Abstract
Objective
To compare the hospital length of stay (LOS) between rivaroxaban and warfarin in hospitalized acute stroke patients with non-valvular atrial fibrillation (NVAF) in Japan.
Methods
This was a retrospective, observational study using a Japanese hospital claims database. Data of NVAF patients who were started on oral anticoagulant (OAC) treatment during hospitalization were extracted and LOS-OAC (period from the initiation of index OAC therapy to the end of hospitalization or censoring date) and medical costs were compared between rivaroxaban and warfarin treatments. To compare LOS-OAC, a time-to-event analysis was performed using the Kaplan–Meier method. The analysis period was from April 2012 to December 2015.
Results
This study included 773 rivaroxaban users and 1077 warfarin users. After the propensity score matching, 546 patients for each treatment constituted the matched cohorts. Although the rivaroxaban users had a similar LOS-OAC to warfarin users (median, 18 vs. 19 days, p = .657) in the matched cohorts, 3 days shorter LOS-OAC was observed in the rivaroxaban users (median, 17 vs. 20 days, p = .043) after IPTW adjustment. Subgroup analysis by the severity of stroke after IPTW adjustment demonstrated that rivaroxaban users had a shorter LOS-OAC than warfarin users among patients with mild (median, 10 vs. 14 days) and moderate stroke severity (22 vs. 27 days), but not among those with severe stroke severity (26 vs. 25 days).
Limitations
It is not possible to say that the only confounder was stroke severity and therefore other possible known and unknown confounders could not be ruled out.
Conclusions
The rivaroxaban users had a 3-day shorter LOS-OAC after IPTW-adjustment. Using rivaroxaban was associated with 4–5 days shorter LOS-OAC than using warfarin in patients with mild or moderate stroke, though treatment selection did not have a large impact in patients with severe stroke.
Introduction
The prevalence of atrial fibrillation (AF) increases as people age. In Japan, the estimated number of patients with AF was 0.72 million in 2005, which is projected to exceed 1 million in 2030 with the aging of the populationCitation1. AF is a well-known important risk factor for stroke, particularly severe or fatal stroke and its recurrenceCitation2–5.
Non-vitamin K antagonist OACs (NOACs), also known as target-specific OACs, directly inhibit the activity of thrombin (dabigatran) or coagulation factor Xa (rivaroxaban, apixaban, and edoxaban). NOACs have several advantages over warfarin, a common vitamin K antagonist, such as fewer drug-drug interactions, no major dietary restrictions, and a lower intracranial bleeding riskCitation6. Thus, NOACs have come to be widely used in patients with non-valvular AF (NVAF) for stroke prevention as an alternative to warfarin. The use of NOACs in NVAF patients has also been increasing in Japan, with a gradual decrease in the use of warfarinCitation7. Indeed, the Japanese guidelines for the management of stroke 2015 (Supplement 2017) recommend the preferential use of NOACs because of fewer serious hemorrhagic complicationsCitation8.
Outside Japan, several studies have reported that the use of NOACs is beneficial also from a health economic perspective, showing that anticoagulation therapy with NOACs in hospitalized NVAF patients resulted in shorter hospital length of stay (LOS)Citation9–11 and lower healthcare costsCitation12,Citation13 than with warfarin. These findings may be explained by NOACs’ rapid onset of action and their lack of the requirement for routine prothrombin time-international normalized ratio (PT-INR) monitoring. Routine PT-INR monitoring is required for warfarin to ensure adequate anticoagulationCitation6,Citation14. When NVAF patients are hospitalized for acute ischemic stroke, the selection of OACs may be one of the factors affecting their LOS.
