Abstract
Aims
Respiratory syncytial virus (RSV) is a common cause of respiratory infection in infants and severe infection can result in hospitalization. The passive immunization, palivizumab, is used as prophylaxis against RSV, however, use in the UK is restricted to populations at high risk of hospitalization. This study assesses the cost-effectiveness (CE) of palivizumab in premature infants with and without risk factors for hospitalization (congenital heart disease [CHD], bronchopulmonary dysplasia [BPD]).
Methods
A decision tree model, based on earlier CE analyses, was updated using data derived from targeted literature reviews and advice gained from a Round Table meeting. All costs were updated to 2019 prices. One-way and probabilistic sensitivity analyses were performed to assess the degree of uncertainty surrounding the results.
Results
Palivizumab is dominant (i.e. clinically superior and cost saving) when used in premature infants born ≤35 weeks gestational age (wGA) without CHD or BPD and aged <6 months at the start of the RSV season, infants aged <24 months with CHD and infants aged <24 months requiring treatment for BPD within the last 6 months.
Limitations
One-way sensitivity analysis suggests that these results are highly sensitive to the efficacy of prophylaxis, number of doses, impact of long-term respiratory sequalae, rate of hospitalization and mortality due to RSV. A conservative approach has been taken toward long-term respiratory sequalae due to uncertainty around epidemiology and etiology and a lack of recent cost and utility data.
Conclusions
Palivizumab prophylaxis is cost-effective in preventing severe RSV infection requiring hospital admission in a wider population than currently recommended in UK guidelines. Prophylaxis in premature infants born <29 wGA, 29–32 wGA and 33–35 wGA without CHD or BPD aged <6 months at the start of the RSV season is not funded under current guidance, however, prophylaxis has been demonstrated to be cost-effective in this analysis.
Introduction
Respiratory syncytial virus (RSV) is a common seasonal respiratory infection occurring between October and March in the UK. Almost all infants will have been infected by RSV by the age of 2 yearsCitation1 and for most children, the infection will be mild and self-limiting. However, in some high-risk populations, including premature infants, infants with bronchopulmonary dysplasia (BPD), also known as chronic lung disease, congenital heart disease (CHD) and/or a weakened immune system, RSV infection is more likely to be severe.Citation2 Severe RSV infection can lead to acute lower respiratory tract infection (LRTI) and bronchiolitis, which results in hospitalization and in some cases to admission to intensive care units (ICU).Citation3
The disease burden associated with RSV is considerable; in children aged <5 years RSV is responsible for nearly twice as many GP consultations and nearly five-times as many admissions to hospital as influenza, at substantial costCitation4 and is a leading cause of hospitalization.Citation5 In England, RSV infection in children aged <5 years leads to an estimated 352,570 GP consultations, 26,400 hospitalizations and 25 deaths in hospital per average season.Citation4
Data from Scotland on almost all births recorded by NHS Scotland between 2001 and 2011 reveals that 2.1% of children are hospitalized at least once with confirmed RSV in the first 2 years of life, with a median length of stay in hospital of 2 days (interquartile [IQR] range 1–4 days) and a median 5 day (IQR 2–8 days) stay in ICU.Citation6 RSV hospitalizations accounted for 6.2% of all in-patient bed days, which rose to 14.2% during the peak months of the RSV season (December–January), equating to over 1,400 hospitalizations and nearly 5,500 bed days each year. More recent data using the same source (2009–2012) restricted to singleton births, with 3-year follow-up, found that 3.05% of children were hospitalized in the first 3 years of life, half of the hospitalizations occurred during the first 6 months of life.Citation7 Data from the Scottish 2000–2011 cohort found that RSV hospital admission rates for infants born at <29 wGA were significantly higher than for infants with a longer gestation: < 29 wGA 12.8%; 29–32 wGA 8.2%; 33–35 wGA 4.6% and ≥36 wGA 1.9% (all p < 0.0001).Citation6
An abstract presented at the 2020 European Society for Paediatric Infectious Disease meeting reveals that not only were infants born at <29 wGA at higher risk of hospitalization, they also spent longer in hospital (median 5 days, IQR 2–9 days) and were more likely to be admitted to ICU than later term babies (13.4% versus 10.1% for 29–35 wGA versus 3.6% for ≥36 wGA).Citation8
Health care costs for RSV in children aged <5 years in England have been estimated at £54 million a year.Citation4 The majority of costs (68%, £37 million) are due to hospital admission (including admissions to ICU).Citation4 Given that RSV is a seasonal virus in the UK, most admissions occur in the winter, meaning that RSV accounts for >75% of infants admitted to hospital between November and January inclusive.Citation4 This is a considerable challenge to the NHS, given the increased pressure on admissions during the winter months.
RSV infection in premature infants, who are born during a critical period of lung development and with reduced pulmonary function, may impact on long-term respiratory function.Citation9 There is an increasing body of evidence supporting an association between hospitalization due to RSV infection in infancy and recurrent wheezing and asthma during childhood.Citation10–17 A systematic review of 41 studies and subsequent meta-analysis found a significant association between early RSV infection and the development of childhood recurrent wheeze from birth to 12 years and the development of asthma in children aged 6–12 years at follow-up.Citation18 Recent data using the NHS Scotland dataset revealed that asthma rates and use of asthma medication at 18 year follow-up was significantly higher in those children who had been hospitalized with confirmed RSV than in those without RSV.Citation14
Data from two studies using English Hospital Episode Statistics (HES) data reveals that admission for bronchiolitis associated with RSV is linked with an increased risk of hospital admission for wheeze and asthma in children aged up to 5 and 8 years.Citation15,Citation16 A retrospective cohort analysis using HES data from 2008, revealed that infants with a bronchiolitis admission associated with RSV within the first year of life were almost three-times more likely to be admitted to hospital for respiratory conditions before age 5 years than those without an admission for bronchiolitis in infancy (adjusted HR 2.95, 95% CI 2.85–3.05). The increase in risk was driven by admissions for asthma and wheeze.Citation15 A similar study, which used HES data from 2007, with 8-year follow-up found that children with RSV-associated bronchiolitis admission in the first year of life were more than twice as likely to be admitted to hospital for wheeze over the subsequent 8 years than infants admitted for urinary tract infection (RR 2.3, 95% CI 2.0–2.6). Urinary tract infection was chosen as the comparator since it is a common reason for paediatric hospital admission.Citation16 The study used length of stay of the initial bronchiolitis admission as a proxy for severity of disease; not surprisingly the risk of later admission for wheeze increased with disease severity.
