Abstract
Objective
To assess the cost-effectiveness of pazopanib versus sunitinib as a first-line treatment for patients with metastatic renal cell carcinoma (mRCC) from an Italian National Health Service perspective, considering the evolving Italian landscape in terms of new reimbursement agreements trend.
Methods
This analysis is an update of the previously published cost-effectiveness analysis to incorporate recent 2019 costs and additional changes regarding drug discounting. A partitioned-survival analysis model with three different health states (progression-free survival, post-progression survival, and dead) was utilized. Outcomes included progression-free life years, post-progression life years, overall life years, quality-adjusted life years (QALYs), and costs calculated for both treatments. Cost-effectiveness was assessed in terms of incremental costs per QALY gained and the net monetary benefit (NMB) of pazopanib versus sunitinib. In the base case analysis, a time horizon of 5 years was used and future costs and QALYs were discounted at a 3% annual discount rate. An impact of methodological and parameter uncertainly on base case results was evaluated using probabilistic and deterministic sensitivity analyses.
Results
In the base case, pazopanib had higher QALYs (+0.060) at lower costs (−€5,857) versus sunitinib, hence it dominated sunitinib. At willingness-to-pay thresholds of €30,000 and €50,000 per QALY, the NMB with pazopanib were €7,647 and €8,841 per patient, respectively, versus sunitinib. The probability that pazopanib is cost-effective versus sunitinib was estimated to be 97.5% at a cost-effectiveness threshold of €20,000, 95.4% at a threshold of €30,000, and 90.2% at a threshold of €50,000 per QALY. Cost-effectiveness results were robust to changes in key parameter values and assumptions as demonstrated by deterministic sensitivity analyses.
Conclusions
Pazopanib is likely to represent a cost-effective treatment option compared with sunitinib as a first-line treatment for patients with metastatic RCC in Italy.
Introduction
Renal cell carcinoma (RCC) typically originates in the lining of the tubules of the kidney (renal parenchyma)Citation1 and consists of approximately 90% of all renal cancersCitation2. There are three major histologic subtypes, namely clear-cell (ccRCC; accounting for 80–90% of RCC), papillary (pRCC; 10–15% of RCC), and chromophobe (chRCC; 4–5% of RCC), although the more recent WHO classification recognizes more than 20 different histotypes. Approximately one-third of the patients are initially diagnosed with advanced or metastatic disease (mRCC)Citation2. Smoking, obesity, and hypertension are the most strongly associated risk factors for RCCCitation3–5. An age-standardized incidence rate of kidney cancer was reported as 4.5 cases per 100,000 people as per 2018 GLOBCAN, with the highest rates observed in North America (11.7), followed by Western Europe (9.8), and Australia/New Zealand (9.2)Citation6. In Italy, age-standardized incidence rates were reported as 8.7 per 100,000 peopleCitation7.
Treatment for RCC is highly individualized, and it varies depending on a range of factors including tumor stage or location, the spread of disease, renal function, comorbidities, and overall health statusCitation8. The first-line treatment of mRCC has evolved rapidly in the past decadeCitation9,Citation10. Growing understanding of the molecularly altered pathways for cancer led to the development of targeted therapies that have rapidly replaced these initial therapies for mRCC patientsCitation11. Treatment decisions are generally based on the risk profile of mRCC patients determined using the risk score developed by the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC)Citation12.
Due to the seminal genetic alterations found in the vast majority of RCCs, that is, mutation, deletion or hypermethylation of the VHL tumor suppressor gene, this tumor is a highly angiogenesis-dependent malignancy, characterized by the hyperproduction of vascular endothelial growth factor (VEGF) and other pro-angiogenic cytokines; thus, tyrosine kinase inhibitors (TKIs) targeting VEGF and its receptors (VEGFRs) still remain the mainstay of therapy for patients with mRCCCitation8,Citation13,Citation14. TKIs include pazopanib, sunitinib, cabozantinib, tivozanib, axitinib, and sorafenib. For mRCC patients with a favorable risk profile, pazopanib and sunitinib are strongly recommended first-line treatments by key treatment guidelines like National Comprehensive Cancer Network (NCCN)Citation13, European Association of Urology (EAU)Citation14, and European Society for Medical Oncology (ESMO)Citation8. Other active treatments include the mammalian target of rapamycin (mTOR) inhibitors and immune checkpoint inhibitors such as programmed death receptor 1/programmed death receptor ligand 1 (PD-1/PD-L1) inhibitors and cytotoxic T lymphocytes antigen 4 (CTLA-4) inhibitorsCitation11. summarizes currently available first-line treatment options for mRCC patients.