Ever-increasing healthcare expenditure is a serious social issue in present-day JapanCitation15, and the burden will further increase with the rapid aging of the population. AF is considered one of the diseases that contribute to this important problem. From this perspective, a single-center study from Japan previously reported that anticoagulant therapy with NOACs in NVAF patients hospitalized for cardiogenic embolism resulted in shorter LOS and lower medical costs than that with warfarinCitation16. Another study reported that initiating dabigatran therapy in hospitalized NVAF patients resulted in a 2.5-day-shorter LOS than warfarin therapyCitation17. However, reports about NOACs from a health economic perspective are still rare in Japan, and to the best of our knowledge, no study has specifically examined the health economic benefits of rivaroxaban compared with warfarin. Therefore, in this study, LOS of hospitalized NVAF patients was compared between patients receiving rivaroxaban and those receiving warfarin using a large-scale hospital claims database in Japan.
Methods
Study design
This was a retrospective, observational hypothesis-generation study to compare the LOS of OAC-naïve NVAF patients between those who started rivaroxaban treatment and those who started warfarin treatment after hospitalization for acute ischemic stroke. The Medical Data Vision (MDV; Tokyo, Japan) database on health claims data and administrative data of 22 million patients in 338 hospitals in Japan were analyzed in this study. This study was conducted based exclusively on anonymized patient claims data without any patient recruitment, and thus it involved no violation of patients’ privacy. The study was conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by Health Outcome Research Institute, Chiba Japan on March 19, 2018.
Data source and the Japanese health-care system
The MDV database is based on health claims data and administrative data of Japanese hospitals that participate in the diagnostic procedure combination/per-diem payment system (DPC/PDPS). In these DPC hospitals, hospitalization costs are reimbursed based on a combination of the DPC/PDPS and a fee-for-service payment system, with the former being applied to items such as basic hospitalization fees, laboratory tests, diagnostic imaging, and medications (with some exceptions) and the latter to other items such as surgery, anesthesia, and rehabilitationCitation18. The database includes both inpatient and outpatient data, including patient demographics (age, sex), diagnoses, prescriptions, medical procedures, tests performed, department visited, and medical costs (costs calculated based on a fee-for-service payment system only). Laboratory test results are also available for patients from a limited number of hospitals.
The MDV database includes data of over 22 million people from 338 DPC hospitals, covering approximately 20% of all DPC hospitals in Japan. The distribution of demographic characteristics in this database is similar to the general Japanese population. The validity of this database for epidemiological research in NVAF has been demonstratedCitation19, and it was used in recent studiesCitation17,Citation20.
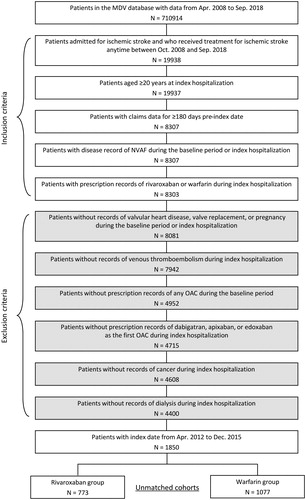
Patient extraction
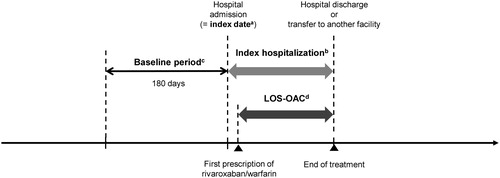
Patients with a diagnosis record of ischemic stroke and also with a record of any of ischemic stroke treatments at hospitalization between April 2012 and December 2015 were identified: (1) rt-PA or endovascular therapy on the first day of index hospitalization (=index date), or (2) medications (urokinase, heparin, argatroban, ozagrel sodium, low-molecular weight dextran, glyceol, mannitol, or edaravone) or surgery for ischemic stroke within the first to the third day of the index hospitalization. The first hospitalization during the study period was defined as the index hospitalization. Patients had to be 20 years or older at the index date, have data for ≥180 days pre-index date (=baseline period), have a disease record of NVAF during the index hospitalization or the 180-day pre-index period, and have a prescription record of rivaroxaban or warfarin during the index hospitalization (Supplementary Table 1) ().
