Abstract
Aims
Long-acting (LA) recombinant FVIII (rFVIII) products with extended dosing intervals have been developed for the treatment of hemophilia A; however, no direct head-to-head trial has been conducted to compare the efficacy of these products.
Materials and methods
A systematic literature search was conducted to identify published Phase III clinical trials of prophylactic LA rFVIII treatment in previously treated patients aged ≥12 years, with moderate-to-severe hemophilia A (endogenous FVIII levels ≤2%). Studies that did not meet these criteria, or did not report the included outcomes, were excluded. Bleeding rates and consumption were extracted and summarized; only data for the dosing frequencies indicated in the US product labels (which are similar to those indicated in the European Medicines Agency labels) were included.
Results
Five articles met the inclusion criteria; these studies only included patients with severe hemophilia A. Treatment length, reported outcomes and dose (range: 20–65 IU/kg) varied between studies. Median annualized bleeding rate (ABR) (IQR) reported in the relevant studies was 1.14 (0.00–4.30), rVIII-SingleChain 2 or 3 times weekly; 1.6 (0.0–4.7), rFVIIIFc 2 times weekly followed by every 3–5 days; 1.9 (0.0–5.8), BAX855 2 times weekly; 1.18 (0.00–4.25), N8-GP every 4 days; 1.9 (0.0–5.2) and 4.1 (2.0–10.6), BAY 94-9027 2 times weekly for the cohort who experienced >1 or <1 bleed in the study run-in phase, respectively. Median spontaneous ABR was 0.0 across studies reporting relevant data. Reported consumption was comparable among all LA products.
Limitations
The primary limitation of this systematic review was the variation in study design and not all studies reported all desired outcomes, which limited the quantity of data available.
Conclusions
This systematic review identified pivotal trial data for LA rFVIII products. Real-world evidence is needed to understand how these products perform in clinical practice.
Introduction
Hemophilia A is an X-linked bleeding disorder characterized by a deficiency or absence of functional coagulation factor VIII (FVIII) resulting in uncontrolled or prolonged bleeding episodesCitation1. Bleeding into the joints can cause progressive joint damage and it has been shown that as little as one joint bleed can cause irreversible damageCitation2. The primary aim of treatment is to prevent and treat bleeding episodes using replacement FVIII either episodically or by scheduled prophylaxis; many patients with severe hemophilia initiate prophylaxis at an early ageCitation1,Citation3. Prophylactic replacement FVIII treatment can reduce the risk of joint bleeds, therefore reducing morbidity and chronic disabilityCitation4. The relatively short half-life of traditional FVIII products (8–12 h) means frequent infusions are required for prophylaxis, placing a significant burden on the patient and caregiverCitation1.
Frequent dosing can be a burden for patients and can negatively affect their quality-of-life, potentially resulting in a lack of treatment adherence that can compromise treatment effectivenessCitation5,Citation6. Maintaining FVIII trough levels above 1% is associated with a reduction in bleeding rate and so is often a target for prophylactic therapy. Several recombinant FVIII (rFVIII) products are currently available for the treatment of patients with hemophilia A; however, the pharmacokinetics of standard-acting products are similar to endogenous FVIII, and therefore require infusions 3–4 times weekly to maintain these target trough levels. To overcome this, long-acting (LA) rFVIII products were produced either by increasing half-life compared with full-length rFVIII resulting in improved pharmacokinetic profiles, or by extending the in vivo effect of the product thereby enabling an extended dosing intervalCitation7. A variety of methods have been employed to extend the dosing interval of FVIII products including; single-chain technology (rVIII-SingleChain, AFSTYLAFootnotei)Citation8, Fc-fusion (rFVIIIFc, ELOCTATEFootnoteii)Citation9, and polyethylene glycol (PEG) conjugation (BAY 94-9027, JIVIFootnoteiii; BAX 855, ADYNOVATEFootnoteiv; N8-GP, ESPEROCTFootnotev)Citation10–12.
A systematic review and indirect comparison of rFVIIIFc with standard-acting products found reduced bleeding rates, factor consumption, and dosing frequency with rFVIIIFc prophylaxisCitation13; however, there have been no similar comparisons for long-acting products. There is a need to understand the differences in efficacy and consumption between available LA rFVIII products in order to enable better clinical treatment decisions for patients with hemophilia A. This manuscript aimed to systematically review the evidence from Phase III clinical trials evaluating the use of LA rFVIII products for prophylaxis in patients with hemophilia A, specifically focusing on bleeding rates and factor consumption.
Methods
In this systematic literature review, we summarized bleeding rates and factor consumption reported in adult and adolescent patients in published Phase III trials for LA rFVIII products available for the prophylactic treatment of hemophilia A.
