Abstract
Objective
Non-small cell lung cancer (NSCLC) accounts for 80–90% of all lung cancer cases and is usually associated with a poor prognosis. However, targeted therapy with first and second generation tyrosine kinase inhibitors (TKIs) has so far improved progression-free survival of epidermal growth factor receptor (EGFR) mutant NSCLC patients. Osimertinib, a third generation EGFR TKI has recently shown improved overall survival of 6.8 months in previously untreated EGFR mutant NSCLC patients. We assessed the cost-effectiveness of osimertinib versus standard EGFR TKIs (gefitinib or erlotinib) as first-line treatment for advanced or metastatic EGFR mutant NSCLC patients in Singapore.
Methods
A partitioned survival model with three health states (progression-free, progressive disease, and death) was developed from the Singapore healthcare payer perspective. Survival curves based on the overall trial population from the FLAURA trial were extrapolated beyond trial period over a 10-year time horizon to estimate the underlying progression-free survival and overall survival parametric distributions. Health state utilities were derived from the literature and direct costs were sourced from public healthcare institutions in Singapore. Deterministic and probabilistic sensitivity analyses were conducted to explore the impact of uncertainties and assumptions on cost-effectiveness results.
Results
Compared with first or second generation TKI, osimertinib had a base-case incremental cost-effectiveness ratio (ICER) of SG$418,839 (US$304,277) per quality-adjusted life year gained. One-way sensitivity analysis showed the ICER was most sensitive to time horizon and variations in progression-free utility values. Scenario analyses showed that a 50% reduction in the cost of osimertinib was still associated with a high ICER that was unlikely to be deemed cost effective.
Conclusions
Osimertinib is not cost effective as a first-line treatment compared to standard EGFR TKIs in advanced EGFR mutant NSCLC patients in Singapore. The findings from our evaluation, alongside other considerations including the lack of survival benefit in the Asian subgroup of the FLAURA trial, will be useful to inform policy makers on funding decisions for NSCLC treatments in Singapore.
1. Introduction
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer-related mortality worldwideCitation1. In Singapore, lung cancer accounts for approximately 11% of all cancer cases and 22% of cancer-related deathsCitation2. Non-small cell lung cancer (NSCLC) comprises 80–90% of all lung cancer cases, of those a majority are diagnosed with advanced or metastatic diseaseCitation3,Citation4.
The identification of epidermal growth factor receptor (EGFR) sensitizing mutations, which plays an important role in carcinogenesis and progression of lung adenocarcinomas has changed the course of treatment in recent years. While EGFR mutation is observed in 10–20% of white patients, the incidence increases to 45–50% among Asian patientsCitation5.
First and second-generation standard EGFR tyrosine kinase inhibitors (TKIs), such as erlotinib, gefitinib and afatinib have demonstrated better efficacy compared to standard chemotherapy, with a meta-analysis of six randomized trials showing prolonged progression-free survival (PFS) (median PFS: 9.6–13.1 months) compared with chemotherapy (median PFS: 4.6–6.9 months)Citation6. There is however no evidence of an advantage in overall survivalCitation7. In local clinical practice, first and second-generation EGFR TKIs are routinely prescribed for first-line treatment of advanced NSCLC with EGFR mutations, which is in line with the Singapore Cancer Network (SCAN) clinical guidelines for advanced NSCLCCitation8.
Despite this, acquired resistance to EGFR TKIs develops and most patients progress after 9–12 months of treatment. The most common mechanism of acquired resistance is the secondary exon 20 T790M point mutation that occurs between 40 and 60% of the patientsCitation9,Citation10.
Osimertinib, a third generation EGFR TKI, is an irreversible TKI that selectively inhibits both EGFR-sensitizing mutations and EGFR T790M resistanceCitation11,Citation12. In a pooled analysis of two phase II studies, osimertinib demonstrated clinical efficacy among patients who possess T790M mutation after progression from first-line EGFR TKI therapyCitation13,Citation14. A confirmatory phase III trial showed that osimertinib demonstrated an increase in response rate and PFS over platinum-based chemotherapy for the same patient populationCitation15.