Figure 1. Study timeframe and definitions of periods examined. aIndex date is the first day of index hospitalization. Patients with an index date and discharge date within the analysis period (Apr 2012 to Dec 2015) were analyzed in this study. bBaseline period is defined as the 180-day pre-index period. cLOS-OAC is defined as the period from the initiation of index OAC therapy to the end of hospitalization or to the censoring date. LOS-OAC is censored at discontinuation of rivaroxaban/warfarin, switching to another OAC, death, transfer without data for its destination, or when a ≥8-day treatment gap cannot not be confirmed. LOS-OAC: length of stay-oral anticoagulant.
Patients were excluded if they had a record of: (1) valvular heart disease during the baseline period or the index hospitalization; (2) valve replacement, venous thromboembolism, pregnancy, cancer, or dialysis during the index hospitalization; (3) any OAC prescription during the baseline period; (4) dabigatran, apixaban, or edoxaban as the first OAC prescription during the index hospitalization; or (5) warfarin or rivaroxaban with any other anticoagulant as the first anticoagulant prescription during the index hospitalization.
Stroke severity index (SSI)
The SSI is a proxy indicator for stroke severity at admission in patients hospitalized for acute ischemic stroke based on information available from patient claims data such as care and procedures given to these patients during hospitalizationCitation21. The SSI was developed to address the problem of stroke outcome studies using a claims database, which often lack severity data. This severity index has been externally validatedCitation21 by its correlations with the National Institutes of Health Stroke Scale (NIHSS)Citation22, an international assessment tool for clinical severity in patients with acute stroke. In the present study, the SSI was calculated using data during stroke treatment (defined as the period from the index date to the first record of rivaroxaban or warfarin prescription) for items corresponding to the following seven predictors (respective weights are shown in parentheses) and a constant (9.6804): airway suctioning (3.5083); bacterial sensitivity test (1.3642); general ward stay (−5.5761); intensive care unitICU stay (4.1770); nasogastric intubation (4.5809); osmotherapy (2.1448); and urinary catheterization (1.6569). The SSI is interpreted as follows: mild, SSI ≤5; moderate, 5< SSI ≤12; and severe, SSI >12Citation23.
Study outcomes
The primary outcome was the hospital LOS, defined as the period from the initiation of index OAC therapy to the end of hospitalization (LOS-OAC). The secondary outcomes were: (1) time from the index date to the first date of rivaroxaban or warfarin prescription; (2) fee-for-service-based costs incurred during the LOS-OAC; (3) location after discharge; and (4) modified Rankin Scale (mRS) at discharge.
Statistical analysis
To compare the LOS-OAC between rivaroxaban and warfarin group, a time-to-event analysis was performed using the Kaplan–Meier method. The difference in the time-to-event distribution between those groups was assessed using the log-rank test.
To account for potential confounding covariates for patients treated by rivaroxaban or warfarin, two propensity score methods were used: propensity score matching and inverse probability of treatment weighting (IPTW). Propensity scores were calculated using a multivariate logistic regression model that incorporated various baseline patient characteristics, including stroke severity based on SSI. Propensity score matching was performed using 1:1 greedy matching with a ± 0.2 caliper. IPTW weights were used to adjust Kaplan–Meier curves and Cox proportional hazard models in IPTW analysis. Standardized differences for patient characteristics were calculated to assess the balance of these characteristics between groups. Characteristics with a standardized difference <10% were considered to be well balancedCitation24.
Then, subgroup analysis according to the SSI category (mild, moderate, severe) was performed between the treatment cohorts after adjustment by IPTW method as we suspected the presence of heterogeneity among subgroups with different severity level. Additionally, we explored the associations of other factors on LOS-OAC using a multivariate Cox proportional hazard model stratified by SSI.
Secondary outcomes were descriptively summarized in the unmatched cohorts.
In addition, for exploratory purposes, LOS-OAC was also analyzed stratified by hospital bed number and location after discharge.
The significance level was set at p < .05 (two-sided), but note that the analyses were not intended for hypothesis testing. Analyses were performed using SAS Release 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.4.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients’ characteristics
A total of 1850 patients were identified, of whom 773 rivaroxaban users and 1077 warfarin users constituted (). After the propensity score matching, a total of 1092 patients constituted the matched cohorts (546 patients for each treatment).