Search strategy and study selection
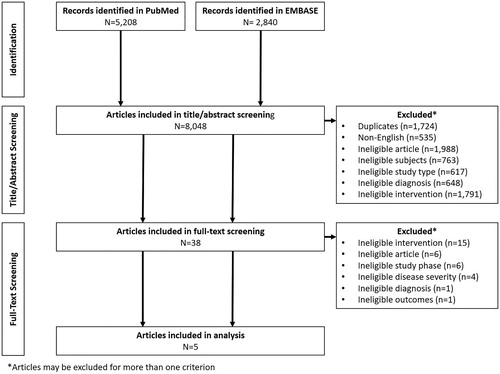
A systematic literature search was conducted in both PubMed and EMBASE according to PRISMA guidelines on August 21, 2020. Search terms were designed to select publications according to the patient population and treatment administered; these were then combined into a search string (). Search terms were limited to articles published in English, with a date range of 1966 (PubMed) or 1968 (EMBASE) to present. Further filters were applied to the EMBASE search to eliminate articles not published in scientific journals and those from the MEDLINE database. All publications retrieved were assessed against predefined inclusion/exclusion criteria to identify Phase III clinical trials of prophylactic LA rFVIII treatment in previously treated patients aged ≥12 years, diagnosed with moderate-to-severe hemophilia A (endogenous FVIII levels ≤2%) (). As this review was not a direct head-to-head comparison, various prophylaxis dosing regimens were implemented across products; to limit the potential for bias, for studies with more than one prophylaxis schedule, only data for the dosing frequencies indicated in the US product labels were included. Eligible studies had to report at least one of the following outcome measures; annualized bleeding rate (ABR), spontaneous ABR (AsBR), joint ABR (AjBR), or rFVIII consumption (for rVIII-SingleChain data on file were used). In all studies, patients must have received at least one dose to be included in the efficacy analysis. In order to compare studies more effectively, only Phase III trials were included as these are more likely to have similar sample sizes and study designs due to required regulatory approvals. Initial screening was conducted on the title and abstract of all search results using the pre-defined inclusion/exclusion criteria; those articles passing the initial screening were subjected to a full-text review using the same criteria to determine eligibility.
Table 1. Literature search terms.
Table 2. Inclusion and exclusion criteria.
Data extraction and management
The relevant data from all included publications were collected, with data retrieved according to the desired outcome measures. Data were extracted using a standardized data extraction form and any missing data were requested from corresponding authors.
Results
The literature search identified a total of 8,048 articles, 38 of which passed the initial title and abstract screen (). Following a full-text review, five articles met the inclusion criteria. These five studies only included patients with severe or moderately severe hemophilia A, with each study investigating one of the following LA rFVIII products; rVIII-SingleChain (AFSTYLA; CSL Behring)Citation8, rFVIIIFc (ELOCTATE; Sanofi Genzyme)Citation9, BAX 855 (ADYNOVATE; Takeda)Citation12, N8-GP (ESPEROCT; Novo Nordisk)Citation11, and BAY 94-9027 (JIVI; Bayer)Citation10 ().
Table 3. Study characteristics of included articles.
Study characteristics of the included trials are reported in . Patient populations were comparable; mean patient age ranged from 28.0–33.1 years and all patients had endogenous FVIII levels of <1%. Median treatment duration ranged from 32.1–299 weeks; however, it should be noted that two of the five studies did not report treatment duration (rVIII-SingleChain and BAX 855). The number of patients in the intent to treat (ITT) population (total number enrolled) in each study were 173 (175) for rVIII-SingleChainCitation8, 165 (165) for rFVIIIFcCitation9, 138 (138) for BAX 855Citation12, 186 (186) for N8-GPCitation11, and 132 (134) for BAY 94-9027Citation10; the proportion of the ITT population treated with prophylaxis during the studies were 84.4%, 86.1%, 87.0%, 94.1%, and 86.4%, respectively. Four studies reported the proportion of patients who received prophylaxis treatment prior to enrollment; 40% for rVIII-SingleChainCitation8, 53% for rFVIIIFcCitation9, 72% for BAX 855Citation12, and 80% for N8-GPCitation11. Two studies reported pre-study prophylactic regimens in which the majority of patients were dosing ≥3 times weeklyCitation8,Citation9. Data were included for prophylactic regimens described in the US product label; the dose and regimen varied between studies: rVIII-SingleChain, 20–50 IU/kg 2 or 3 times weekly (n = 126)Citation9; rFVIIIFc, 25 IU/kg on day 1, 50 IU/kg on day 4, followed by 25–65 IU/kg every 3–5 days (n = 117)Citation10; BAX 855, 45 ± 5 IU/kg 2 times weekly (n = 120)Citation13; N8-GP, 50 IU/kg every 4 days (n = 175)Citation12; BAY 94-9027, 30–40 IU/kg 2 times weekly (n = 24, separated into two cohorts)Citation11 (). Details of bleeding rates and consumption of the five prophylactic LA rFVIII products are reported in . Not all studies reported every outcome measure. Median ABR (IQR) reported in the relevant studies ranged from 1.14 (0.00–4.30) for rVIII-SingleChain 2 or 3 times weeklyCitation8 to 4.1 (2.0–10.6) for BAY 94-9027 2 times weekly for patients who experienced >1 bleed in the study run-in phaseCitation10. Mean ABR was similar across the three studies reporting data; 3.32 for rVIII-SingleChainCitation8, 3.7 for BAX 855Citation12, and 3.04 for N8-GPCitation11. Median AsBR values were 0 in the four studies which reported it. Mean AsBR was comparable in the two studies that reported data. Three studies reported median AjBR which were also comparable. Mean AjBR was reported in only one study (BAX 855; 1.8)Citation12.