A recent landmark phase III trial, the FLAURA trial, compared osimertinib with standard EGFR TKIs erlotinib and gefitinib on treatment-naïve patients with advanced or metastatic NSCLC and EGFR mutationCitation16. As a first line therapy, osimertinib significantly improved PFS compared to standard EGFR TKIs [18.9 months vs. 10.2 months; HR, 0.46; 95% confidence interval (CI), 0.37–0.57; p < 0.001]. Interim data on overall survival (OS) at 25% maturity showed an indication of favouring osimertinib, albeit not reaching statistical significance (HR 0.63). The final analysis for overall survival (median follow-up of 27–35.8 months, 58% maturity), reported a lower hazard ratio of 0.80 (95% CI, 0.64–1.00; p = 0.046), with absolute gain of 6.8 months in favour of osimertinib. An exploratory analysis on post-progression outcomes from the FLAURA trial showed that the PFS benefit for osimertinib continues to persist beyond first disease progressionCitation17. Median second progression-free survival (PFS2) was not reached (95% CI, 23.7 – not calculable (NC)) in the osimertinib treatment arm while it was 20 months (95% CI, 18.2 – NC) for the standard EGFR TKI arm (HR, 0.58; 95% CI 0.44–0.78; p = 0.0004).
Osimertinib was recently granted market approval by the Health Sciences Authority, Singapore, for first line treatment of locally advanced or metastatic NSCLC patients with EGFR mutation. A comparative analysis of effectiveness and costs is nonetheless required to inform drug subsidy funding decisions. Therefore, the purpose of this study is to evaluate the cost-effectiveness of osimertinib compared with standard EGFR TKIs (erlotinib or gefinitib) for first line treatment of locally advanced or metastatic EGFR mutant NSCLC patients in Singapore.
There are no price controls on drugs at the point of marketing authorization in Singapore. Any patients who can afford to pay for a drug have access as soon as it is made commercially available by the company. To improve affordability of treatment, subsidies and financial assistance are provided to eligible patients treated at public healthcare institutions, for drugs listed on the Standard Drug List and the Medication Assistance Fund. Notably, there is no fixed cost-effectiveness threshold in Singapore, as the decision to subsidize a health technology is informed by multiple factors besides cost-effectiveness, such as comparative effectiveness, unmet need and budget impact.
2. Methods
2.1. Model structure and outcomes
2.1.1. Model structure
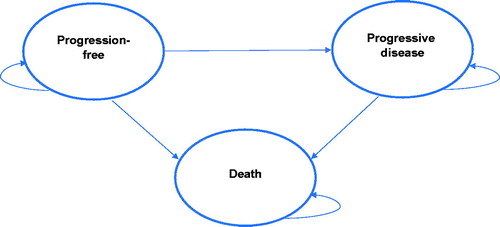
The cost-effectiveness of osimertinib compared with standard EGFR TKIs was assessed using a partitioned survival model (PSM) developed in Microsoft Excel 2013 (Microsoft Corp, Redmond, WA). The model consists of three mutually exclusive health states: progression-free (PF), progressed-disease (PD), and death (). All patients in the model were assumed to enter the model in the PF health state. Because the modelled health states represent the sequence of events patients may undergo during their course of treatment, patients may either transition to the PD or death state, or remain in the PF state after every cycle. Progression to health states from PF to PD or death is irreversible.
Figure 1. Treatment pathway for EGFR-mutated advanced non-small cell lung cancer. Abbreviations. NSCLC, non-small cell lung cancer; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor.
A time horizon of 10 years and a cycle length of 1 week (with a half-cycle correction) were used in the model.