Figure 2. Patient extraction from the database. NVAF: non-valvular atrial fibrillation; OAC: oral anticoagulant.
The patient characteristics of the unmatched cohorts and matched cohorts are summarized in . In the unmatched cohorts, the rivaroxaban group contained slightly fewer elderly patients aged ≥75 years than the warfarin group (70.0 vs. 79.4%). The rivaroxaban group had a one-point lower median score for the modified CHADS2 (2 vs. 3), and had a three-point lower median SSI than the warfarin group (6.3 vs. 9.3). In both groups, about 20% of patients had a history of stroke, but there were fewer patients with a history of congestive heart failure in the rivaroxaban group (30.7 vs. 41.8%). For the treatment of stroke leading to the index hospitalization, 80.1% of patients in the rivaroxaban group were on edavarone, in contrast to 60.2% in the warfarin group. Whilst, between the matched cohorts, patient characteristics were below 10% for all factors with a standardized difference; after matching, the median age was 81 years, and about 75% were ≥75 years in both cohorts. Both cohorts had a median score of 2 for the modified CHADS2, and a median SSI of 7.9 (moderate stroke severity). However, more importantly, the distribution of stroke severity (SSI) in each cohort was altered by propensity score matching although most of patient characteristics were not affected, and the matched population presented a higher degree of severity (SSI) for rivaroxaban group (Supplementary Table 2).
Table 1. Baseline characteristics of patients in the unmatched and matched cohorts.
Primary outcome
In the unmatched cohorts, patients receiving rivaroxaban had a 5-day shorter LOS-OAC than those receiving warfarin (median [95% CI], 16 [15 − 18] days vs. 21 [20 − 23] days, p < .0001; ). Although patients receiving rivaroxaban had a similar LOS-OAC to those receiving warfarin (18 [16 − 21] days vs. 19 [17 − 21] days, p = .657; ) in the matched cohorts, a 3-day shorter LOS-OAC (17 [17 − 19] days vs. 20 [19 − 21] days, p = .043; ) for rivaroxaban was observed after IPTW adjustment.
Figure 3. Kaplan–Meier curves for time to the end of LOS-OAC by treatment group. (a) Unmatched cohort. Median (95% CI) LOS-OAC in rivaroxaban vs. warfarin users: 16.0 (15.0‒18.0) days vs. 21.0 (20.0‒23.0); p < .0001, (b) Matched cohort. Median (95% CI) LOS-OAC in rivaroxaban vs. warfarin users: 18.0 (16.0‒21.0) days vs. 19.0 (17.0‒21.0); p = .657, (c) IPTW adjustment. Median (95% CI) LOS-OAC in rivaroxaban vs. warfarin users: 17.0 (17.0‒19.0) days vs. 20.0 (19.0‒21.0); p = .043. LOS-OAC: length of stay-oral anticoagulant.
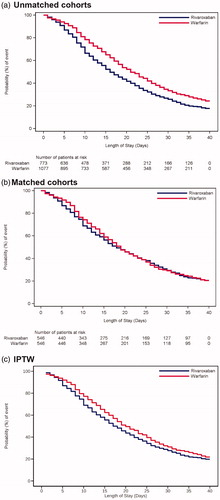
The results of LOS-OAC in the unmatched cohorts and after IPTW adjustment were similar whilst the result in the matched cohorts was different from the others. The most plausible cause of the difference observed was the altered distribution of stroke severity (SSI) in each matched cohort by propensity score matching. In the matched cohorts, the specific population was excluded and the matched population presented a higher degree of severity (SSI) for rivaroxaban group. Hence, considering the generalizability, adjustment by IPTW was adopted in the primary analysis in this study.
