals who did not receive a transplant due to organ discard were assumed to either die within the same year or stay on the waiting list. Gola et al.Citation20 estimated that costs for these individuals is approximately £20,066 per patient.
Analysis
Cumulative estimates of costs and effectiveness were estimated using Monte Carlo simulation (10,000 iterations) for both the intervention and comparator. Deterministic sensitivity analyses were conducted to test the impact of varying the values of key parameters used in the model.
Probabilistic sensitivity analysis (PSA) was performed to map the parameter uncertainty. To conduct the PSA, probabilistic distributions were assigned to each input variable in the model and these were used to randomly select new values within their plausible range. The distributions for each variable are included in . Each new randomly sampled set of values based on these distributions/ranges was used in the model to give a set of results based on the randomly selected input values. This process was repeated for 10,000 iterations and the results of each iteration were recorded to produce a distribution of results from the model. The probability of the intervention being cost-saving describes the percentage of iterations within the PSA where the incremental cost was negative. Similarly, the probability of being cost-effective represents the percentage of iterations within the PSA that the incremental cost-effectiveness ratios (ICERs) fall below the willingness-to-pay (WTP) threshold.
Table 2. Health-related quality-of-life.
Table 3. Cost input parameters.
Results
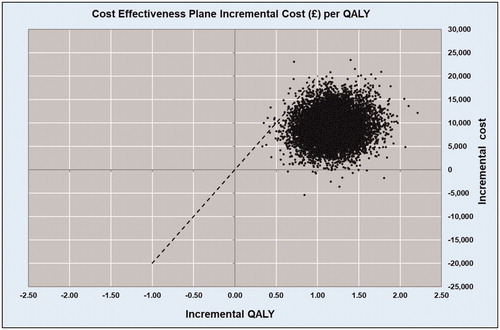
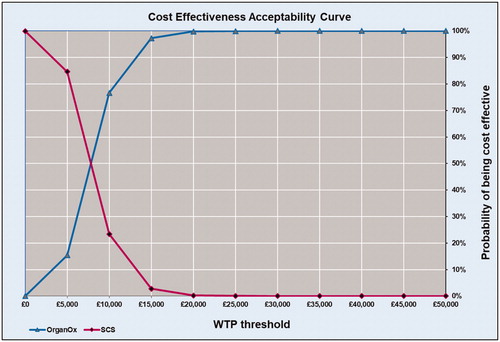
Total and incremental costs, QALYs, ICERs, and probabilities that each strategy is cost-effective at various WTP thresholds are presented in . Probabilistic results from the Monte Carlo simulation, in the form of a cost-effectiveness scatterplot and a cost-effectiveness acceptability curve, are presented in and .
Base case analysis
Over the lifetime time horizon, liver transplantation with grafts which had been preserved and perfused in normothermic conditions with OrganOx metra was more costly and more effective than SCS. The total costs per patient were £37,370 vs £46,711 and the total effectiveness per patient was 9.09 QALYs vs 10.27 QALYs for SCS and OrganOx metra groups, respectively (). The estimated ICER was £7,876 per each QALY gained.
Table 4. Base-case probabilistic results over the time horizon.
Although the total cost of OrganOx metra for the cohort as a whole was higher compared to SCS, with a total cost over the entire time horizon of £20.1 million vs £16.1 million, this was due to the extra available transplantable grafts compared to SCS. Grafts which had been preserved and perfused with OrganOx metra were 50% less likely to be discarded; this resulted in increased availability of transplantable grafts and consequently increased post-transplantation costs (). From the 432 patients which constituted the model’s cohort, 381 patients received a transplant in the intervention group and 328 in the comparator group. These additional 54 livers that were transplanted in the intervention group led to increased transplantation costs, since more patients received transplants, as well as increased costs of ICU stays, RRT, and follow-up care costs. Individuals in the SCS group mainly incurred costs resulting from end stage liver disease and AEs. Furthermore, more EAD events were observed in the SCS group.
Table 5. Base-case deterministic results over the time horizon.
Over the time horizon, OrganOx metra resulted in 653 more life years lived and 512 more QALYs gained for the entire cohort compared to SCS. This translates to approximately 1.51 extra life years and 1.19 QALYs gained per patient. The Monte Carlo simulation showed that the intervention NMP with OrganOx metra has a 99% probability of being cost-effective at a £20,000 WTP threshold ( and ).