Table 1. Overview of current options for first-line treatment of mRCC.
More recently, a combination therapy with two immune checkpoint inhibitors – nivolumab (PD-1 inhibitor) and ipilimumab (CTLA-4 inhibitor) – was shown to be effective in the first-line setting in the phase III CheckMate-214 studyCitation15. In this study, treatment-naive patients with mRCC treated with nivolumab plus ipilimumab had significantly improved the co-primary endpoints of objective response rate (ORR) and overall survival (OS) compared with sunitinib in patients with the intermediate or poor-risk profile. Therefore, this combination is strongly recommended treatment for intermediate or poor-risk patients in different guidelines like NCCN, EAU, and ESMOCitation8,Citation13,Citation14. Apart from this therapy, approved cabozantinib (TKI) is also recommended for the intermediate and poor-risk RCC patients by these guidelines; however, this recommendation is weaker, being supported by the results of a relatively small randomized phase II trial.
Recently few trials reported encouraging results for the combination of immune checkpoint and VEGF inhibitors in patients with previously untreated mRCC, cementing the strategy of using a combination of drugs from these two different classes. The keynote-426 trial demonstrated that a combination of pembrolizumab (PD-1 inhibitor) plus axitinib (TKI) was superior to sunitinib regardless of the risk, with an acceptable safety profileCitation16. Furthermore, the Javelin Renal-101 trial demonstrated avelumab (PD-L1 inhibitor) plus axitinib (TKI) to be more efficacious than sunitinib for all risk group patients. Recently NCCN recommended both combinations – pembrolizumab plus axitinib and avelumab plus axitinib – for favorable as well as intermediate/poor risk patientsCitation13.
For favorable-risk patients, pazopanib and sunitinib still remain the most commonly used first-line treatmentsCitation8,Citation13,Citation14. Head-to-head comparison between pazopanib and sunitinib as a first-line treatment for mRCC has been conducted in two randomized phase III trials. The COMPARZ trial was the non-inferiority study that included 1,110 mRCC patientsCitation17,Citation18. The primary endpoint of non-inferior PFS with pazopanib versus sunitinib was met (median, 843 vs 9.5 months; hazard ratio [HR], 1.05; 95% confidence interval [CI], 0.90–1.22). The comparative efficacy of pazopanib and sunitinib was established by the secondary efficacy endpoints of ORR (31 vs 25%, respectively; p = .03) and median OS (28.3 vs 29.1 months, respectively; p = .24). In a post-hoc analysis of COMPARZ trial, one potential difference in efficacy between sunitinib and pazopanib was the time to respond in patients achieving a partial or complete response, which was numerically shorter for pazopanib versus sunitinib (11.9 vs 17.4 weeks)Citation19. The randomized, cross-over, double-blind, phase III PISCES study revealed differences in patient preference between first-line pazopanib and sunitinib in patients with mRCCCitation20. A significantly greater proportion of patients preferred pazopanib compared with sunitinib (70 vs 22%; p < .001), with the most common reasons being a better overall quality of life (QoL) and less fatigue. In Italy, both drugs are hospital products (Class H) and are fully reimbursed by the NHS as per the label without any restriction.
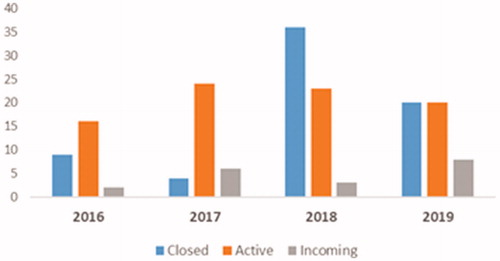
Apart from clinical efficacy, safety, quality of life, and patient preference, the cost could be one of the major drivers in selecting one treatment over the other. Therefore, economic analyses of treatment choices are becoming increasingly important to both payers and prescribers in order to determine the most cost-effective treatments for patients. Our previous analysis assessed the cost-effectiveness of pazopanib versus sunitinib as a first-line treatment for patients with mRCC from an Italian National Health Service perspectiveCitation21. Recently there has been a change in the negotiation agreements for pazopanib and sunitinib in Italy. For both treatments, related AIFA registry and the associated managed entry agreements (MEAs) have been closed, and are replaced with the application of a new confidential discountCitation22,Citation23. This is in line with the recent AIFA trend to close the monitoring registers and replace the MEAs with confidential discounts, in the more general perspective of a greater simplification ()Citation24. Considering these changes, the present analysis is an update of the previous modelCitation21 to incorporate recent 2019 costs and additional changes regarding the discounting of both treatments.
Figure 1. AIFA Registry closing trend (2016–2019). Source: AIFA RegistryCitation24.