Palivizumab is a humanized IgG1κ monoclonal antibody directed to an epitope in the A antigenic site of the fusion protein of RSV. The antibody targets the fusion protein of RSV responsible for fusing the virus and the host cell and therefore prevents the virus from entering the host cell.Citation3,Citation19 It is a passive immunization, rather than a vaccine, and provides short-term protection against RSV.Citation2 At present, palivizumab is the only available prophylaxis against RSV, although a number of vaccines directed at different patient populations (pregnant women, infants, older people) are under development.
Palivizumab is indicated for the prevention of serious LRTI requiring hospitalization caused by RSV in children at high risk for RSV disease. High risk of RSV disease is defined as children born at ≤35 weeks gestational age (wGA) and <6 months of age at the onset of the RSV season, children aged <2 years and requiring treatment for BPD within the last 6 months and children aged <2 years with haemodynamically significant CHD.Citation19 Palivizumab is administered as an intramuscular injection at a dose of 15 mg/kg of body weight. Up to five doses are given at monthly intervals during anticipated periods of RSV risk in the community.Citation19
The efficacy of palivizumab in reducing admissions is supported by two randomized placebo-controlled clinical trials: IMpact-RSV trial in 1,502 premature infants or infants with BPDCitation20and a trial in 1,287 young children with haemodynamically significant CHD.Citation21 In both studies, use of palivizumab significantly reduced the rates of hospital and high dependency (HDU)/ICU admissions, length of stay and use of supplementary oxygen versus placebo. Long-term studies with 6-year follow-up have demonstrated a significant reduction in recurrent wheeze in children treated with palivizumab.Citation11,Citation12,Citation22 The evidence for a reduction in childhood asthma is less clear cut, with some studies showing a significant reduction in both wheeze and asthmaCitation22,Citation23 and others showing only a significant reduction in wheeze.Citation11,Citation12
In the UK, the Joint Committee on Vaccination and Immunization (JCVI) issued guidance on the use of palivizumab in 2010.Citation3 This guidance limits the use of palivizumab to specific high-risk populations based on cost-effectiveness (CE) analysis carried out by the West Midlands Health Technology Assessment (HTA) CollaborationCitation24,Citation25 (). This guidance is more restrictive than in other developed countries; a recent consensus from developed countries recommends palivizumab across a wider patient population, including premature infants without associated BPD or CHD.Citation10
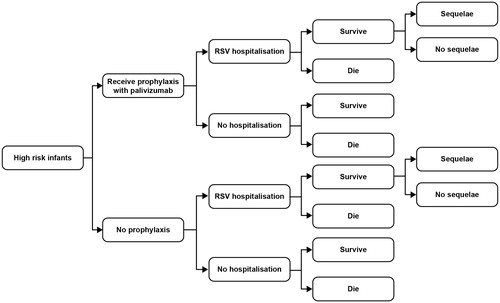
Figure 1. High-risk patient populations endorsed by JCVI for palivizumab in existing guidance.Citation3

The original work which underpinned the JCVI recommendations was carried out a decade ago. The aim of this work was to assess the CE of palivizumab in premature infants (with and without BPD or CHD) and update inputs into the model, considering recent data around long-term respiratory sequalae and palivizumab dosage.
Methods
Targeted literature review
We carried out a targeted literature review using PubMed, Embase and heoro.com databases to identify health economic analyses for the use of palivizumab in RSV prophylaxis in September 2018. We excluded health economic analyses carried out in the USA as fundamental differences in healthcare delivery and funding make it very difficult to extrapolate findings to a European context.
Our search identified nine potentially relevant publications. Three of these related to various iterations of the JCVI (West Midlands HTA) model carried out by Wang et al.Citation24–26 A further study was an evolution of this approach, funded by AbbVie and published in 2013.Citation27 We also identified five other studies: one from the UK,Citation4 two from Spain,Citation28,Citation29 one from the NetherlandsCitation30 and one from Canada.Citation31 These publications used approaches that were either at odds at those adopted by JCVI or simply reinforced the conclusions of the two key models, therefore, we did not consider these models further and focused on the West Midlands HTA model carried out by Wang et al.Citation24–26 and the updated Bentley et al.Citation27 model.
Round table meeting
We held a Round Table meeting to inform our modeling approach and gain an understanding of real-world practice in the UK. The meeting members included clinicians currently managing infants with RSV (Consultant Neonatologists [n = 3], Consultant in Paediatric Intensive Care, Consultant Respiratory Paediatrician, Consultant General Paediatrician), a Quality Improvement Manager, a nurse currently managing RSV and a health economist. A questionnaire was issued prior to the meeting to gain understanding of attitudes around the current JCVI guidelines, other potential high-risk groups, the CE of prophylaxis and to validate inputs into the economic modeling (inclusion of respiratory sequalae, number of doses of palivizumab, vial sharing). There was considerable debate around the inputs into the economic model and a consensus was reached for each risk factor after discussion.
Outline of the economic model
Comparison of the West Midlands HTACitation24,Citation25,Citation26 and the Bentley et al.Citation27 models, together with Round Table discussion allowed us to develop the most robust model possible. Information provided by the Round Table participants allowed us to validate our assumptions with current UK practice. Inputs were updated where possible.
Five separate patient populations were included in the economic modeling: premature infants without BPD or CHD (born at <29 wGA, 29–32 wGA and 33–35 wGA) and aged <6 months at the start of the RSV season, infants (aged <24 months) with BPD and infants (aged <24 months) with haemodynamically significant CHD.