Sensitivity analysis
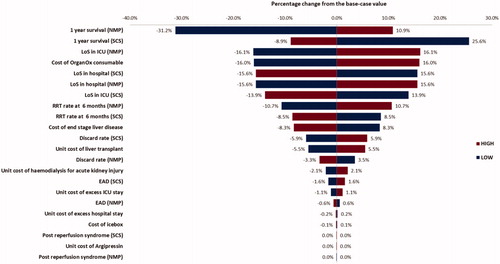
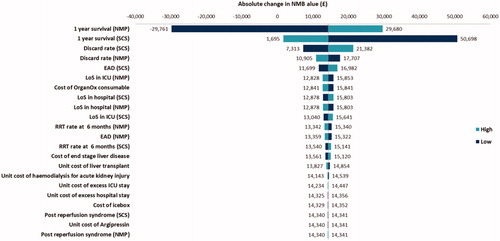
The uncertainty surrounding the parameters was explored through one-way sensitivity analysis by altering each parameter’s value by ±25%. The results from the analyses are presented as a Tornado diagram in and and showed that ±25% changes on the survival rates for both strategies, discard rates for SCS, LoS for OrganOx, and the unit cost of hemodialysis for RRT had the most impact on the estimated cost saving ( and ).
Discussion
To our knowledge, this is the first economic evaluation examining the cost-effectiveness of using ex- situ NMP for liver transplantation. Our results suggest that NMP with OrganOx metra is a cost-effective intervention for liver transplantation when compared to the current standard of SCS.
A similar UK analysis examining the cost-effectiveness of in-situ NRP for liver transplantation showed that NRP was less costly and led to better outcomes including fewer post-transplant complications and survival compared to standard DCD retrievalCitation9. In contrast to our results, the study showed that the cost per surviving patient was lower in the NRP group compared to the standard retrieval group (£33,556 vs £50,586). There are several significant differences between this study and our analysis: the time horizon of the study was only 1 year and costs associated with the management of the patient after transplantation such as the administration of immunosuppressants and follow-up costs were not considered.
Our economic analysis was largely informed by the results from a single clinical studyCitation5 with a sample size of 334 livers. The study showed very promising results with fewer discard, EAD, PRS, and AE rates for the NMP group compared to the SCS group. Other complications such as primary non-function, hepatic artery thrombosis, and ischemic cholangiopathy are quite common in liver transplantation and have a detrimental effect on the outcome of the transplanted liver requiring immediate re-transplantation. In that study, only a few events of these complications occurred, leading to non-statistically significant results; thus these complications as well as the possibility of re-transplantation were not considered in our model. The study on which this analysis was based was not primarily designed to assess discard rate, which emerges as one driver of the ICER. The study was open label and it is therefore possible that the decision to discard a liver was in some part dependent on whether NMP or SCS was being used. However, results from our deterministic sensitivity analysis show that a 25% increase or decrease in discard rate would not have changed the overall conclusion. In an extreme scenario, we also modelled an increase in the discard rate by 50% in the OrganOx metra arm (i.e. from 12% to 18%). This increased the estimated ICER from €7,905 to €11,202 per QALY, still well below any conventional WTP threshold.
Due to the short follow-up period of the trial no statistically significant differences were observed in the survival rates between groups. In order to strengthen our analysis and extrapolate the results to the longer time horizon, we supplemented our model with survival rates from NHSBT dataCitation3 and an additional US studyCitation11. Five-year survival rates were obtained from 397 DCD livers which were analyzed by NHSBT during the period of 2011–2013. The 1-, 2-, and 5-year survival rates were 92%, 89%, and 79%, respectively. Additionally, different survival rates were used for those patients who experienced an EAD event after transplantation as it has been shown that EAD events are associated with worse survival outcomes after liver transplantationCitation11–13.
Our model also considered costs of follow-up care, including the administration of immunosuppressants for the avoidance of graft rejection and visits to specialists and GPs for follow-up after transplantation. However, the choice of the dose for the immunosuppressants depends on various factors including the weight of the patientCitation19. Similarly, the frequency of follow-up visits depends on the health status of the individual after the transplantation and not on a standardized schedule. In our analysis we have used an average number of both of these inputs based on assumptions, though it is possible that these values were overestimated or underestimated. We addressed this limitation by conducting both deterministic and probabilistic sensitivity analyses.
This model explored the cost-effectiveness of the use of NMP of livers using OrganOx metra over a 20-year time horizon. The technology was found to be more expensive and more effective than the current standard of care of SCS. The increased costs of OrganOx metra were mainly due to the costs incurred from the greater availability of transplantable livers, including liver transplantation costs, AE costs, RRT costs, and follow-up care costs.
The results of this analysis demonstrate major implications of the adoption of this technology. DCD livers represent a large source of organs but only half of the available livers are transplanted due to their high-risk nature and possible complicationsCitation3. OrganOx metra has the potential to increase organ utilization from this high-risk group; this would have a significant impact on waiting list mortality since more livers will be available for transplantation.
For this economic evaluation, we adhered to the best practice guidelines recommended by NICECitation23. We conducted a range of probabilistic and deterministic sensitivity analyses to address uncertainty in the inputs that were used in our model. This analysis represents the first evaluation of OrganOx metra and similar portable technologies in the UK context for the transplantation of livers.