2.1.2. Treatment strategies
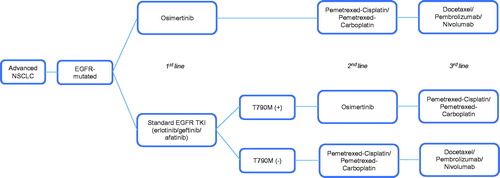
The model compared two treatment arms in a population of locally advanced or metastatic NSCLC patients with EGFR mutation. In the osimertinib arm, patients first receive osimertinib (80 mg daily; first-line), followed by platinum doublet chemotherapy (pemetrexed-cisplatin or pemetrexed-carboplatin; second-line), chemotherapy/immunotherapy (docetaxel, pembrolizumab, or nivolumab; third-line), and then best supportive care (BSC). In the standard EGFR TKI arm, patients receive erlotinib (150 mg daily) or gefinitib (250 mg daily) as first-line treatment. After progression, treatment strategies differed based on T790M status. For T790M-positive patients, they will receive osimertinib (second-line), platinum doublet chemotherapy (pemetrexed-cisplatin or pemetrexed-carboplatin; third-line), followed by BSC. T790M-negative patients will receive platinum doublet chemotherapy (pemetrexed-cisplatin or pemetrexed-carboplatin; second-line), chemotherapy/immunotherapy (docetaxel, pembrolizumab, or nivolumab), and then followed by BSC. The treatment strategies were reflective of the local clinical practice in the management of EGFR mutant NSCLC patients in Singapore, after consultation with local clinical experts. An overview of the treatment algorithm is depicted in .
2.1.3. Outcomes
The analysis was conducted from the Singapore healthcare system perspective. Health outcomes of interest include overall life years (LYs), quality-adjusted life years (QALYs), costs of intervention and comparator treatments, and incremental cost-effectiveness ratios (ICERs). Costs and outcomes were discounted at 3% per annumCitation18.
2.2. Data and sources
2.2.1. Clinical efficacy data
Using results from the FLAURA trial, the area under curve (AUC) was used to derive the mean time patients spent in each health state. To do so, individual patient data (IPD) was recovered by digitizing the Kaplan–Meier (KM) curves of OS and PFS from the FLAURA trial using WebPlotDigitizer developed by RohatgiCitation19. The OS and PFS curves for the overall trial population were used. An algorithm developed by Guyot et al.Citation2 was then applied to reconstruct the IPD. A composite KM curve with parametric extrapolation approach was employed to model the PFS and OS curves for each treatment arm (osimertinib and standard EGFR-TKI). KM data up to 21 months for PFS curves and 52 months for OS curves were used (these time points were selected because the survival probabilities are expected to be statistically uncertain due to the low numbers at risk beyond these time points), followed by fitting various parametric survival distribution (Weibull, exponential, log-normal, log-logistic, Gompertz, and generalized gamma). Using goodness-of-fit data based on Akaike Information Criterion (AIC) value and assessment of clinical plausibility by local clinical experts, Weibull was selected as the parametric survival distribution to model the extrapolation for all survival curves, with the exception of the Gompertz distribution chosen to model OS extrapolation in the standard EGFR-TKI.
The time spent in PD was determined by taking the difference in the AUCs of the PFS and OS curves. Patients who entered the PD health state were assumed to have progressed to second line treatments. The time to discontinuation of study treatment (TDT), PFS2, and time to first subsequent therapy (TFST) curves were extracted by utilising post-progression outcomes data from the FLAURA trialCitation17. The TDT and PFS2, curves were extrapolated using 21 months of KM data and fitted with the selected parametric model based on assessment of clinical plausibility. Time on treatment is based on the modelled TDT curves which were used to inform treatment costs. Duration in second-line treatment was calculated using the differences in AUCs between PFS2 and TFST curves. Time spent in PD beyond second line treatments was calculated by subtracting the duration in second-line treatment from total time spent in PD. A summary of the time spent receiving each line of treatment in the PD health state is shown in .
Table 1. Time spent receiving each treatment during PD health state.
2.2.2. Adverse events
Adverse events (AEs) classified grade ≥3 were included in the model given their potential impact on patients’ quality of life, and this was informed by local clinical experts. The incidence of AEs was sourced from the FLAURA trial.
2.2.3. Costs
Only direct costs were incorporated into the model. These include drug costs, mutation testing, disease management and monitoring, BSC and costs of AEs (). The costs of drugs were estimated from the weighted average of selling price across public health institutions in Singapore.