Methods
Model structure
A partitioned-survival analysis model with three mutually exclusive health states – alive with no progression (progression-free survival; PFS), alive with progression (post-progression survival; PPS), and dead – was used to calculate the expected costs, life-years, and quality-adjusted life-years (QALYs) for treatment-naive patients receiving pazopanib and sunitinib for mRCC. The population of interest was assumed to be the same as that in the COMPARZ trialCitation17 and to represent a typical population of patients with mRCC. The proportion of patients in each health state overtime was calculated based on survival functions for PFS and OS obtained from the COMPRAZ trial. PPS was calculated as the difference between OS and PFS. Costs and health-related quality of life (HRQoL) were assumed to be conditioned on treatment and expected time in the progression-free and post-progression disease states. The cycle duration of the model was kept as 1 week to accommodate the 4-week cycle for pazopanib and the 6-week cycle for sunitinib, and thus eliminating the requirement for a half-cycle correction. This is the adaption of the previous model assessing the cost-effectiveness of pazopanib and sunitinib in mRCC patients in ItalyCitation21.
Model inputs
Clinical inputs
Survival data were obtained from the COMPARZ trial, wherein long-term PFS and OS data were reported for direct comparison between pazopanib and sunitinibCitation18. In the base case, OS for pazopanib and sunitinib to 60 months (the last time point for which OS was available for both treatments based on the 30 September 2013, data cut-off) was obtained based on Kaplan–Meier (KM) curves. The PFS data was not available for 60 months, but was available for 38.8 and 37.5 months, respectively, for pazopanib and sunitinib. Therefore, the PFS data up to 38 months was based on KM curves as available from the trial, and data from 38 ∼ 60 months was estimated based on Weibull survival functions fit to individual patient data from the COMPARZ trial using accelerated failure time regression. Investigator-assessed PFS, rather than independent review committee (IRC)-assessed PFS, was used because the former is more likely to accurately reflect the assessment of disease progression during the course of routine clinical practice. The λ and γ values for PFS and OS are shown in .
Table 2. Model inputs.
Utilities
A preference-based measure of HRQoL like EuroQol-5 Dimensions (EQ-5D) was not included in the COMPARZ trial. Hence, mean utility values for both treatments during PFS were estimated by combining data on the incidence and duration of adverse events (AEs) from COMPARZ trial with a regression equation relating the presence of AEs to utility valuesCitation25. The regression equation was estimated using data from the pivotal phase III randomized controlled trial of pazopanib compared with placebo in patients with mRCC (VEG105192 trial)Citation26. Generalized linear model regression was used, with patients defined as clusters. The regression equation had EQ-5D utility values as the dependent variable and baseline patient characteristics (age, sex, prior treatment, and performance status), the presence of AEs, and treatment group as independent variables. The AEs were characterized by grade (grades 1–2 vs grade 3 and greater). Patient-level data from COMPARZ were utilized for baseline patient characteristics and for the incidence and duration of AEs. Using the regression equation, utility values were estimated for every day of the pre-progression follow-up period for all patients in COMPARZ trial. Mean utility values for PFS were then estimated for each treatment group using Kaplan–Meier Sample Average (KMSA) methodsCitation27. Standard errors (SEs) for utility values were obtained by bootstrapping (see for utility inputs in the model).
Patients were not followed consistently after progression in the VEG10592 trial. Therefore, it was not possible to estimate utility values for the post-progression state using data from this study. Post-progression utility values for both treatments were therefore assumed to be 0.5509, based on the reported utility value for best supportive care after the termination of second-line therapy in a cost-effectiveness analysis of sunitinib, which was based on data from the phase III trial of sunitinibCitation28. In the PFS state, utilities are different for both pazopanib (0.7089) and sunitinib (0.6832). Hence, different disutilities for post- vs pre-progression survival were applied for pazopanib (−0.1580) and sunitinib (−0.1323) to achieve the same post-progression utilities of 0.5509 for both treatments ().
Cost inputs
Direct costs were considered in the analyses, which included the medication costs for pazopanib and sunitinib, routine follow-up care, other treatment-related costs (medical resource utilization [MRU]), and terminal care costs in Italy. Consumer price index (CPI) data sourced from the I.Stat website (http://dati.istat.it/?lang=en), a key source to get Italian statistics, was used to adjust for inflation. All costs were inflated to 2019 costs using the CPI factor of 1.028 (2014–2019).