Model structure
The West Midlands HTA model was a simple decision analytic tree (). In scenario analyses, the model was extended to include the cost and quality adjusted life year (QALY) consequences of long-term respiratory sequelae (principally wheeze and asthma) in RSV-infected children. The Bentley et al. model used a very similar underlying structure to the West Midlands model, the key difference was the inclusion of management costs and utility impact for respiratory sequelae in RSV infected children for 2 years after admission in the base case. Both models use a lifetime time horizon.
During the Round Table meeting all participating clinicians agreed that RSV is likely to increase the likelihood of respiratory conditions in later childhood. Based on this expert input, inclusion of respiratory sequalae in the base case was deemed to be appropriate and therefore we used the model structure defined in Bentley et al., which is described in detail in Bentley et al.Citation27
Incremental cost effectiveness ratio (ICER) and net monetary benefit (NMB) were used as summary statistics. NMB is calculated as (benefit x CE threshold) − cost. This scales health outcomes and use of resources to costs, allowing comparisons to be made without ratios (such as in ICERs). A CE threshold of £30,000 was used. Incremental NMB (INMB) measures the difference in NMB between alternative interventions, a positive INMB indicates that the intervention is cost-effective compared with the alternative at the given CE threshold. INMBs are more informative when ICERs are negative, i.e. either less effective and more expensive or more effective and less costly (dominant).Citation32
Core inputs
Details of the rationale for the original data sources in the model can be found in the original publication, Bentley et al.Citation27 lists the sources for the main clinical and cost inputs, updated since the original publication of the model wherever possible from information gathered via a pragmatic review of the literature. The definitions of BPD and CHD used in this model reflect the marketing authorization for palivizumab since the data for these populations are taken from the pivotal trials for palivizumab (). Therefore, the BPD and CHD populations within the model are wider than those recommended by the current JCVI recommendations, which restrict access to palivizumab to a higher risk population ().
Table 1. Clinical and cost inputs of original Bentley et al model and the updated model.
Number of doses
In the original modeling carried out by the West Midlands HTA groupCitation24–26 and Bentley et al.Citation27 the number of palivizumab doses was assumed to be five (range 2–6) reflecting the Summary of Product Characteristics (SPC) for palivizumab. However, in this iteration of the model we chose to assume a dose of 3.7 reflecting the seasonality of infection and real-world evidence.Citation3,Citation10,Citation33–37
The JCVI guidelines recommend that palivizumab is given from the beginning of the RSV season (October) and that infants in hospital should only receive palivizumab on discharge from the neonatal unit.Citation3 Recent consensus guidelinesCitation10 state that children born during the RSV season will require fewer doses than those born before the season, which is confirmed by work we carried out around the seasonality of RSV for this publication. A proportion of eligible at-risk infants will be born within the RSV season and/or be on neonatal units during the RSV season and will not be eligible for palivizumab.
We have carried out some analyses to investigate this assumption, looking specifically at babies born <29 wGA. Our work estimates the mean number of doses for babies born <29 wGA and aged <6 months at the onset of the RSV season at 3.8 doses when discharged at 36 weeks and 3.7 doses when discharged at 40 weeks. See for the workings for predicted doses for babies born <29 wGA, aged <6 months of age at the onset of the RSV season and discharged at 36 weeks. This data was estimated using 2017 birth data from the Office for National Statistics for the United Kingdom (birth rates, number of births at each gestation) and our calculation excludes babies already eligible for palivizumab under current JCVI guidance.Citation38
Table 2. Predicted dose of palivizumab for babies born <29 wGA, aged <6 months of age at the onset of the RSV season (October) and discharged at 36 weeks.
Our dosage estimates are consistent with an audit carried out during the September 2001 to March 2002 RSV season. The audit included 190 infants receiving palivizumab across the UK and revealed that the mean number of doses was 3.7 per patient. In this audit, 9.5% of infants received only one dose, 10% two doses, 17.4% three doses, 23.2% four doses, 36.3% five doses and 3.7% six doses.Citation33
More recent real-world evidence from around the world confirms the UK data. Doses varied across the studies, and were 3.5 dosesCitation34 or 4 doses in Brazil,Citation35 3.7 doses in KoreaCitation36 and 3.8 doses in Latin America.Citation37
The Round Table felt that this was a reasonable reflection of current practice and that it would be appropriate to use 3.7 doses in the base case.
Seasonality of RSV infection, real world evidence and clinical evidence at the Round Table meeting all suggest that on average fewer than 5 doses are used in clinical practice and that the likely mean number of doses is around 3.7. It is important to note that in this case a lower dose would not lead to reduced efficacy since patients are only dosed during the RSV season when they are at risk of infection.
Body weight and cost of treatment
Palivizumab is supplied in 50 mg and 100 mg vial sizes at costs of £306.64 and £563.64, respectively. Treatment is administered monthly at a rate of 15 mg/kg. Advice from the Round Table meeting was that vial sharing would not be good medical practice and would be unlikely to occur. Therefore, the costs of treatment are subject to a quantum effect due to vial size. Thus, any child weighing less than 3.3 kg will incur a cost of £306.64 per dose while a child weighing more than 3.4 kg will require a higher dose vial at almost double the price. Weight gain over the RSV season period will usually result in cost escalation. For a very low birthweight baby with multiple comorbidities starting treatment early, it is conceivable that five doses could be given for a total maximum cost of £1,533. On the other hand, an older child, with greater growth velocity and starting treatment later, the cost of treatment could be much higher.
Regression methods (ordinary least squares) were used to approximate the relationship between weight (y) and chronological age (x), using goodness of fit criteria. The resulting equation y = 0.0083×x3 − 1.9512×x2 + 191.01×x + 3869.4 was subsequently used to estimate the relative increase in infant weight at monthly intervals from the initial dose.Citation27 Body weight at the initial dose was taken from the two pivotal trials for palivizumab (Feltes et al.Citation21 and IMpact-RSVCitation20).
RSV-related respiratory sequelae
In the original Bentley et al. model, data on the risk of RSV-related respiratory sequelae (RSV-associated respiratory morbidity) were taken from two cohort studies by Greenough et al.Citation39,Citation40 and a study by Shefali-Patel et al.Citation41 The studies showed that there was an increase in healthcare resource use attributable to respiratory sequelae for 2 years and a decrease in quality of life for 5 years. This was used as the base case in our modeling, with the costs updated to 2019 prices.