Table 2. Model inputs (base case).
Tissue biopsy cost was added to all patients who enter the model to account for EGFR mutation testing. Among patients who received first-line standard EGFR TKI therapy, it was assumed that 65% of them, based on local clinician inputs, would progress to receive second-line treatments and thus were subject to T790M testing. Subsequently, patients who were tested as T790M negative (assumed to be 50%) were subject to another tissue biopsy.
Administration fees such as chair time and preparation fees were administered for each cycle of chemotherapy or immunotherapy which was assumed to be of 3 weeks duration. Local oncologists were consulted for frequency of outpatient consultation visits and disease monitoring tests such as computerized tomography-thorax (CT) scan, liver function test, full blood count and renal panel. The associated costs were derived from public healthcare institutions.
Patients entering BSC were assumed to receive care either at home or in hospice centres. Expert opinion was used to assume the distribution of patients across each setting (∼60% in home care; ∼40% in hospice centre).
Cost of grade ≥3 AEs was incorporated into the model only if the AEs require inpatient hospitalization (i.e. interstitial lung disease). Hospitalization costs were sourced from the Ministry of Health’s casemix data which include hospital admission charges and treatment costs from public healthcare hospitals.
2.2.4. Utility values
In the absence of local data, health state utility values were obtained from an international, prospective health-related quality of life (HRQoL) survey using the EQ-5D instrument on patients (mean age 64.7 years) with advanced NSCLC receiving first-, second- or subsequent-line treatments or best supportive careCitation20. The utility values were consistent with an earlier cost-effectiveness analysis evaluating tyrosine kinase inhibitors for metastatic NSCLC in SingaporeCitation21. Utilities were weighted by the proportion of time spent in each health state stratified by treatment arms. Disutilities associated with AEs were sourced from a UK studyCitation22 (). The disutility associated with interstitial lung disease was omitted as the underlying cause could not be definitively determined to be treatment induced or disease related.
2.3. Sensitivity analyses
One-way sensitivity analyses (OWSA) were conducted to explore the impact of uncertain model parameters on the ICER. Each parameter was varied independently from the lower to the upper range of the 95% confidence interval.
A probabilistic sensitivity analysis (PSA) was also performed whereby probabilistic distributions were selected in accordance to the nature of the variable. Survival functions for PFS and OS were sampled from multivariate normal distributions using the Cholesky matrix of the Weibull distribution. In implausible iterations where PFS was greater than OS, PFS was set to be equal to OS. Utility values were assumed to have a beta distribution (continuous distribution confined within interval 0 and 1). 15,000 Monte Carlo simulations were performed to generate ICER outcomes reported in a scatterplot. A cost-effectiveness acceptability curve (CEAC) was also generated to evaluate the probability of osimertinib being cost-effective over a range of willingness-to-pay (WTP) thresholds.
2.4. Additional scenario analyses
Additional scenario analyses were conducted to examine the impact of utilising different parametric distributions for extrapolation of survival curves, utility weights, and pricing adjustments on the base case ICER results. Instead of fitting a Gompertz model on the observed KM data for the OS curve of the standard EGFR TKI arm, a Weibull model was used for extrapolation. A joint fitting model was also explored by applying a Weibull parametric distribution using observed KM data for OS and the whole KM data for PFS. Analysis using the interim data from the FLAURA trial (progression-free median follow up of 9.7–15 months) was also performed as a basis for assessing the predictive performance of survival extrapolations vis-à-vis the observed final OS data.
3. Results
3.1. Base-case analysis
In the base-case with a time horizon of 10 years, treatment with osimertinib was associated with an increased 0.319 QALYs at an incremental cost of SG$133,633. This resulted in an incremental cost per QALY gained of SG$418,839 whilst the incremental cost per LY gained was SG$338,709 (). The model’s base-case analysis projected a mean PF duration of 21.2 months for the osimertinib arm and 12.7 months for standard EGFR TKI (erlotinib or gefitinib) arm. The projected mean duration spent in PD was 19.4 months and 23 months for osimertinib and standard EGFR TKI respectively.