In addition, among factors considered in SSI, airway suctioning, ICU stay and nasogastric intubation were expected to be important confounders associated with both drug choice in this study and LOS, as patients with those factors cannot administer drugs orally and tend to be moved to long-term care beds after discharge of the index hospitalization. In terms of grinding process of the tablet and the drug cost, those patients are likely to be treated with warfarin. Hence, to address potential heterogeneity by SSI, subgroup analysis based on the SSI after IPTW adjustment was performed.
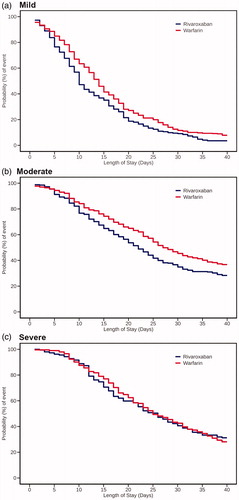
The result of the subgroup analysis based on the SSI to assess the association between LoS and anticoagulant therapy after adjustment with IPTW was shown in . In patients with mild stroke (SSI ≤ 5), rivaroxaban users had a 4-day shorter LOS-OAC than warfarin users (median [95% CI], 10 [10‒11] days vs. 14 [13‒14] days). Patients with moderate stroke (5< SSI ≤ 12) had longer LOS-OAC than those with mild stroke, with rivaroxaban users having a 5-day shorter LOS-OAC than warfarin users (22 [20‒24] days vs. 27 [26‒30] days). Patients with severe stroke (SSI > 12) again had longer LOS-OAC than those with mild stroke; however, the LOS-OAC did not differ between rivaroxaban and warfarin users (26 [23‒28] days vs. 25 [23‒29] days).
Figure 4. Kaplan–Meier curves for time to the end of LOS-OAC in each treatment group stratified by stroke severity. Stroke severity was stratified according to the SSI as follows: mild, SSI ≤5; moderate, 5< SSI ≤12; and severe, SSI >12. Median (95% CI) LOS-OAC in rivaroxaban vs. warfarin users: 10.0 (10.0‒11.0) vs. 14.0 (13.0‒14.0) days for mild stroke; 22.0 (20.0‒24.0) vs. 27.0 (26.0‒30.0) days for moderate stroke; and 26.0 (23.0‒28.0) vs. 25.0 (23.0‒29.0) days for severe stroke. LOS-OAC: length of stay-oral anticoagulant; SSI: stroke severity index.
To explore the association of other factors with LOS-OAC, a multivariate stratified regression analysis by SSI category was performed, using LOS-OAC as the outcome and variables with p < .05 on univariate analysis as explanatory variables (). In patients with mild stroke, rivaroxaban was significantly associated with shorter LOS-OAC (adjusted HR: 1.30, 95% CI: 1.09‒1.55), and age ≥75 years (0.69, 95% CI: 0.48‒0.98) and modified CHA2DS2-VASc score ≥2 (high stroke risk) (0.62, 95% CI: 0.41‒0.94) were associated with longer LOS-OAC. In patients with moderate stroke, rivaroxaban (adjusted HR: 1.23, 95% CI: 1.01‒1.51) and hospital with large number of beds (vs. <200 beds) were associated with shorter LOS-OAC (2.06, 95% CI: 1.20‒3.55 for 200‒499 beds; 2.40, 95% CI: 1.38‒4.17 for ≥500 beds). In patients with severe stroke, no variables were significantly associated with LOS-OAC.
Table 2. Hazard ratios for LOS-OAC in the univariate Cox proportional hazard model and multivariate regression analysis stratified by stroke severity.
For exploratory purposes, LOS-OAC was analyzed stratified by hospital bed number and location after discharge (). Patients at hospitals with more beds had shorter LOS-OAC (median, 17 days for ≥500 beds, 20 days for 200‒499 beds, and 26 days for <200 beds). Patients who were discharged home or to outpatient care had a 9-day shorter LOS-OAC compared with those transferred to another institution or department (median, 12 vs. 21 days).
Table 3. LOS-OAC stratified by hospital bed number and location after discharge.