Recent data provide evidence that RSV-related sequelae have a clinical impact for longer than 2 years, however, data on healthcare resource is lacking. In order to explore the impact of RSV sequalae up to age 7 years we used data from Sigurs et al.Citation42,Citation43 as a scenario analysis with costs updated to 2019 prices.
Model validation
We repeated the model validation as used in the original Bentley et al.Citation27 paper: a one-way sensitivity analysis and a probabilistic sensitivity analysis (PSA).
In the one-way (univariate) sensitivity analysis all model parameters were individually varied between their minimum and maximum values, based on the 95% confidence interval for all parameters. Results are presented as Tornado diagrams with the ten parameters with the greatest impact on the INMB included.
The PSA was performed using Monte Carlo simulation techniques (5,000 Monte Carlo simulations) allowing all parameters to be varied simultaneously within a plausible range. Beta distributions were used for the probability of RSV hospitalization, prophylaxis efficacy, mortality and baseline utility scores for RSV and non-RSV hospitalization. Gamma distributions were used for number of doses and all costs.
Result-base case
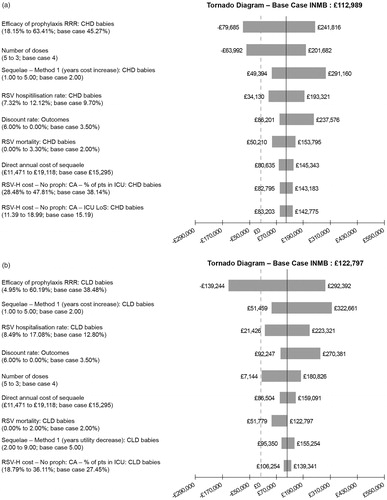
The results for the base case are presented in and show the total costs and QALYs with palivizumab prophylaxis and no prophylaxis based on 100 infants within each subgroup. Prophylaxis with palivizumab against severe RSV infection requiring hospital admission is both clinically superior and cost saving in all the groups which we assessed: premature infants born ≤35 wGA without CHD or BPD and aged <6 months at the start of the RSV season, in infants with CHD and in infants with BPD, demonstrating that the ICER is dominant (ranging from −£3,422 to −£44,629) . The INMB are positive, ranging from £112,989 to £221,881 depending on the population, confirming the CE of prophylaxis with palivizumab.
Table 3. Summary of results per hundred infants from the base case model.
Scenario analyses
Two scenario analyses were performed – one looking at increasing the number of doses of palivizumab from 3.7 to 5 doses and another assuming that the impact of respiratory sequalae on cost and utilities continues until age 7 years ( and ).
Table 4. Summary of results per hundred infants, scenario five doses of palivizumab, costs of respiratory sequelae for 2 years and a decrease in utility due to respiratory sequelae for 5 years.
Table 5. Summary of results per hundred infants, scenario respiratory sequalae (costs and utilities) continue until age 7 years, 3.7 doses of palivizumab.
One-way (univariate) sensitivity analysis
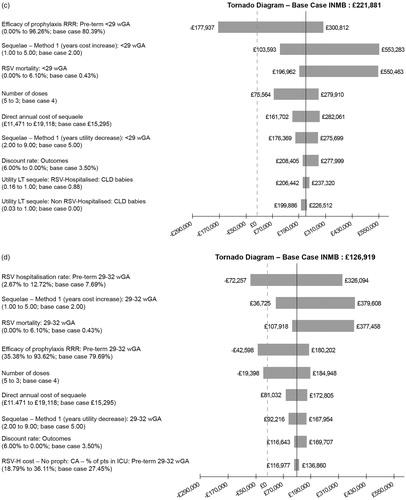
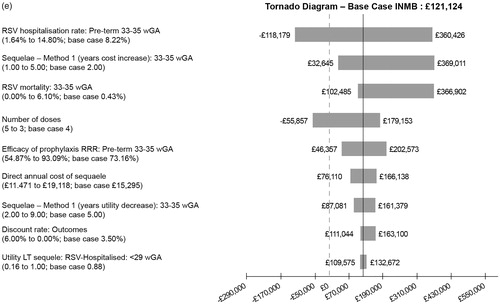
The one-way (univariate) sensitivity analysis demonstrates how the INMB (based on a CE threshold of £30,000) is affected by varying parameters, as illustrated in . Each tornado diagram in contains the ten most important parameters for each subgroup. The efficacy of prophylaxis, number of doses, duration of long-term respiratory sequalae, rate of hospitalization and mortality due to RSV are the most important parameters. In infants with comorbidities or very premature babies the most important factor is the efficacy of prophylaxis, whereas in premature babies born at 29–35 wGA hospitalization rate dominates.
Figure 3. One-way (univariate) sensitivity analysis: tornado diagrams. (a) CHD infants, base case INMB. (b) BPD infants, base case INMB. (c) Infants born at <29 wGA without BPD or CHD, base case INMB. (d) Infants born at 29–32 wGA without BPD or CHD, base case INMB. (e) Infants born at 33–35 wGA without BPD or CHD, base case INMB.
Probabilistic sensitivity analysis
The results of the PSA are shown in for two CE thresholds (£20,000 and £30,000). At the £30,000 threshold, palivizumab has a greater than 50% probability of being cost-effective in all patient groups. At the £20,000 threshold palivizumab has a greater than 50% probability of being cost-effective in all patient groups except premature infants aged 33–35 wGA (49.4%).
Table 6. Probability that palivizumab is cost-effective for different CE thresholds.
Discussion
This economic analysis indicates that palivizumab is cost-effective for infants at high risk of hospitalization due to RSV with ICERs well below the £30,000 threshold. Palivizumab is dominant (i.e. both clinically superior and cost saving) with a positive INMB when used in premature infants born ≤35 wGA without CHD or BPD (including infants born ≤39 wGA) and aged <6 months at the start of the RSV season, infants with CHD and in infants with BPD.