Table 3. Summary of costs and benefits of osimertinib versus standard EGFR TKI, base-case analysis.
3.2. Sensitivity analyses
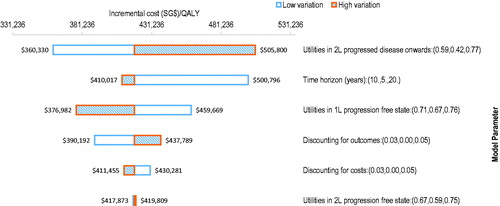
OWSA demonstrated that the ICER was most sensitive to the variations in the utility values for the PF health state during second-line treatment and time horizon of the model (). While an increase in the time horizon from 10 to 20 years only decreased the ICER to SG$409,955 inputting a time horizon of 5 years into the model increased the ICER to SG$500,917. Using a lower variation of the utility value in the second line PD health state lowered the ICER to SG$360,330 while a higher variation of the utility value increased the ICER to SG$505,800. Altering the discounting for costs had little impact on the ICER result.
Figure 3. One-way sensitivity analyses tornado diagram. Abbreviations. QALY, quality-adjusted life-year; 1L, first-line; 2L, second-line.
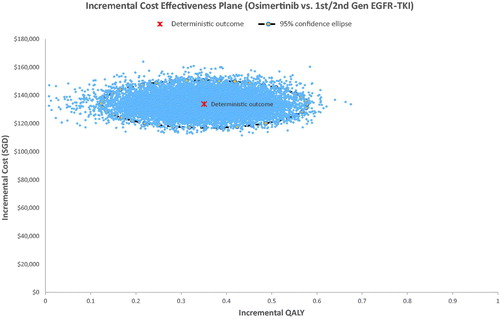
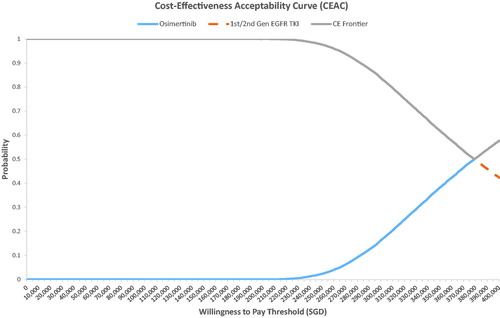
The PSA result was congruent with the base-case analysis, where almost all the simulation results showed that osimertinib was costlier and more effective than the standard EGFR TKI arm (). The probabilistic ICER for osimertinib versus standard EGFR TKI was SG$381,730 per QALY. CEAC showed that osimertinib had a 0% probability of being cost effective at a willingness-to-pay (WTP) threshold of SG$100,000 per QALY and only became the cost-effective option beyond a WTP threshold of SG$3,80,000 per QALY ().
3.3. Additional analyses
The results of additional scenario analyses are summarized in . Applying the Weibull parametric fit to the tail end of the OS KM curve for the standard EGFR TKI generated a higher ICER of SG$517,651 compared to the base-case scenario ICER of SG$418,839. Conversely, applying a joint fitting approach using Weibull produced a lower ICER of SG$337,380. However, none of the scenario analyses generated an ICER of an accepted cost-effectiveness range in the Singapore context. A price reduction of 50% for osimertinib corresponded to an ICER of SG$1,62,013.
Table 4. Summary of costs and benefits in additional scenario analyses.
4. Discussion
In Singapore, targeted therapy with EGFR TKIs is a common first-line treatment for patients with EGFR-mutated advanced or metastatic NSCLC, which is also concordant with international guidelines. Osimertinib, a third generation EGFR TKI has been increasingly used as first-line treatment due to recent findings of increased clinical benefit and improved safety profile compared to previous generation EGFR TKIs. To our best knowledge, this is the first study to evaluate the cost-effectiveness of osimertinib in the first line treatment of advanced and/or metastatic NSCLC in the Singapore setting.