The unit cost of pazopanib is €57.53 per 400-mg tablet and sunitinib is €195.02 per 50-mg tablet in Italy. After applying mandatory discounts for each drug of 5% (9.75% combined), discounted prices were estimated to be €51.92 per 400-mg tablet for pazopanib and €176.01 per 50-mg tablet for sunitinibCitation29. Dosages for pazopanib and sunitinib were assumed to be the same as per the per-protocol dosages in the COMPARZ trialCitation29. In the previous publication, costs of sunitinib and pazopanib were further discounted to account for the risk-sharing and pay-for-performance agreements with the NHSCitation21. Considering that now both drugs have in place only a confidential and not published discount, discounting was not considered for this analysis update.
In the current analysis, it has been assumed that the patient remains on the treatment until disease progression or death. However, patients can discontinue treatment before progression/death and this could have a resultant impact on treatment costs. Hence the costs of medication in the model were adjusted for treatment discontinuation or dose intensity by multiplying the projected costs by the ratio of the mean actual cumulative dose received in the COMPARZ trial using the mean planned cumulative dose over all days of follow-up; this ratio was 67.69% for pazopanib and 67.49% for sunitinib. In the model, patients were assumed to receive a prescription for pazopanib and sunitinib at the beginning of every 28-day and 42-day treatment cycle, respectively. Any medication supplied but not taken was assumed to be discarded. Thus, the full cost of a prescription was assumed to be incurred on the first day of each treatment cycle. Administration and dispensing costs for pazopanib and sunitinib were assumed insignificant and were not considered.
Additionally, other costs related to MRU were included to account for the differences in non-study MRU between pazopanib and sunitinib in the COMPARZ trial. These costs were calculated based on monthly rates of non-study MRU from COMPARZ with unit cost estimates from published or publicly available sources in Italy. Costs of managing adverse events were not considered explicitly in the model as it is assumed that differences between pazopanib and sunitinib in these costs are reflected in the differences in the costs of other services associated with pazopanib and sunitinib treatment based on MRU data collected during COMPARZ trial.
Costs of treatment initiation (€596.29; one-time cost), pre-progression routine care and follow-up (€85.47/month), and post-progression supportive care (€85.47/month) were estimated based on Capri et al.Citation29 A one-time cost of death (€7,032.42) was assumed to constitute 20.7 days of hospice careCitation30. The SEs of the cost estimates were assumed to be 25% of the mean estimates.
To arrive at the total cost of treatment, medication costs, resource utilization costs, one-time cost of treatment initiation, the cost for pre-progression routine care and follow-up, the cost for post-progression supportive care and one-time terminal care (death) costs were summed up. summarizes the different cost inputs used in the model.
Analyses
Base case analyses
For both treatments, expected progression-free life years (PFLYs), post-progression life years, overall life years, quality-adjusted life years (QALYs), and costs (medications, dispensing and administration, routine follow-up, other costs, and total costs) were calculated. A time horizon of 5 years (60 months) was used in the base case, consistent with the maximum duration of follow-up for OS in the COMPARZ trial. Both costs and QALYS were discounted using an annual discount rate of 3%. Cost-effectiveness was expressed in terms of the incremental cost per QALY gained with pazopanib versus sunitinib. In addition, incremental net monetary benefit (NMB) of pazopanib versus sunitinib was evaluated. There is no published threshold value for assessing cost-effectiveness in Italy. The willingness-to-pay (WTP) threshold for cost-effectiveness was defined alternatively as €20,000, €30,000 and €50,000 per QALY gained.
Sensitivity analyses
Both probabilistic and deterministic sensitivity analyses were conducted. Probabilistic sensitivity analyses involved simultaneous sampling from estimated probability distributions of model parameters to obtain 1,000 sets of model input estimates and then generating costs and QALYs for each set of inputsCitation31. For each comparison, simulation results were plotted on the cost-effectiveness plane, and cost-effectiveness acceptability curves were generated for pazopanib versus sunitinib to identify the proportion of simulations in which pazopanib was preferred given various levels of WTP threshold values for cost per QALY gained.
The impact of changing assumptions concerning key model parameter values on the incremental cost-effectiveness and NMB of pazopanib versus sunitinib was explored through one-way sensitivity analyses, which incorporated a variety of different scenarios with variations in the time horizon, PFS, OS, relative dose intensity, administration/dispensing costs, monthly costs, decrements in utility, and discount rates.
Results
Base-case analyses
mRCC patients treated with pazopanib had 1.613 QALYs at the total costs of €55,721, whereas patients treated with sunitinib had 1.553 QALYs at the total costs of €61,578 (). Pazopanib-treated patients had higher QALYs (+0.060) at the lower costs (−€5,857) compared with sunitinib, hence pazopanib dominated sunitinib. Both progression-free QALYs (+0.020) and post-progression QALYs (+0.039) were higher for pazopanib vs sunitinib. Reduced medication costs (€30,602 for pazopanib vs €35,472 for sunitinib) and other treatment-related costs (estimated based on MRU data from the COMPARZ trial; €2,907 for pazopanib vs €3,942 for sunitinib) mainly contributed to the lower total costs for with pazopanib vs sunitinib ().