As shown in the base case (), QALY differences are small between palivizumab treated and untreated populations, whereas cost differences are considerable. CE is driven by factors that affect costs rather than clinical effectiveness. Indeed, the sensitivity analyses revealed that results for the subgroups are affected by the clinical effectiveness of prophylaxis (which itself impacts on hospitalization and therefore cost), number of doses, duration of long-term respiratory sequalae and rate of hospitalization. This is not unexpected, a systematic review of 28 economic evaluations of palivizumab published in 2019, identified RSV hospitalization reduction rates and the cost of palivizumab as two of the most influential factors.Citation44
Cost effective populations for palivizumab
This analysis suggests that the population of infants in whom palivizumab is cost effective may be wider than the current JCVI guidelinesCitation3 which restricts use to high-risk infants with BPD or CHD and immunocompromised children (SCID).
As part of the Round Table meeting, participants were asked to complete a questionnaire prior to attending the meeting. All nine responders considered that current JCVI guidance did not cover all at risk infants. They suggested that infants born at <29 wGA without BPD or CHD were at high risk of severe RSV infection and subsequent hospitalization. This population was ranked as third in need for prophylaxis behind infants with BPD or CHD by six of the nine participants. One participant felt that very premature infants born at <29 wGA without BPD or CHD were at the greatest need for prophylaxis. Other specific populations mentioned by the clinical experts as at high risk of severe RSV were premature infants without BPD or CHD, twins and multiples, infants with Down syndrome or other chromosomal abnormalities, infants with muscular dystrophy/neuromuscular diseases and those from homes with other risk factors e.g. parental smoking, high air population, overcrowding.
The questionnaire results are echoed by recent consensus guidelines which recommend the use of palivizumab in infants with BPD or CHD, premature infants born at <29 wGA without comorbidities and aged ≤9 months at the start of the RSV season and in premature infants aged 29–31 wGA without comorbidities and aged ≤6 months at the start of the RSV season (all high quality, 1a, grade A evidence).Citation10 At a lower evidence threshold, the guidelines also point out that children with Down syndrome aged ≤ 24 months, those with cystic fibrosis aged ≤ 12 months or ≤ 24 months with manifestations of severe lung disease or weight <10th percentile and those with neuromuscular disease ≤ 24 months could benefit from prophylaxis with palivizumab. Although it should be noted that palivizumab is not currently indicated for these conditions.
Two studies provide clinical trial evidence for a benefit with palivizumab in premature infants without BPD or CHD. Firstly, a post-hoc analysis of the pivotal IMpact-RSV-RSV trial demonstrated an 80% reduction in admissions for RSV in very premature infants (<29 wGA) without BPD receiving palivizumab prophylaxis compared with no prophylaxis, 2.0% versus 10.0% admission rate, n = 142. An 80% reduction was also seen in infants born at 29–32 wGA (1.6% versus 7.7%, n = 373) and 73% reduction in infants born at 33–35 wGA (2.2% versus 8.2%, n = 209).Citation45
A study carried out in US and Canada assessed the effectiveness of palivizumab in preventing hospital admission using a test-negative case–control design.Citation46 The study showed that palivizumab was effective in preventing admission for infants ≤6 months of chronologic age without BPD or CHD if they were born at <29 wGA or 29–35 wGA in 66% and 74% of cases, respectively.
This is echoed by our analysis, which demonstrated cost-effectiveness in premature infants born at ≤29 wGA, a group which is currently at high unmet need.
Economic evidence comes from work in Canada and the USA, which looked at the impact of restricting the use of palivizumab on hospital admissions for RSV. Data from Quebec in Canada compared hospitalization rates for RSV during 2 years in which palivizumab prophylaxis was used and the subsequent 2 years in which palivizumab was not funded for premature infants born at 33–35 wGA.Citation47 There was a significant increase in the incidence of hospitalizations due to RSV/LRTI between the two periods. Admission rates rose from 3.3% in the period in which palivizumab was used to 4.19% in the period without palivizumab. Furthermore, those infants hospitalized during the period without palivizumab had a longer stay in ICU (mean 5.9 days versus 7.0 days in paediatric ICU and mean 4 days versus 26.6 days in neonatal ICU), required more days of mechanical ventilation (4.8 versus 6.1 days) and oxygen supplementation (4 versus 4.4 days), with clear cost consequences.
Similar data from the US considered RSV hospitalizations in premature infants before and after the 2014 American Academy of Pediatrics (AAP) guidance on the use of prophylaxis which recommended against the use of palivizumab in infants born at 29–34 wGA unless the infant had an underlying health condition, including BPD or CHD.Citation48 The study compared the rate ratios for RSV hospitalization in premature (29–34 wGA) and full term (>36 wGA) infants. During the period in which palivizumab was recommended, the rate ratio (rate of hospitalization for RSV in premature infants compared to rate of hospitalization for RSV in term infants aged <6 months, rate ratio >1 indicates higher rates of RSV hospitalization in the premature cohort) ranged from 1.6 to 3.4 depending on the setting (commercial, Medicaid, age <3 months, age 3–6 months). However, after the change in guidance, the rate ratios rose to between 2.6 and 5.6. The risk of admission for infants born 29–34 wGA was significantly higher (p < 0.0001) after the guidance change, regardless of setting.
A US-based study assessed the impact of RSV in infants born at 29–35 wGA and aged <1 year who did not receive palivizumab due to restrictive AAP guidelines.Citation49 The study included 702 infants hospitalized due to RSV, of whom 42% were admitted to ICU and 20% required mechanical ventilation. Hospital length of stay in the overall cohort was 5 days (range 3–10 days) and ICU length of stay was 6 days (range 3–12 days). Younger infants (29–32 wGA) required the most intervention, with 68% admitted to ICU and 44% requiring mechanical ventilation, length of stay for younger infants were 6 days (range 3–14 days) and 8 days (3–14 days), respectively.