Table 4. Details of bleeding rates and consumption in LA rFVIII studies identified by the systematic review.
Reported prophylactic consumption was comparable among all products. Median yearly prophylactic consumption was reported in one study, 4282.9 IU/kg for rVIII-SingleChainCitation8, and mean yearly consumption was reported in three studies; 4472.5 IU/kg for rVIII-SingleChainCitation8, 4845 IU/kg for N8-GPCitation11, 4497.8 IU/kg for BAY 94-9027 for patients who experienced >1 bleed in the study run-in phaseCitation10, and 3341.1 IU/kg for BAY 94-9027 for patients who experienced <1 bleed in the study run-in phaseCitation10. rFVIIIFc reported mean and median weekly prophylactic consumption (77.9 and 85.4 IU/kg, respectively), which was converted to yearly values to enable comparisons with the other products (4050.8 and 4440.8 IU/kg, respectively)Citation9. Median yearly prophylactic consumption for BAX 855 (4546 IU/kg) was calculated from the reported median dose per infusion (44.6 IU/kg) and the median number of infusions per week (1.96)Citation12.
Discussion
In view of the lack of head-to-head clinical trials, this review presents data from pivotal trials of different LA rFVIII products. The results of this systematic review demonstrate that currently available LA rFVIII products have comparable efficacy and consumption. While bleeding rates and consumption for rVIII-SingleChain 2 or 3 times weekly (n = 126) are data on file, results for these are very close to those published in the pivotal study for all prophylaxis patients (n = 146)Citation8.
Some patients using prophylaxis with LA products during these trials still experienced breakthrough bleeding. ABRs and AsBRs were similar across studies reporting relevant data. Of the labeled dosing regimens indirectly compared in this study, rVIII-SingleChain dosed at 20–50 IU/kg 2 or 3 times weekly and N8-GP dosed at 50 IU/kg every 4 days displayed the lowest ABR compared with the labeled dosing regimens in other studies reporting relevant data. rVIII-SingleChain showed equivalent results in terms of bleeding rates compared with the other products in this review. N8-GP had comparably low mean and median ABR; however, this was the only product to report a median AjBR greater than 0.0 of the three studies which reported it.
The decision to switch to a less frequent dosing regimen should be based on a number of factors including bleeding phenotype, activity levels, patient lifestyle, and individual pharmacokinetic data. Additionally, patients should be well-controlled on their previous regimen before reducing dosing frequency. Recently, real-world data reported by Simpson et al.Citation14 and Olivieri et al.Citation15 showed that patients switching from prophylaxis with prior FVIII products to rVIII-SingleChain could reduce their dosing frequency whilst maintaining or improving efficacy. The reduced dosing frequency and improved pharmacokinetic profile of rVIII-SingleChain (lower clearance, higher mean residence time, and larger area under the curve) compared with standard-acting rFVIII products demonstrates that it shares characteristics with other LA products, making it an effective treatment for hemophilia A whilst reducing consumption, leading to potential cost savingsCitation7,Citation8,Citation14,Citation16.
Of the studies with consumption data available, BAY 94-9027 reported the lowest yearly consumptionCitation10. Real-world evidence is needed to confirm such data for this recently launched product.
The primary limitation of this systematic review was the variation in study design; the substantial variation in reported treatment dose and prophylaxis regimen potentially weakens any comparisons made between products. As this review was an indirect comparison, various dosing regimens were implemented across the different studies. The dosing regimens selected for comparison in this study were in line with those recommended in the US product label, enabling a comparison of regimens likely to be used in clinical practice. While this is a significant limitation with regards to the strength of the conclusions from this study, it is an expected limitation of this type of comparison. Additionally, in the absence of a head-to-head comparison, which is unlikely to occur in these marketed products, the data provided here provides a comparative guide as to the expected efficacy and consumption of these products, as recommended in the product label. The comparison between products was also limited in that not all studies reported all desired outcomes. In addition, this review is limited by the small number of studies identified, with only one study found for each of the LA rFVIII products; this limited the quantity of data available for comparison. The rarity of hemophilia A and the low number of patients worldwide, eligible and willing to participate in clinical trials, limits the amount of available data; it may be valuable to carry out a similar review with expanded criteria, for example, including real-world data. As is common among systematic reviews and literature searches, another limitation is the potential for publication bias, although the methods described in this study were selected to reduce the impact of such bias.