Conclusion
OrganOx metra is a portable device which is used during liver transplantation to preserve and maintain the donated liver for up to 24 h prior to transplantation. The device, although more costly than the current standard method of SCS, yields important clinical benefits for patients and society. While this analysis has some limitations, due to a lack of longer follow-up data, it suggests that OrganOx metra could aid in the utilization of high-risk livers and increase the availability of transplantable organs which will have an impact on waiting list mortality.
Transparency
Declaration of funding
This report is independent research funded by OrganOx Company.
Declaration of financial/other interests
MT has no conflicts of interest that are directly relevant to the content of this article. Device Access (MJ, AM, JA, and MBH) received funds from OrganOx Company during the conduct of the study.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed that they are a minor shareholder in Organox and that the transplant center they work at was the highest recruiter into the trial published in Nature, whose data has been used as the basis of this economic assessment. The reviewers have no other relevant financial relationships or otherwise to disclose.
Acknowledgements
None reported.
Notes
i Transmedics OCS is a trademark of TransMedics, Inc., Andover, MA, USA.
References
- EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–485.
- British Liver Trust. The alarming impact of liver disease in the UK: facts and statistics. 2019 [cited 2020 April]. Available from: https://britishlivertrust.org.uk/wp-content/uploads/The-alarming-impact-of-liver-disease-FINAL-June-2019.pdf
- NHS Blood and Transplant. 2019. Organ Donation and Transplantation Activity Report [2018; 2020 April 19]. Available from: https://www.odt.nhs.uk/statistics-and-reports/annual-activity-report/
- Bellini MI, Nozdrin M, Yiu J, et al. Machine perfusion for abdominal organ preservation: a systematic review of kidney and liver human grafts. J Clin Med. 2019;8(8):1221.
- Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557(7703):50–56.
- Brockmann J, Reddy S, Coussios C, et al. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250(1):1–6.
- Akateh C, Beal EW, Whitson BA, et al. Normothermic ex-vivo liver perfusion and the clinical implications for liver transplantation. J Clin Transl Hepatol. 2018;6(3):276–282.
- OrganOx Limited. [cited 2019 October]; Available from: http://www.organox.com/
- Scottish Health Technologies Group. The cost effectiveness of organ retrieval using in situ normothermic regional perfusion (NRP) for liver transplantation. England: The National Institute for Health and Care Excellence; 2019.
- The National Institute for Health and Care Excellence. Guide to the methods of technology appraisal 2013. NICE 2013 [cited March 2019]. Available from: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781
- Lee DD, Croome KP, Shalev JA, et al. Early allograft dysfunction after liver transplantation: an intermediate outcome measure for targeted improvements. Ann Hepatol. 2016;15(1):53–60.
- Ekser B, Mangus R, Fridell J, et al. Impact of Early Allograft Dysfunction (EAD) and its components on patient survival following liver transplantation (LT): long-term outcomes up to 10 years. [abstract]. Am J Transplant. 2016;16(suppl 3). [cited 2020 Aug 14]. Available from: https://atcmeetingabstracts.com/abstract/impact-of-early-allograft-dysfunction-ead-and-its-components-on-patient-survival-following-liver-transplantation-lt-long-term-outcomes-up-to-10-years/
- Guo Z, Zheng D, He X, et al. Impact of early allograft dysfunction on graft and patient outcomes after liver transplantation. [abstract]. Am J Transplant. 2017;17(suppl 3). [cited 2020 Aug 14]. Available from: https://atcmeetingabstracts.com/abstract/impact-of-early-allograft-dysfunction-on-graft-and-patient-outcomes-after-liver-transplantation/
- Ratcliffe J, Longworth L, Young T, et al. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transpl. 2002;8(3):263–270.
- Department of Health and Social Care. NHS Reference costs. 2018 [cited April 2020]; Available from: https://improvement.nhs.uk/resources/reference-costs/
- British National Formulary. 2019 [cited 2019 October]; Available from: https://www.bnf.org/
- NHS. Dialysis. How it’s performed. 2019 [cited October 2019]. Available from: https://www.nhs.uk/conditions/dialysis/what-happens/
- Ramsay C, Pickard R, Robertson C, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess. 2012;16(41):1–313.
- Moini M, Schilsky ML, Tichy EM. Review on immunosuppression in liver transplantation. World J Hepatol. 2015;7(10):1355–1368.
- Gola A, David S, Greenslade L, et al. Economic analysis of costs for patients with end stage liver disease over the last year of life. BMJ. 2015;5:110.
- Bond M, Pitt M, Akoh J, et al. The effectiveness and cost-effectiveness of methods of storing donated kidneys from deceased donors: a systematic review and economic model. Health Technol Assess. 2009;13(38):iii–iv, xi–xiv, 1–156.
- Curtis L, Burns A. Unit Costs of Health and Social Care 2017, Personal Social Services Research Unit, University of Kent, Canterbury. 2017. Available from: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2017/
- NICE. Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence; 2013.