Other economic analyses have demonstrated CE of palivizumab in premature infants with or without BPD or CHD.Citation44 Specific evidence for CE in premature infants is provided by two studies, one using England-based Hospital Episode Statistics (HES) data in which palivizumab was found to be cost-effective in extremely premature infants (≤27 wGA) and premature infants (28–36 wGA)Citation50 and one from Mexico which found that palivizumab was dominant in infants born at <29 wGA and cost-effective in those born at 29–32 wGA.Citation51
The English analysis carried out by Thomas et al. used a cost benefit approach utilizing HES data to quantify hospital costs for admission for RSV (infants with BPD, infants with CHD, infants born at <28 wGA, infants born at 28–36 wGA, all other admissions).Citation50 The study considered potential benefits of prophylaxis with palivizumab, estimated as the relative risk reduction in hospital admissions with palivizumab multiplied by the actual costs of hospitalization due to RSV (taken from HES) and indirect costs (evaluation of the loss of life [as lost earnings] and the reduced quality of health that results from RSV infection) for each subgroup. The direct costs of prophylaxis (cost of palivizumab and cost of administration of palivizumab) were subtracted from the avoidable costs to give net benefits, and then the net benefit per infant estimated. The benefit to cost ratio was then calculated by the costs of palivizumab, benefit to cost ratio of >1 indicated cost savings. Using this approach, prophylaxis with palivizumab was cost-effective in infants with BPD, infants born at <28 wGA and infants born at 28–36 wGA. Prophylaxis with palivizumab was not cost-effective in infants with CHD or other RSV-related admissions due to the absolute population size; with drug costs outweighing benefits in these populations.
Indeed, in the HES analysis detailed above, most admissions for RSV (80%) were in infants not defined as high risk.Citation50 This pattern is confirmed by data which considered all singleton children born in Scotland between October 2009 and September 2012 with 3-year follow-up. The study found that although the risk of hospitalization was higher in premature infants, with or without BPD or CHD, the number of hospitalizations due to confirmed RSV was actually highest in infants born >37 wGA (84.2% of admissions due to RSV).Citation7 Vaccines currently under development may provide a cost-effective approach to protection against RSV in the wider populations.
Strengths and limitations
The main strength of this economic analysis is that it builds on previous models used by the JCVI to inform their recommendations for use of palivizumab. The original work which informed the JCVI decision was carried out by Wang et al.Citation24,Citation25 and updated to include long-term respiratory sequalae by Bentley et al.Citation27
The model uses data from the pivotal trials for palivizumab and as much UK-based data as possible. The inputs into the model have been updated where available and validated by UK clinicians working in the field. However, it was challenging to update all the data inputs.
Data was identified to update RSV hospitalization rates for infants with CHD and infants with BPD from a study which used HES data from 296,618 individual birth records from 2007 to 2008 which were linked to hospital admission in the first year of life.Citation52 However, the study identified RSV admissions using ICD-10 codes which included admissions for acute bronchiolitis due to RSV and infection by organisms other than RSV. Therefore, we chose to use the original data from the pivotal trials for palivizumab in which RSV infection was confirmed as the reason for hospital admission, which may be conservative. Recent (2019) real world data was also identified for the efficacy of prophylaxis with palivizumab in preventing hospital admission, however, we chose not to use this data because the data was from BrazilCitation34 and TaiwanCitation53 and so not considered generalizable. Furthermore, because the baseline risk of hospitalization was taken from the pivotal trials for palivizumab, we believe that the efficacy of prophylaxis with palivizumab should also be taken from the same data source.
Data was updated for mortality rates of infants hospitalized due to RSV disease. We identified two large systematic reviews in infants with RSV and CHDCitation54 and with BPD.Citation55 The systematic reviews found mortality rates of 0–3.3% in CHD infants and 0–2% in BPD infants. We were concerned that these figures differed from those in the original model and sought UK clinical advice. Clinical advice suggested that we used 2% as the mortality rate for both CHD and BPD infants.
The main limitation of this work is the uncertainty around the epidemiology of long-term respiratory sequalae. Increasing data support a link between the development of long-term respiratory problems such as asthma and wheeze and severe RSV infection.Citation10–14,Citation16–18 It does not seem unreasonable to assume that RSV infection in premature infants, who are born before the lungs are fully developed and with decreased pulmonary function, would result in long-term respiratory issues. However, we cannot be sure that the long-term respiratory problems are as a result of RSV infection or as a result of premature lung development. The members of the Round Table meeting believed that severe RSV infection contributed to early lung problems but considered that the evidence was not compelling enough to extend the impact of respiratory sequalae out to more than 7 years.
This work differs from earlier work by Wang et al.Citation24,Citation25 and Bentley et al.Citation27 since the base case considers a lower number of doses of palivizumab (3.7 doses versus 5 doses). We believe that this is an appropriate approach for a number of reasons: it reflects real world evidence in the UKCitation33 and world-wide,Citation34–37 advice from UK clinicians supports this approach and the seasonality of the virus means that patients born in season and those in neonatal ICU in season will require fewer doses.Citation10
Conclusions
This updated health economic analysis suggests that palivizumab prophylaxis is cost-effective in premature infants born <29 wGA, 29–32 wGA and 33–35 wGA without CHD or BPD aged <6 months at the start of the RSV season and in premature infants with CHD or BPD aged <2 years. Cost-effectiveness is achieved because prophylaxis with palivizumab is cost saving in these populations compared with no prophylaxis and results in an improvement in QALY, leading to a dominant ICER. The INMB are positive confirming the CE of prophylaxis with palivizumab. This is a wider population than recommended in the current JCVI guidelines,Citation3 which restricts palivizumab to premature infants with CHD or BPD and children aged <2 years with severe immunodeficiency.
A key driver in achieving CE in our model is the number of doses of palivizumab: we used a mean of 3.7 doses rather than the 5 doses suggested in the product licence. This change reflects the seasonality of infection, hospitalization of premature infants (who will not require prophylaxis whilst in hospital) and real-world evidence in infants treated with palivizumab. Since patients only receive prophylaxis with palivizumab during the RSV season when they are at risk of RSV infection, appropriate dosing for a proportion of these patients will be less than 5 doses.
Transparency
Declaration of funding
AbbVie participated in the development of the methodology and funded a Round Table meeting to inform the modeling approach.
All funding for the work went to JB Medical Limited to update and run the economic model and provide medical writing support following input from AbbVie, Advisory Board insights and literature review.