Conclusion
This systematic review identified pivotal trial data for LA rFVIII products. These data showed that LA rFVIII products can provide adequate protection from bleeding with similar consumption in patients with hemophilia A. Given the lack of direct head-to-head trials of rFVIII products, this review provided a summary of the efficacy and consumption data of different LA rFVIII products from relevant pivotal trials. Further comparative real-world data are needed to assess how these products perform in clinical practice.
Transparency
Declaration of funding
This study was funded by CSL Behring LLC, King of Prussia, PA, USA.
Declaration of financial/other interests
LG: consultancy for CSL Behring, Novo Nordisk, Octapharma, Roche and SOBI; SY: employee of CSL Behring; MCS: no conflicts of interest; VB: consultancy for Bayer, Bioverativ/Sanofi Genzyme, CSL Behring, Novartis and Shire.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Previous presentations
The results of this literature search were presented as a poster at ISTH 2019 and EAHAD 2020.
Acknowledgements
The authors thank Katie Lisle of Meridian HealthComms, Plumley, UK for providing medical writing support, which was funded by CSL Behring LLC, King of Prussia, PA, USA in accordance with good publication practice (GPP3).
Notes
i AFSTYLA is a registered trademark of CSL Behring, Germany.
ii ELOCTATE is a registered trademark of Sanofi Genzyme, USA.
iii JIVI is a registered trademark of Bayer, USA.
iv ADYNOVATE is a registered trademark of Takeda, Japan.
v ESPEROCT is a registered trademark of Novo Nordisk, USA.
References
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1.
- van Vulpen LFD, Holstein K, Martinoli C. Joint disease in haemophilia: pathophysiology, pain and imaging. Haemophilia. 2018;24(Suppl 6):44–49.
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544.
- Fischer K, van der Bom JG, Mauser-Bunschoten EP, et al. The effects of postponing prophylactic treatment on long-term outcome in patients with severe hemophilia. Blood. 2002;99(7):2337–2341.
- Wiley RE, Khoury CP, Snihur AWK, et al. From the voices of people with haemophilia A and their caregivers: challenges with current treatment, their impact on quality of life and desired improvements in future therapies. Haemophilia. 2019;25(3):433–440.
- Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677–1686.
- Escobar M, Santagostino E, Mancuso ME, et al. Switching patients in the age of long-acting recombinant products? Expert Rev Hematol. 2019;12(sup1):1–13.
- Mahlangu J, Kuliczkowski K, Karim FA, et al. Efficacy and safety of rVIII-SingleChain: results of a phase 1/3 multicenter clinical trial in severe hemophilia A. Blood. 2016;128(5):630–637.
- Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317–325.
- Reding MT, Ng HJ, Poulsen LH, et al. Safety and efficacy of BAY 94-9027, a prolonged-half-life factor VIII. J Thromb Haemost. 2017;15(3):411–419.
- Giangrande P, Andreeva T, Chowdary P, et al. Clinical evaluation of glycoPEGylated recombinant FVIII: efficacy and safety in severe haemophilia A. Thromb Haemost. 2017;117(2):252–261.
- Konkle BA, Stasyshyn O, Chowdary P, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(9):1078–1085.
- Iorio A, Krishnan S, Myren KJ, et al. Indirect comparisons of efficacy and weekly factor consumption during continuous prophylaxis with recombinant factor VIII Fc fusion protein and conventional recombinant factor VIII products. Haemophilia. 2017;23(3):408–416.
- Simpson ML, Desai V, Maro GS, et al. Comparing factor use and bleed rates in U.S. hemophilia A patients receiving prophylaxis with 3 different long-acting recombinant factor VIII products. J Manag Care Spec Pharm. 2020;5:1–9.
- Olivieri M, Sommerer P, Maro G, et al. Assessing prophylactic use and clinical outcomes in hemophilia A patients treated with rVIII-SingleChain and other common rFVIII products in Germany. Eur J Haematol. 2020;104(4):310–317.
- Klamroth R, Simpson M, von Depka-Prondzinski M, et al. Comparative pharmacokinetics of rVIII-SingleChain and octocog alfa (Advate(®) in patients with severe haemophilia A. Haemophilia. 2016;22(5):730–738.