Secondary outcomes
summarizes the results of secondary outcome analyses. Time from index date to the initiation of rivaroxaban or warfarin treatment was similar between the two groups with a median length of 4 days for both. The median costs during the LOS-OAC were about JPY94,000 lower for rivaroxaban users than for warfarin users. The distribution of locations after discharge was similar between rivaroxaban and warfarin users; almost half of patients were transferred to outpatient care (50.6 and 43.6%), and about 40% were transferred to another institution. For functional status at discharge, there were slightly fewer rivaroxaban users with severe disability (15.7 vs. 19.6%).
Table 4. Time to treatment initiation, medical costs, location after discharge, and functional status at discharge (in unmatched cohorts).
For exploratory purposes, location after discharge was examined stratified by hospital bed number (Supplementary Table 3). Irrespective of hospital bed number, around 30% of patients were transferred to outpatient care at the same hospital. The proportions of patients who were transferred to another institution were much larger in hospitals with ≥200 beds than in those with <200 beds (around 40% for both 200‒499 beds and ≥500 beds vs. 17.2% for <200 beds).
Discussion
To the best of our knowledge, this was the first study to compare hospital LOS in NVAF patients between rivaroxaban and warfarin admitted for acute ischemic stroke in real-world clinical settings in Japan.
In the unmatched cohorts, the rivaroxaban group had a smaller proportion of elderly patients aged ≥75 years, lower median CHADS2 score, and lower median SSI compared with those treated with warfarin, which presumably reflected the treatment selection bias in actual clinical settingsCitation7,Citation25.
It was shown that rivaroxaban users had a 5-day shorter LOS-OAC than warfarin users in the unmatched cohorts and a 3-day shorter LOS-OAC than warfarin users after IPTW adjustment, though no difference was shown when comparing to warfarin users in the matched cohorts. Based on the results of two propensity score matching methods and biased SSI distribution observed in the matched cohorts, IPTW was found to be more appropriate in terms of generalizability. In addition, from a clinical perspective, the subgroup analysis according to SSI was performed as SSI included some important known confounders, and that we suspected the presence of heterogeneity on LOS-OAC according to SSI level.
The result of the subgroup analysis according to SSI after IPTW adjustment showed that regardless of drug selection, patients with mild stroke were more likely to have shorter LOS-OAC than those with a moderate or severe stroke which corroborated the previous finding that stroke severity is a significant predictor of LOSCitation26,Citation27. Regarding the comparison between the drugs, the LOS-OAC was similar between rivaroxaban and warfarin users in patients with severe stroke whilst rivaroxaban users had a 4-day shorter LOS-OAC than warfarin users in the mild stroke severity subgroup, and a 5-day shorter LOS-OAC in the moderate stroke severity subgroup. It was also shown that the use of rivaroxaban was significantly associated with shorter LOS-OAC in patients with mild and moderate stroke severity.
The observed 4 to 5 day difference in LOS between treatments was similar to the results of previous studies comparing NOACs and warfarin in JapanCitation16,Citation17. From the consistent result among these studies, it can be hypothesized that NOACs would contribute in part to the shorter LOS although the observed time difference seemed rather small considering that it usually takes about one week to achieve a stable PT-INR with a maintenance dose of warfarin.
It was also suggested that LOS-OAC was affected not only by the OAC selection but also by other factors, which might differ depending on the level of stroke severity, i.e. patient characteristics including old age (≥75 years) and high stroke risk (modified CHA2DS2-VASc score ≥2) for patients with mild stroke severity and environmental factors including more hospital beds (≥200 beds). Based on the result of the location after discharge stratified by hospital bed number, hospitals’ treatment policies/systems, such as care coordination, could be assumed to be factors associated with LOS-OAC for patients with moderate stroke severity.
The potential advantage of rivaroxaban over warfarin in terms of hospitalization costs was shown in the study; rivaroxaban users had lower costs during LOS-OAC than warfarin users by a median of approximately JPY94,000, probably reflecting their shorter LOS.