Table 3. Base case results.
Progression-free life years were slightly lower for pazopanib vs sunitinib (−0.014), but post-progression life years were higher for pazopanib (+0.071). This resulted in slightly higher overall life years (+0.058) for pazopanib compared with sunitinib ().
The NMB with pazopanib were €7,647 and €8,841 per patient versus sunitinib at WTP thresholds of €30,000 and €50,000 per QALY, respectively.
Sensitivity analyses
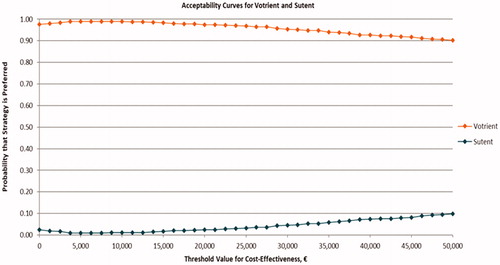
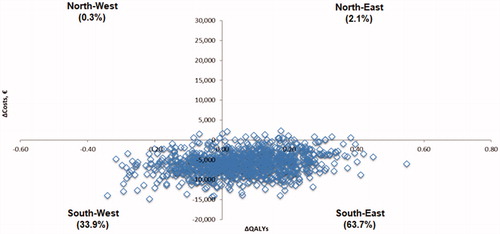
The results of the probabilistic sensitivity analyses are displayed on the cost-effectiveness plane in . The 95% credible interval of the difference in costs for pazopanib versus sunitinib ranged from −€11,386 to −€137. The 95% credible interval of the difference in QALYs for pazopanib versus sunitinib was −0.213 to 0.306. In 63.7% of simulations, pazopanib was projected to have more QALYs and lower costs compared with sunitinib (pazopanib dominant, south-east quadrant); whereas the opposite (sunitinib dominant, north-west quadrant) was expected in only 0.3% of simulations (). The probability that pazopanib was cost-effective versus sunitinib was 97.5%, given a threshold value of cost-effectiveness of €20,000 per QALY gained; 95.4%, given a threshold value of €30,000; and 90.2%, given a threshold value of €50,000 ().
Figure 2. Scatter plot of incremental costs and incremental QALYs for pazopanib versus sunitinib from probabilistic sensitivity analyses. Abbreviation. QALYs: quality-adjusted life years.
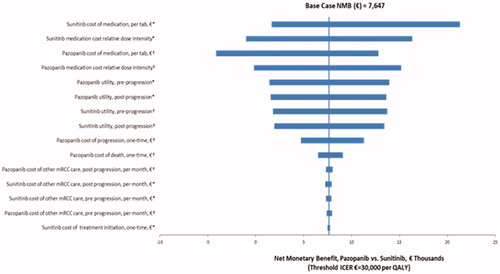
The results of the deterministic sensitivity analyses are presented as a tornado diagram in . In these analyses, the threshold value for cost-effectiveness was defined as €30,000 per QALY. The NMB for pazopanib versus sunitinib was most sensitive to assumptions regarding the medication costs for pazopanib and sunitinib, pre- and post-progression utility values, and other costs like post-progression costs, treatment initiation costs.
Figure 4. Deterministic sensitivity analysis – Tornado diagram for NMB of pazopanib versus sunitinib. *Low value of parameter corresponds to low value of NMB; high value of parameter corresponds to high value of NMB. †Low value of parameter corresponds to high value of NMB; high value of parameter corresponds to low value of NMB.
Discussion
The current study which is an update of previous analysisCitation21 evaluated the cost-effectiveness of pazopanib versus sunitinib as a first-line treatment for mRCC from an Italian healthcare system perspective utilizing PFS, OS, and MRU data from the COMPARZ trial. Utility values were obtained using AEs data from COMPARZ trial and a regression equation based on EQ-5D from the pivotal trial of pazopanib versus placebo in mRCC. In the base-case analysis, pazopanib was projected to yield more QALYs and lower treatment costs compared with sunitinib. Hence pazopanib was found to be a dominant treatment compared with sunitinib in the base case. The benefits of pazopanib on QALYs were derived mainly from the assumed difference in HRQoL in pazopanib patients compared with sunitinib, as well as a projected gain in post-progression survival. The robustness of base case results was assessed in sensitivity analyses. In probabilistic sensitivity analyses, in 63.7% of simulations pazopanib was found to be the dominant treatment, whereas sunitinib was found to be the dominant treatment in only 0.3% of simulations. At different WTP thresholds ranging from €20,000 to €50,000, the probability of pazopanib being cost-effective vs sunitinib was >90%.