Our analysis showed that osimertinib is not a cost-effective treatment given current prices in the local context. The high ICER for osimertinib compared to standard EGFR TKI was largely driven by the high cost of treatment. The lifetime cost of treatment for each patient in the PF state for osimertinib was SG$207,440 compared to SG$48,039 for standard EGFR TKI. While treatment with osimertinib has resulted in a gain of 0.319 QALYs compared to standard EGFR TKI, the increase is unable to offset the high incremental treatment costs at a level likely to be considered cost-effective.
The large incremental difference in the cost per patient in the PF state was driven by both the increased differential drug cost (cost of osimertinib and standard EGFR TKI per 1-week cycle was SG$2,042 and SG$633 respectively) and by the longer duration of the PF state associated with osimertinib compared with standard EGFR TKI (21.2 months versus 12.7 months). Scenario analyses investigating different pricing scenarios revealed that even a reduction of 50% in the cost of osimertinib only lowered the ICER to SG$1,62,013 which is unlikely to be considered cost effective in the Singapore setting.
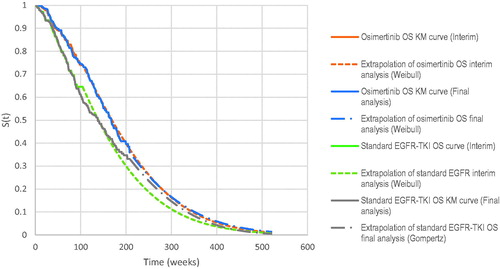
Our results are consistent with other published cost-effectiveness (CE) studies in other countries comparing first-line treatment of osimertinib with standard EGFR TKI in EGFR-mutated advanced and/or metastatic NSCLC patients. Aguiar et al.Citation23 reported ICERs/QALY of at least US$220,000 and US$162,000 in the United States (U.S) Medicare system and Brazilian private health system respectively. Wu et al.Citation24 compared first-line osimertinib with first-generation EGFR TKIs (erlotinib and gefitinib) from a public payer’s perspective in the U.S. and China and showed ICERs/QALY of US$327,601 and US$43,034 respectively. The substantially lower ICER obtained for China was due to the reduced healthcare costs compared to the U.S. A cost-effectiveness study comparing first-line osimertinib with gefitinib and afatinib from a public payer perspective in Canada obtained an ICER/QALY of $223,123 CADCitation25. Most of these CE studies, however, have used the OS and PFS curves from the interim analysis of FLAURA. Nonetheless, using the same interim data, our analysis generated a comparable ICER of SG$373,768 per QALY, which is about SG$45,000 lower than current base-case. A common finding among these published studies was that the high ICERs were due in large part to the relative high drug cost of osimertinib, which was in line with our study results. Our base-case leveraged on the updated final OS data, with extrapolation predicting a 5-year OS of 24% and 22% for the osimertinib and standard EGFR-TKI arms respectively. With the earlier data cut off, the extrapolated 5-year OS for osimertinib was similar but the survival for patients on standard EGFR-TKI was worse at 17% (). In other words, projections from the more mature data suggest a convergence in the survival outcome between the standard EGFR-TKI arm and osimertinib arm over time, leading to a reduction in the magnitude of the OS benefits conferred by osimertinib. This was concordant to the observed KM data in the final data analyses for FLAURA which showed an appreciably higher hazard ratio of 0.80 (95% CI 0.64–1.00) compared to the interim dataset’s hazard ratio of 0.63 (95% CI 0.45–0.88).
Figure 6. Kaplan–Meier and extrapolated curves for overall survival. Abbreviations. EGFR, epidermal growth factor; TKI, tyrosine kinase inhibitor.
By varying input parameters, OWSA was carried out to assess key drivers of the model. Our analysis showed that the model was most sensitive to the time horizon, albeit only to the lower variation of the parameter which was set at 5 years. Our base case time horizon was 10 years, which was consistent with other published CEAsCitation23–25 and also allowed for all QALYs to be fully captured. Shortening the time horizon to 5 years resulted in an increased ICER of SG$500,917.