Declaration of financial/other relationships
ON is affiliated with The University of Manchester and has received Speakers’ fees from AbbVie for lectures at industry organized Symposium for attendance at advisory board meetings. ON is a joint investigator on GlaxoSmithKline and Janssen sponsored RSV Vaccine trials.
AB is a director in Mtech Access Limited and received fees from AbbVie for development of the original economic model and attendance at an advisory board meeting.
KM is an employee of AbbVie Limited.
MH is an employee of AbbVie Limited.
DP is an employee of AbbVie Limited.
ENK is an employee of JB Medical Limited.
JB is a director of JB Medical Limited.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed that they are an author on several of the papers referenced by this paper, studies which were also funded by AbbVie. They have also acted as an expert advisor and given lectures for AbbVie. The reviewers have no other relevant financial relationships or otherwise to disclose.
Author contributions
KM, JB, MH, DP and AB were involved in the conception and design of this work, ENK carried out economic modeling, AB provided health economic input, ON provided clinical input and all authors revised and approved the manuscript for publication. All authors agree to be accountable for all aspects of the work.
Acknowledgements
Tricia Dixon of JB Medical Limited provided medical writing support.
References
- Henderson FW, Collier AM, Clyde WA Jr, et al. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534.
- Joint Committee on Vaccination and Immunisation. Joint Committee on Vaccination and Immunisation Statement on immunisation for Respiratory Syncytial Virus.
- Public Health England. Immunisation against infectious disease (Green Book). Chapter 27a Respiratory syncytial virus. 2015.
- Cromer D, van Hoek AJ, Newall AT, et al. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: a modelling and cost-effectiveness analysis for England. Lancet Public Health. 2017;2:e367–e374.
- Figueras-Aloy J, Manzoni P, Paes B, et al. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among preterm infants without chronic lung disease or congenital heart disease. Infect Dis Ther. 2016;5:417–452.
- Thwaites R, Buchan S, Fullarton J, et al. Clinical burden of severe respiratory syncytial virus infection during the first 2 years of life in children born between 2000 and 2011 in Scotland. Eur J Pediatr. 2020;179:791–799.
- Hardelid P, Verfuerden M, McMenamin J, et al. The contribution of child, family and health service factors to respiratory syncytial virus (RSV) hospital admissions in the first 3 years of life: birth cohort study in Scotland, 2009 to 2015. Euro Surveill. 2019;24:1800046.
- Thwaites R, Coutts J, Fullarton J, et al., editors. Burden of respiratory syncytial virus hospitalisations (RSVH) over the first 2 years of life in children born <29 weeks' gestational age (WGA) European Society for Paediatric Infectious Diseases. Virtual meeting, 2020 Oct 26–29.
- Kugelman A, Colin AA. Late preterm infants: near term but still in a critical developmental time period. Pediatrics. 2013;132:741–751.
- Luna M, Manzoni P, Paes B, et al. Expert consensus on palivizumab use for respiratory syncytial virus in developed countries. Paediatr Respir Rev. 2020;33:35–44.
- Carbonell-Estrany X, Perez-Yarza EG, Garcia LS, et al.; IRIS (Infección Respiratoria Infantil por Virus Respiratorio Sincitial) Study Group. Long-term burden and respiratory effects of respiratory syncytial virus hospitalization in preterm infants-the SPRING study. PloS One. 2015;10:e0125422.
- Scheltema NM, Nibbelke EE, Pouw J, et al. Respiratory syncytial virus prevention and asthma in healthy preterm infants: a randomised controlled trial. Lancet Respir Med. 2018;6:257–264.
- Fauroux B, Simoes EAF, Checchia PA, et al. The burden and long-term respiratory morbidity associated with respiratory syncytial virus infection in early childhood. Infect Dis Ther. 2017;6:173–197.
- Coutts J, Fullarton J, Morris C, et al. Association between respiratory syncytial virus hospitalization in infancy and childhood asthma. Pediatr Pulmonol. 2020;55:1104–1110.
- Skirrow H, Wincott T, Cecil E, et al. Preschool respiratory hospital admissions following infant bronchiolitis: a birth cohort study. Arch Dis Child. 2019;104:658–663.
- Marlow R, Finn A, Henderson J. Assessing the association between bronchiolitis in infancy and recurrent wheeze: a whole English birth cohort case-control study. Thorax. 2019;74:503–505.
- Henderson J, Hilliard TN, Sherriff A, et al. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol. 2005;16:386–392.
- Shi T, Ooi Y, Zaw EM, et al.; RESCEU Investigators. Association between respiratory syncytial virus-associated acute lower respiratory infection in early life and recurrent wheeze and asthma in later childhood. J Infect Dis. 2020;222:S628–S633.
- AbbVie Ltd. Summary of product characteristics: Synagis 100 mg/ml solution for injection. 2018.
- Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. The IMpact-RSV Study Group. Pediatrics. 1998;102:531–537.
- Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–540.
- Mochizuki H, Kusuda S, Okada K, et al.; Scientific Committee for Elucidation of Infantile Asthma. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. Six-year follow-up study. Am J Respir Crit Care Med. 2017;196:29–38.
- Igde M, Kabasakal H, Ozturk O, et al. Palivizumab prophylaxis, respiratory syncytial virus and subsequent development of asthma. Minerva Pediatr. 2018;70:252–259.
- Wang D, Cummins C, Bayliss S, et al. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assess. 2008;12:iii, ix-x, 1–86.
- Wang D, Bayliss S, Meads C. Palivizumab for immunoprophylaxis of respiratory syncytial virus (RSV) bronchiolitis in high-risk infants and young children: a systematic review and additional economic modelling of subgroup analyses. Health Technol Assess. 2011;15:iii–iv, 1–124.
- Wang D, Cummins C, Bayliss S, et al. The clinical and cost-effectiveness of Immunoprophylaxis against respiratory syncytial virus with palivizumab in children. Birmingham: Department of Public Health and Epidemiology West Midlands Health Technology Assessment Group University of Birmingham; 2007.
- Bentley A, Filipovic I, Gooch K, et al. A cost-effectiveness analysis of respiratory syncytial virus (RSV) prophylaxis in infants in the United Kingdom. Health Econ Rev. 2013;3:18.