The difference was approximately 13% of the median costs during LOS-OAC in the warfarin group and the influence of the cost reduction would benefit patients and have positive impact on the national health care expenditure in Japan.
The present result added to previous evidence suggesting such economic benefits of NOACs compared with warfarin in the acute care of hospitalized NVAF patientsCitation11,Citation16,Citation17.
This study has several limitations. First, the patients in this study may not be fully representative of the target population in Japan because the DPC hospitals included in the MDV database were not selected at random. However, the use of this database, one of the largest databases available, provided a real-world picture of the target population with a relatively large sample sizeCitation19. Second, original definitions for SSI based on a Taiwanese study were adapted to Japanese claims data, and this index has not been validated for use in Japanese claims data studies yet. Third, the possibility of the results having been affected by known and unknown confounders other than stroke severity cannot be ruled out. Fourth, data in this hospital-based database could not be tracked across different hospitals. Because of this, baseline medications, e.g. might have been underreported in this study. Fifth, the study period of this study is limited prior to the introduction of endovascular therapy using a catheter in Japan considering the recent transition phase of the treatment for ischemic stroke in Japan. As catheter therapy began to spread since 2016 and is still expanding, further assessment post expansion of endovascular therapy will be needed in the future. Finally, in the analysis of medical costs, only initial hospitalization costs were compared, meaning that costs incurred during subsequent outpatient care or those incurred at another institution after discharge were not taken into account. Thus, the observed economic benefit should be interpreted with this point in mind.
Conclusions
The rivaroxaban users had a 3-day shorter LOS-OAC after IPTW-adjustment. According to the stroke severity level, using rivaroxaban was associated with a 4–5-day shorter LOS-OAC than using warfarin in patients with mild or moderate stroke, though treatment selection did not have a large impact on LOS-OAC in patients with severe stroke. Reduction of hospitalization cost was also showed in the rivaroxaban users.
Transparency
Declaration of funding
This work was supported by Bayer Yakuhin, Ltd. The funding body was involved in the design of the study, interpretation of data, writing the manuscript and decision to publish the findings.
Declaration of financial/other relationships
TY has received research funds and/or lecture fees from Daiichi Sankyo, Bayer Yakuhin, Bristol-Myers Squibb, Pfizer, Nippon Boehringer Ingelheim, Ono Pharmaceutical, and Toa Eiyo. MK and FO are employees of Bayer Yakuhin, Ltd. NY was an employee of Bayer Yakuhin, Ltd. TL is an employee of Clinical Study Support, Inc.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All authors contributed to the study design and data analysis or interpretation, and critically reviewed and revised the manuscript. All authors approved the final version to be published, and agreed to be accountable for all aspects of the work.
Supplementary Table 3
Download MS Word (27.5 KB)Supplementary Table 2
Download MS Word (26.9 KB)Supplementary Table 1
Download MS Excel (23 KB)Acknowledgements
Medical writing assistance was provided by Yuri Haga of Clinical Study Support, Inc.
Data availability statement
The data that support the findings of this study are available from the corresponding author, FO, upon reasonable request.
References
- Inoue H, Fujiki A, Origasa H, et al. Prevalence of atrial fibrillation in the general population of Japan: an analysis based on periodic health examination. Int J Cardiol. 2009;137(2):102–107.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(8):983–988.
- Appelros P, Nydevik I, Seiger Å, et al. Predictors of severe stroke: influence of preexisting dementia and cardiac disorders. Stroke. 2002;33(10):2357–2362.
- Lin H-J, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation: the Framingham Study. Stroke. 1996;27(10):1760–1764.
- Lai SM, Alter M, Friday G, et al. A multifactorial analysis of risk factors for recurrence of ischemic stroke. Stroke. 1994;25:958–962.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–e76.
- Toyoda K, Arihiro S, Todo K, et al. Trends in oral anticoagulant choice for acute stroke patients with nonvalvular atrial fibrillation in Japan: the SAMURAI-NVAF study. Int J Stroke. 2015;10:836–842.