Another key driver of the model was the utility values used in the PF state, which were extracted from published literature due to the absence of local utility data for advanced and/or metastatic NSCLC. Moreover, there was a dearth of published data on Asian-specific utility weights for the different health states during NSCLC disease progression. We thus adapted utility values from a multinational HRQoL survey on advanced NSCLC patients, and applied AE-associated disutility values sourced from a UK study. Although extracted from another published studyCitation26, utility values in the PF state were also one of the key drivers in models of similar published cost-effectiveness studiesCitation24.
Scenario analyses showed that when using the joint fitting approach where the shape parameter for both treatment arms was jointly estimated, the resultant ICER/QALY was reduced to SG$337,380. The lower ICER value was primarily driven by the increased OS benefit from osimertinib treatment. The hazard ratio applied was assumed to hold true for the entire duration of the time horizon. Such an assumption contributes to higher uncertainty associated with the resultant ICER, especially in light of FLAURA’s final data set suggesting a converging trend in the OS benefits between osimertinib and the standard EGFR-TKI arms, challenging the constant proportional hazards assumption.
The results of our economic model must be interpreted within the context of our study limitations. Firstly, the published HRQoL studies where we extracted our utility data from were not representative of the Singapore population as the study populations were predominantly from western countries. The HRQoL study by Chouaid et al.Citation20 is a multinational study in hospitals across Europe, Canada, Australia, and Turkey, while Nafees et al.Citation22 published a UK HRQoL study which had 9% Asian representation. Although Nafees et al.Citation26 later published an international study on NSCLC health state utilities that included Asian countries such as China, Taiwan, and South Korea, the cultural differences from physician-patient interactions in those settings resulted in utility values that were not representative of our NSCLC population in Singapore.
Secondly, because we did not have access to individual patient data from the FLAURA trial, digitization of the published survival curves was utilized to reconstruct survival data. Extrapolation of the survival data was required to estimate OS and PFS curves over a 10-year time horizon. We had to examine the variability of extrapolated survival curves from candidate parametric functions, namely Weibull, exponential, log-normal, log-logistic, Gompertz, and generalized gamma distributions. For OS, we selected Weibull and Gompertz (for the osimertinib and standard EGFR TKI curves respectively) as our base case after assessing the parametric functions’ goodness-of-fit using AIC, visual inspection of the curves against actual empirical data, and local clinical expert opinion.
Thirdly, we did not incorporate cost data for significant AEs that did not require hospitalization, which in this case were paronychia and rash that would require treatment in an outpatient setting. Although proportions of patients who had paronychia were similar across treatment arms, there were more patients in the standard EGFR TKI arm who reported having grade ≥3 rash in the FLAURA trial. Excluding the cost of dermatology consultations may have overestimated the ICER. However, given the low proportion of reported grade ≥3 rash and local clinical expert opinion that only half of those would seek outpatient treatment with a dermatologist, we expect the omission of these cost data to be negligible.
Further limitations stemmed from the nature of cost-effectiveness analysis, which carries inevitable assumptions of certain homogenous transition probabilities that deviate from the heterogeneous dynamics of the real world setting. In this context, we assumed that all patients who entered the model underwent tissue biopsy, all patients who progressed to second-line treatment in the standard EGFR TKI arm underwent T790M testing, and all patients who tested negative in T790M testing had undergone repeat tissue biopsy. In addition, we did not take into account biopsy complication rates, which we assumed to be uncommon. Our proposed treatment algorithm, although rigid, represented the most realistic treatment pathway for EGFR-mutated advanced NSCLC patients in the local clinical setting. Lastly, the analysis was carried out using data from the overall trial population and not the Asian subgroup, to which the results would have been more generalizable to Singapore’s context. The reason for this was that only the OS, and not the PFS curves, for the Asian subgroup were available from the published final data set. Hence for consistency, the model only utilized data from the entire trial population. The final OS curves for the Asian subgroup revealed no statistically significant survival benefits for osimertinib [hazard ratio of 1.00 (95% CI 0.75–1.32)]Citation27 and would likely have increased the base-case ICER substantially owing to the expected lower QALY gains, if any, in comparison to the incremental QALYs estimated in the main population.