- Sanchez-Luna M, Burgos-Pol R, Oyaguez I, et al. Cost-utility analysis of palivizumab for respiratory syncytial virus infection prophylaxis in preterm infants: update based on the clinical evidence in Spain. BMC Infect Dis. 2017;17:687.
- Schmidt R, Majer I, Garcia Roman N, et al. Palivizumab in the prevention of severe respiratory syncytial virus infection in children with congenital heart disease; a novel cost-utility modeling study reflecting evidence-based clinical pathways in Spain. Health Econ Rev. 2017;7:47.
- Blanken MO, Frederix GW, Nibbelke EE, et al.; Dutch RSV Neonatal Network. Cost-effectiveness of rule-based immunoprophylaxis against respiratory syncytial virus infections in preterm infants. Eur J Pediatr. 2018;177:133–144.
- McGirr AA, Schwartz KL, Allen U, et al. The cost-effectiveness of palivizumab in infants with cystic fibrosis in the Canadian setting: a decision analysis model. Hum Vaccin Immunother. 2017;13:599–606.
- Messori A, Trippoli S. The results of a pharmacoeconomic study: incremental cost-effectiveness ratio versus net monetary benefit. Heart. 2017;103:1746–1746.
- Thwaites R, Edwards K, Buchan S. Reducing the burden of respiratory syncytial virus: an audit of the use of palivizumab prophylaxis in the UK during the September 2001–March 2002 RSV season. J Neonatal Nurs. 2008;14:116–123.
- de Souza RP, Ribeiro ALR, de Menezes SAF, et al. Incidence of respiratory syncytial virus infection in children with congenital heart disease undergoing immunoprophylaxis with palivizumab in Pará state, north region of Brazil. BMC Pediatr. 2019;19:299–299.
- Monteiro AIMP, Bellei NCJ, Sousa AR, et al. Respiratory infections in children up to two years of age on prophylaxis with palivizumab. Rev Paul Pediatr. 2014;32:152–158.por.
- Kim AY, Jung SY, Choi JY, et al. Retrospective multicenter study of respiratory syncytial virus prophylaxis in Korean children with congenital heart diseases. Korean Circ J. 2016;46:719–726.
- Castillo LM, Bugarin G, Arias JC, et al. One-year observational study of palivizumab prophylaxis on infants at risk for respiratory syncytial virus infection in Latin America. J Pediatr. 2017;93:467–474.
- Office for National Statistics. Number of live births in the United Kingdom (UK) from 2000 to 2017 (in 1,000 births). 2018.
- Greenough A, Alexander J, Burgess S, et al. Health care utilisation of prematurely born, preschool children related to hospitalisation for RSV infection. Arch Dis Child. 2004;89:673–678.
- Greenough A, Cox S, Alexander J, et al. Health care utilisation of infants with chronic lung disease, related to hospitalisation for RSV infection. Arch Dis Child. 2001;85:463–468.
- Shefali-Patel D, Paris MA, Watson F, et al. RSV hospitalisation and healthcare utilisation in moderately prematurely born infants. Eur J Pediatr. 2012;171:1055–1061.
- Sigurs N, Bjarnason R, Sigurbergsson F, et al. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500–505.
- Sigurs N, Bjarnason R, Sigurbergsson F, et al. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161:1501–1507.
- Mac S, Sumner A, Duchesne-Belanger S, et al. Cost-effectiveness of palivizumab for respiratory syncytial virus: a systematic review. Pediatrics. 2019;143:e20184064.
- Notario G, Vo P, Gooch K, et al. Respiratory syncytial virus-related hospitalization in premature infants without bronchopulmonary dysplasia: subgroup efficacy analysis of the IMpact-RSV trial by gestational age group. Pediatr Health Med Ther. 2014;5:43–48.
- Anderson EJ, Carosone-Link P, Yogev R, et al. Effectiveness of palivizumab in high-risk infants and children: a propensity score weighted regression analysis. Pediatr Infect Dis J. 2017;36:699–704.
- Papenburg J, Defoy I, Masse E, et al., editors. Impact of the withdrawal of palivizumab immunoprophylaxis on the incidence of respiratory syncytial virus hospitalizations among infants born at 33 to 35 weeks gestational age in the province of Quebec, Canada (RSV-QC Study). Poster session presented at: International Conference on Clinical Neonatology; 2019; Venice, Italy.
- Goldstein M, Krilov LR, Fergie J, et al. Respiratory syncytial virus hospitalizations among U.S. preterm infants compared with term infants before and after the 2014 American Academy of Pediatrics guidance on immunoprophylaxis: 2012–2016. Amer J Perinatol. 2018;35:1433–1442.
- Anderson EJ, Krilov LR, DeVincenzo JP, et al. SENTINEL1: an observational study of respiratory syncytial virus hospitalizations among U.S. infants born at 29 to 35 weeks' gestational age not receiving immunoprophylaxis. Am J Perinatol. 2017;34:51–61.
- Thomas G. A cost-benefit analysis of the immunisation of children against respiratory syncytial virus (RSV) using the English Hospital Episode Statistics (HES) data set. Eur J Health Econ. 2018;19:177–187.
- Majer I, Pichardo-Pina CA, Sanchez-Casillas JL, et al. Cost-effectiveness of palivizumab in premature infants and children with chronic lung disease in Mexico. Value Health. 2015;18:A834.
- Murray J, Bottle A, Sharland M, et al.; Medicines for Neonates Investigator Group. Risk factors for hospital admission with RSV bronchiolitis in England: a population-based birth cohort study. PloS One. 2014;9:e89186.
- Lin Y-J, Chung C-H, Chi H, et al. Six-monthly palivizumab prophylaxis effectively reduced RSV-associated hospitalization rates of preterm infants in a subtropical area: a population-based cohort study. Pediatr Res. 2019;86:628–634.
- Checchia PA, Paes B, Bont L, et al. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among infants with congenital heart disease. Infect Dis Ther. 2017;6:37–56.
- Paes B, Fauroux B, Figueras-Aloy J, et al. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among infants with chronic lung disease. Infect Dis Ther. 2016;5:453–471.