Compared to the previous analysis, the present analysis has incorporated recent costs with all costs adjusted for inflation to the year 2019. In addition, drug discounting was not considered to keep the analysis up-to-date in line with the recent negotiation trend in Italy where the MEAs have been replaced with the confidential discount. In a previous analysis, pazopanib was associated with higher QALYs and lower costs and dominated sunitinib, and these findings are the same as the current analysis. In a previous analysis, using WTP thresholds of €30,000 and €50,000 per QALY, the NMB with pazopanib were €6,508 and €7,702 per patient, respectively, versus sunitinib. In the current update also, pazopanib had a positive incremental NMB versus sunitinib with NMB of €7,647 and €8,841 per patient, respectively, at WTP thresholds of €30,000 and €50,000 per QALY.
Although a large number of drugs are indicated for first-line treatment for mRCC, very few studies evaluated the cost-effectiveness of different treatments. Few previous old studies (before pazopanib approval for mRCC) assessed the cost-effectiveness of sunitinib compared with treatments like bevacizumab plus IFNα, sorafenib, and IL-2Citation32–34. All these studies reported sunitinib as a cost-effective treatment option for first-line mRCC patients.
Pazopanib was compared with sunitinib in three studies for different country settings, namely Canada, USA, and UKCitation35–37. All these studies employed a partitioned survival model with three health states (alive with no progression, alive with progression, and dead) and obtained clinical information from the COMPARZ trial. The Canadian study was carried out from the Canadian healthcare system perspective and a time horizon of 5 years was usedCitation35. Analyses were conducted for two different scenarios: using list prices for sunitinib and pazopanib and by assuming equivalent pricing for sunitinib and pazopanib. Based on list prices, pazopanib had lower expected costs of CA$10,293 and higher QALYs of 0.059 vs sunitinib, hence pazopanib dominated sunitinib. For equivalent pricing, pazopanib yielded CA$917 in savings compared to sunitinib. Another study conducted from the perspective of the United Kingdom’s National Health Service reported that pazopanib was estimated to provide more QALYs (+0.0565) at a lower cost (−£1,061) compared with sunitinib, hence pazopanib dominated sunitinib in this settingCitation36. A study by Delea et al., conducted from the US healthcare system perspective, supplemented clinical information retrieved from the COMPARZ trial with the PISCES trial, which collected information on patient preference, measured by EQ-5D, as the primary endpoint. Pazopanib had a lower cost of US$6,828 and higher QALYs of 0.090 vs sunitinib, thus it dominated sunitinib in US settingsCitation37. Despite the different national contexts, all these different studies also demonstrated pazopanib as a dominant first-line treatment compared with sunitinib in mRCC patients.
Six studies also compared the overall cost of pazopanib vs sunitinib as first-line therapy in mRCC patients for different countries. Despite the difference in study approaches and the definition of costs, all six studies concluded that pazopanib had a lower cost, with the magnitude of the annual cost difference ranging from less than $7,200 per patient to more than $20,000Citation38–43.
The present analyses have some limitations. This study compared pazopanib and sunitinib only and did not evaluate the cost-effectiveness of other therapies that will be approved in the near future for use as a first-line treatment of mRCC in Italy. Comparisons with these therapies would have required an indirect treatment comparison, which represents a lower level of evidence than the direct comparison of pazopanib versus sunitinib in the COMPARZ trial, which was the primary basis of this evaluation. Future analysis could consider including additional treatments once indirect treatment comparison data become available. As COMPARZ was a multinational trial, the MRU data in the trial may not reflect utilization in all settings.
The estimated benefits of pazopanib on QALYs were obtained from the assumed differences in utility values between patients who received pazopanib vs those receiving sunitinib. Because the COMPARZ trial did not include a preference-based HRQoL assessment, the current analysis estimated utility values by combining data on the incidence and duration of AEs in COMPARZ with a regression model that related AEs to utility values. The estimated difference in mean utility values for pazopanib versus sunitinib based on this approach (0.0257) is less than that used in a previous analysis from a US perspective, which was based on EQ-5D data from the PISCES trial (0.0569)Citation37. Although the difference in utility values for pazopanib and sunitinib in the current study is consistent with the HRQOL data from the COMPARZ trial and patient preferences in the PISCES study, the estimated benefits of pazopanib versus sunitinib on utility values should be interpreted cautiously. The lack of utility data from a randomized comparison represents a limitation of the analysis. In the current analysis, it has been assumed that the patient remains on the treatment until progression. However, patients can discontinue treatment before progression, which has not been considered in the current model.