5. Conclusion
Osimertinib is not considered cost-effective for first line treatment of locally advanced or metastatic EGFR mutant NSCLC patients in the Singapore setting due to its high cost of treatment. Based on our analysis, other older (first or second generation) EGFR TKIs should remain the preferred choice as first line, given their lower cost and the lack of OS benefit with osimertinib in the Asian subgroup as reported in the updated final analyses of the FLAURA trial. The study findings should be supplemented with other considerations such as comparative safety and affordability to inform policy makers on funding decisions concerning NSCLC treatments in Singapore.
Transparency
Declaration of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of financial/other relationships
All authors have no relevant financial or other relationships to disclose.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Authors contributions
All authors have made substantial contributions to the development of the manuscript and have approved the final version submitted.
Acknowledgements
Not applicable.
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953.
- Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol. 2012;12:9.
- Planchard D, Popat S, Kerr K, ESMO Guidelines Committee, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237.
- Besse B, Adjei A, Baas P, ESMO, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. 2014;25:1475–1484.
- Dearden S, Stevens J, Wu YL, et al. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol. 2013;24:2371–2376.
- Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Cin Oncol. 2015;33:1958–1965.
- Lee CK, Davies L, Wu YL, et al. Gefitinib or erlotinib vs chemotherapy for EGFR mutation-positive lung cancer: individual patient data meta-analysis of overall survival. J Natl Cancer Inst. 2017;109.
- Singapore Lung Cancer Workgroup. Singapore Cancer Network (SCAN) guidelines for the use of systemic therapy in advanced non-small cell lung cancer. Ann Acad Med Singapore. 2015;44:449–462.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247.
- Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377:849–861.
- Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061.
- Bollinger MK, Agnew AS, Mascara GP. Osimertinib: a third-generation tyrosine kinase inhibitor for treatment of epidermal growth factor receptor-mutated non-small cell lung cancer with the acquired Thr790Met mutation. J Oncol Pharm Pract. 2018;24:379–388.
- Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study phase II extension component. J Cin Oncol. 2017;35:1288–1296.
- Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–1652.
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung Cancer. N Engl J Med. 2018;378:113–125.
- Planchard D, Boyer MJ, Lee JS, et al. Postprogression outcomes for osimertinib versus standard-of-care EGFR-TKI in patients with previously untreated EGFR-mutated advanced non-small cell lung cancer. Clin Cancer Res. 2019; 25:2058–2063.
- Agency for Care Effectiveness. Drug evaluation methods and process guide. 2019 [updated December 2019]. Available from: www.ace-hta.gov.sg/our-process-and-methods.html#health-technology
- Rohatgi A. WebPlotDigitizer Version 4.1 [cited 2018 Dec 20]. Available from: https://automeris.io/WebPlotDigitizer/
- Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8:997–1003.
- Tan P-T, Aziz MIA, Pearce F, et al. Cost effectiveness analysis of afatinib versus pemetrexed-cisplatin for first-line treatment of locally advanced or metastatic EGFR mutation positive non-small-cell lung cancer from the Singapore healthcare payer’s perspective. BMC Cancer. 2018;18:352.
- Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84.
- Aguiar PN Jr, Haaland B, Park W, et al. Cost-effectiveness of osimertinib in the first-line treatment of patients with EGFR-mutated advanced non-small cell lung cancer. JAMA Oncol. 2018;4:1080–1084.
- Wu B, Gu X, Zhang Q, et al. Cost-effectiveness of osimertinib in treating newly diagnosed, advanced EGFR-mutation-positive non-small cell lung cancer. Oncologist. 2019;24:349–357.
- Ezeife DA, Kirk V, Chew DS, et al. Economic analysis of osimertinib in previously untreated EGFR-mutant advanced non-small cell lung cancer in Canada. Lung Cancer. 2018;125:1–7.
- Nafees B, Lloyd AJ, Dewilde S, et al. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13:e195–e203.
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50.