- The Joint Committee on Guidelines for the Management of Stroke, The Japan Stroke Society. Japanese Guidelines for the Management of Stroke 2015. 2017 Supplement. [cited 2019 Apr 17]. Available from: http://www.jsts.gr.jp/img/guideline2015_tuiho2017.pdf.
- Farr AM, Jing Y, Johnston S, et al. Comparison of hospital length of stay between hospitalized non-valvular atrial fibrillation patients treated with either apixaban or warfarin. Hosp Pract. 2015;43(3):172–179.
- Laliberté F, Pilon D, Raut MK, et al. Hospital length of stay: is rivaroxaban associated with shorter inpatient stay compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin. 2014;30(4):645–653.
- Fonseca E, Sander SD, Hess GP, et al. Hospital admissions, costs, and 30-day readmissions among newly diagnosed nonvalvular atrial fibrillation patients treated with dabigatran etexilate or warfarin. J Manag Care Spec Pharm. 2015;21(11):1039–1053.
- Gilligan AM, Gandhi P, Song X, et al. All-cause, stroke-, and bleed-specific healthcare costs: comparison among patients with non-valvular atrial fibrillation (NVAF) newly treated with dabigatran or warfarin. Am J Cardiovasc Drugs. 2017;17(6):481–492.
- Deitelzweig S, Luo X, Gupta K, et al. Effect of apixaban versus warfarin use on health care resource utilization and costs among elderly patients with nonvalvular atrial fibrillation. JMCP. 2017;23(11):1191–1201.
- Clayville LR, Anderson KV, Miller SA, et al. New options in anticoagulation for the prevention of venous thromboembolism and stroke. Pharm Ther. 2011;36:86–99.
- Sakamoto H, Rahman M, Nomura S, et al. Japan Health System Review. New Delhi: World Health Organization, Regional Office for South-East Asia; 2018.
- Deguchi I, Takao M, Hayashi T, et al. Effect of oral anticoagulation therapy on duration of hospital stay and medical costs in patients with cardiogenic embolism stroke who were discharged to home. Jpn J Stroke. 2016;38(3):155–160.
- Yamashita T, Fukaya T, Kuroki D, et al. Comparison of the length of stay in patients and initiated with dabigatran or warfarin for a concomitant non-valvular atrial fibrillation in real-world Japanese therapeutic practice (SHORT J). Ther Res. 2017;38:377–391.
- Ishii M. DRG/PPS and DPC/PDPS as prospective payment systems. Jpn Med Assoc J. 2012;55:279–291.
- Koretsune Y, Yamashita T, Yasaka M, et al. Usefulness of a healthcare database for epidemiological research in atrial fibrillation. J Cardiol. 2017;70:169–179.
- Koretsune Y, Yamashita T, Yasaka M, et al. Comparative effectiveness and safety of warfarin and dabigatran in patients with non-valvular atrial fibrillation in Japan: a claims database analysis. J Cardiol. 2019;73:204–209.
- Sung S-F, Hsieh C-Y, Kao Yang Y-H, et al. Developing a stroke severity index based on administrative data was feasible using data mining techniques. J Clin Epidemiol. 2015;68:1292–1300.
- Brott T, Adams HP, Jr Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870.
- Sung S-F, Hsieh C-Y, Lin H-J, et al. Validity of a stroke severity index for administrative claims data research: a retrospective cohort study. BMC Health Serv Res. 2016;16:509.
- Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234.
- Fujimoto Y, Kajikawa R, Izutsu N, et al. Choice behavior of attending physicians toward oral anticoagulants for secondary prevention of cardiogenic cerebral embolism. Jpn J Stroke. 2016;38(4):239–244.
- Chang K-C, Tseng M-C, Weng H-H, et al. Prediction of length of stay of first-ever ischemic stroke. Stroke. 2002;33:2670–2674.
- Kang J-H, Bae H-J, Choi Y-A, et al. Length of hospital stay after stroke: a Korean nationwide study. Ann Rehabil Med. 2016;40:675–681.