Finally, another limitation is related to the fact that, when all novel combinations will be made available and reimbursed, the therapeutic space for both pazopanib and sunitinib will be limited to a percentage of mRCC patients with good IMDC prognostic criteria.
Conclusions
The results of this analysis suggest that pazopanib is likely to be a cost-effective treatment option when compared with sunitinib as a first-line treatment of mRCC in Italy in early 2020. In the future, the present analysis can be further updated with new treatment options and including preference-based HRQoL assessment measures from any trial or real-world studies once relevant data become available.
Transparency
Declaration of funding
The study is funded by Novartis Pharmaceutical Corporation, East Hanover, NJ, USA.
Declaration of financial/other interests
SC is an advisor of the major pharmaceutical company, at headquarters level (Europe and US): Ablynx, Alcon, Alexion, Allergan, Amgen, Amicus Therapeutics, Ascendis Pharma, Astellas, AstraZeneca, Audentes Therapeutics, Bayer, Biogen, Bohringer Igelheim, Bristol-Myers-Squibb, Celgene, Chiesi, Dompé, Eisai, Eli Lilly, Gilead, Grunenthal, GSK, Incyte, Janssen, LEOL Pharma, Lunbek, Merck Serono, MSD, Mundi Pharma, Nanobiotix, Novartis, Novo Nordisk, Onyx Pharmaceuticals, Otsuka, Pfizer, Roche, Sanofi, Seattle Genetics, Shire, Takeda, Tesaro, UCB. CP is a consultant and/or Speaker for Novartis, Pfizer, BMS, MSD, Ipsen, EUSA, Eisai, Astra Zeneca, Janssen and General Electrics; Expert Testimony – Pfizer, USA. SC and CP have not received any economic remuneration for their contribution in this analysis. CC, EP, AK, KM, NM, and BR are employees of Novartis.
A peer reviewer on this manuscript has disclosed that they are a salaried employee of a consultancy which receives consulting fees for research projects from pharmaceutical manufacturers. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
All authors contributed to the study design, collection of the data, interpretation of the model results, and critically reviewing the manuscript. All authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. All authors approved the manuscript for the final submission.
Acknowledgements
The authors would like to thank Thomas E. Delea for his contribution to developing the original model which has been adopted in this analysis.
References
- Azeem K, Kollarova H, Horakova D, et al. Genetic syndromes associated with renal cell carcinoma: a review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155(3):231–238.
- Ljungberg B, Campbell SC, Choi HY, et al. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60(4):615–621.
- Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7(5):245–257.
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578.
- Weikert S, Boeing H, Pischon T, et al. Blood pressure and risk of renal cell carcinoma in the European prospective investigation into cancer and nutrition. Am J Epidemiol. 2008;167(4):438–446.
- GLOBOCAN. Fact Sheets. 2018. [cited 2019 Dec 15]. Available from: https://gco.iarc.fr/today/fact-sheets-cancers
- Capitanio U, Bensalah K, Bex A, et al. Epidemiology of renal cell carcinoma. Eur Urol. 2019;75(1):74–84.
- Escudier B, Schmidinger M, Rioux-Leclercq N, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(5):706–720.
- Salgia NJ, Dara Y, Bergerot P, et al. The changing landscape of management of metastatic renal cell carcinoma: current treatment options and future directions. Curr Treat Options Oncol. 2019;20(5):41.
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376(4):354–366.
- Angulo JC, Shapiro O. The changing therapeutic landscape of metastatic renal cancer. Cancers. 2019;11(9):1227.
- Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–148.
- NCCN. NCCN clinical practice guidelines in oncology. Version 2. 2020 kidney cancer. Plymouth meeting, PA: National comprehensive Cancer Network; 2019.
- Ljungberg B, Albiges L, Yasmin AG. European Association of Urology Guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810.
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731.
- Motzer RJ, Hutson TE, McCann L, et al. Overall survival in renal-cell carcinoma with pazopanib versus sunitinib. N Engl J Med. 2014;370(18):1769–1770.
- Tannir NM, Porta C, Gruenwald V, et al. Long-term response and time to response to pazopanib (PAZ) and sunitinib (SUN) in metastatic renal cell carcinoma (mRCC): COMPARZ subanalysis. Clin Oncol. 2017;35(15):4572.
- Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Cin Oncol. 2014;32(14):1412–1418.
- Capri S, Porta C, Delea TE. Cost-effectiveness of pazopanib versus sunitinib as first-line treatment for locally advanced or metastatic renal cell carcinoma from an Italian National Health Service perspective. Clin Ther. 2017;39(3):567–580.
- Votrient Official Journal n.151 – 02.07.2018. [cited 2019 Dec 15]. Available from: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2018-07-02&atto.codiceRedazionale=18A04514&elenco30giorni=false
- Sutent Official Journal n. 43 – 20.02.2019. [cited 2019 Dec 15]. Available from: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2019-02-20&atto.codiceRedazionale=19A01061&elenco30giorni=false
- Agenzia Italiana del Farmaco (AIFA). [cited 2020 Jan 29]. Available from: https://www.aifa.gov.it/registri-e-piani-terapeutici1.
- Hagiwara M, Park J, Delea TE. Utility values among patients (pts) with metastatic renal cell carcinoma (mRCC) receiving first-line treatment with pazopanib (PZ) and sunitinib (SU). J Cin Oncol. 2016;34(15):e21068.
- Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Cin Oncol. 2010;28(6):1061–1068.
- Lin DY, Feuer EJ, Etzioni R, et al. Estimating medical costs from incomplete follow-up data. Biometrics. 1997;53(2):419–434.
- Chabot I, Rocchi A. How do cost-effectiveness analyses inform reimbursement decisions for oncology medicines in Canada? The example of sunitinib for first-line treatment of metastatic renal cell carcinoma. Value Health. 2010;13(6):837–845.
- Capri S, Valenzano G. La valutazione economica di Pazopanib rispetto alle alternative terapeutiche in uso. Ital J Public Health. 2011;8:S32–S50.
- Roggeri D, Saramin C, Terrazzani G, et al. Resource consumption and costs of treating pain in patients affected by cancer in a district of northeast Italy. Pharmacol Res. 2007;56(4):329–334.
- Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500.
- Wu B, Dong B, Xu Y, et al. Economic evaluation of first-line treatments for metastatic renal cell carcinoma: a cost-effectiveness analysis in a health resource-limited setting. PloS One. 2012;7(3):e32530.
- Calvo Aller E, Maroto P, Kreif N, et al. Cost-effectiveness evaluation of sunitinib as first-line targeted therapy for metastatic renal cell carcinoma in Spain. Clin Transl Oncol. 2011;13(12):869–877.
- Benedict A, Figlin RA, Sandstrom P, et al. Economic evaluation of new targeted therapies for the first-line treatment of patients with metastatic renal cell carcinoma. BJU Int. 2011;108(5):665–672.
- Amdahl J, Diaz J, Park J, et al. Cost-effectiveness of pazopanib compared with sunitinib in metastatic renal cell carcinoma in Canada. Curr Oncol. 2016;23(4):e340–e354.
- Amdahl J, Diaz J, Sharma A, et al. Cost-effectiveness of pazopanib versus sunitinib for metastatic renal cell carcinoma in the United Kingdom. PloS One. 2017;12(6):e0175920.
- Delea TE, Amdahl J, Diaz J, et al. Cost-effectiveness of pazopanib versus sunitinib for renal cancer in the United States. J Manag Care Spec Pharm. 2015;21(1):46–54.
- Vogelzang NJ, Pal SK, Ghate SR, et al. Clinical and economic outcomes in elderly advanced renal cell carcinoma patients starting pazopanib or sunitinib treatment: a retrospective medicare claims analysis. Adv Ther. 2017;34(11):2452–2465.
- Vogelzang NJ, Pal SK, Ghate SR, et al. Real-world economic outcomes during time on treatment among patients who initiated sunitinib or pazopanib as first targeted therapy for advanced renal cell carcinoma: a retrospective analysis of medicare claims data. JMCP. 2018;24(6):525–533.
- MacLean E, Mardekian J, Cisar LA, et al. Real-world treatment patterns and costs for patients with renal cell carcinoma initiating treatment with sunitinib and pazopanib. JMCP. 2016;22(8):979–990.
- Racsa PN, Whisman TR, Worley K. Comparing two tyrosine kinase inhibitors for treatment of advanced renal cell carcinoma in Medicare and commercially insured patients. Curr Med Res Opin. 2015;31(10):1933–1940.
- Hansen RN, Hackshaw MD, Nagar SP, et al. Health care costs among renal cancer patients using pazopanib and sunitinib. J Manag Care Spec Pharm. 2015;21(1):37–44.
- Villa G, Hernandez-Pastor LJ. Budget impact analysis of first-line treatment with pazopanib for advanced renal cell carcinoma in Spain. BMC Cancer. 2013;13:399.