Abstract
Aims
V114 is an investigational 15-valent pneumococcal conjugate vaccine (PCV) containing the 13 Streptococcus pneumoniae serotypes in 13-valent PCV (PCV13) plus two additional serotypes. This study quantified the health and economic burden of invasive pneumococcal disease (IPD) and acute otitis media (AOM) caused by V114 types among children in the United States.
Materials and methods
A Markov model estimated the number of V114-type IPD and AOM cases and costs in a hypothetical, unvaccinated US birth cohort over 20 years. Three time periods were analyzed using time-specific epidemiological data to determine the number of IPD and AOM cases associated with all 15 serotypes in V114. The time periods were: (1) pre-PCV7 (1999); (2) pre-PCV13 (2009); (3) post-PCV13 (2017). Costs were estimated from a societal perspective (2018 US dollars) and discounted at 3%.
Results
The model estimated 18,983 IPD cases and 5.4 million AOM cases associated with V114 serotypes pre-PCV7, 4,697 IPD cases and 3.0 million AOM cases pre-PCV13, and 948 IPD cases and 0.2 million AOM cases post-PCV13. Total discounted costs associated with V114 serotypes were $1.7 billion pre-PCV7, $730 million pre-PCV13, and $75 million US dollars post-PCV13.
Limitations
Post-meningitis sequelae, cases of non-bacteremic pneumonia, and direct non-medical costs were not included.
Conclusions
IPD and AOM cases and costs were estimated in a hypothetical US birth cohort followed for 20 years at three time periods. In all three periods, the serotypes targeted by V114 contributed to significant morbidity and costs. New pediatric pneumococcal vaccines must continue to retain serotypes in licensed vaccines to maintain disease reduction while extending coverage to non-vaccine serotypes.
Introduction
Streptococcus pneumoniae is a Gram-positive bacterium that causes both invasive and non-invasive disease manifestationsCitation1. Invasive pneumococcal disease (IPD) manifestations include bacteremic pneumonia, bacteremia without focus, and meningitisCitation1. In the United States (US), the annual incidence of IPD in 2017 was seven cases per 100,000 children aged <5 yearsCitation2. IPD is associated with substantial disease burden, including high case fatality rates (CFRs), and risk of developing meningitis and post-meningitis sequelae (PMS), such as hearing loss or developmental delayCitation1,Citation3.
Non-invasive manifestations of pneumococcal disease include non-bacteremic pneumococcal pneumonia (NBPP) and acute otitis media (AOM)Citation1. Although difficult to diagnoseCitation4, the incidence and burden of NBPP are understood to be considerably higher than those of IPDCitation5,Citation6. AOM also has a higher incidence rate (358.7 cases per 1,000 in US children aged ≤2 years in 2016)Citation7 and is one of the most common causes of medical visits, antibiotic prescriptions, and hearing impairment in childrenCitation8,Citation9. AOM is also associated with substantial medical costsCitation8.
Pneumococcal diseases also lead to considerable indirect costs from productivity losses due to premature disability and death in children ($3.1 billion in 2004 [2007 US dollars]), and work loss in parents caring for children with pneumococcal disease ($914 million in 2004 [2007 US dollars])Citation5.
The introduction of pneumococcal conjugate vaccines (PCVs) has been effective in reducing the burden of pneumococcal diseases. PCV7, which targets seven S. pneumoniae serotypes, was introduced into infant vaccination schedules in 2000Citation10. Its use has led to a substantial reduction in IPD and AOM cases in the USCitation11,Citation12. However, the subsequent increase in disease caused by non-PCV7 serotypes necessitated the development of new vaccines containing additional serotypes to maintain disease reductionCitation13. In 2010, PCV13, which targets 13 S. pneumoniae serotypes, was licensed and recommended for use in infants in the USCitation14. Following PCV13 introduction, the disease caused by non-PCV13 serotypes has increased, while select PCV13 vaccine serotypes, such as serotype 3, continue to persistCitation15–18. New vaccines must therefore continue to retain original PCV serotypes to maintain disease reduction while extending coverage to emerging disease-causing serotypes.
A 15-valent PCV, V114, is currently in clinical developmentCitation19,Citation20. V114 covers all serotypes included in PCV13 (serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F), as well as two additional serotypes, 22F and 33F. Serotypes 22F and 33F are two of the most common non-PCV13 serotypes found in IPD in childrenCitation15, causing 8% and 9% of residual IPD cases in children aged <5 years in the US, respectively, during 2017Citation21. Serotype 33 F has high invasive disease potential compared with other non-PCV13 serotypes and is associated with multidrug resistanceCitation22,Citation23.
The objective of this study was to quantify the health and economic burden of IPD and AOM attributable to the 15 serotypes targeted by V114 in a hypothetical, unvaccinated birth cohort over 20 years in the US. To highlight the importance of maintaining disease reduction while addressing the current disease burden, IPD and AOM cases and costs attributable to V114 serotypes were calculated at three time periods using time-specific epidemiological data to determine the number of IPD and AOM cases associated with all 15 serotypes in V114. The time periods were: (1) pre-PCV7 (1999), (2) pre-PCV13 (2009), and (3) post-PCV13 (2017 or the most current year).
Methods
Analytical approach
This analysis was conducted from a societal perspective using a probabilistic Markov state transition model, which simulated clinical events and costs over a 20-year time horizon.
Model structure
As illustrated in , the model has four health states: no pneumococcal disease, IPD (meningitis or bacteremia, including bacteremic pneumonia), AOM, and death. An unvaccinated birth cohort enters the model and is at risk of developing IPD or AOM. The probability of an infant developing pneumococcal disease varies by age over the time horizon of the model. The model assumes that pneumococcal disease events are mutually exclusive; however, infants can experience both IPD and AOM during each model cycle. Following IPD or AOM episodes, infants can recover and return to the no pneumococcal disease health state. Only infants with IPD face a risk of death (depending on the specific manifestation of IPD and age). The model tracks the birth cohort up to 20 years of age or until death, whichever occurs earlier, with a cycle length of 1 year.
Figure 1. Markov pathway. Abbreviations. AOM, acute otitis media; IPD, invasive pneumococcal disease.
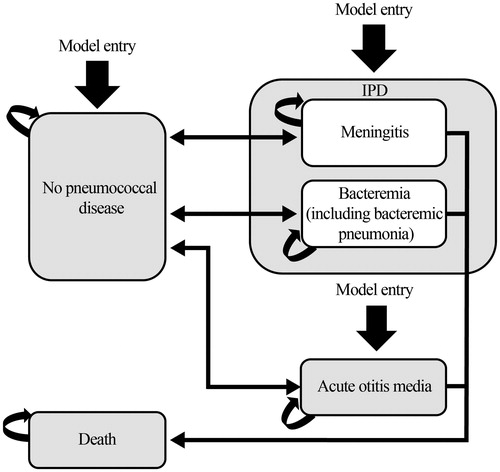
The societal perspective was used in this analysis. Therefore, costs include both direct medical costs and indirect costs from productivity losses in children and parents. The model did not include transmission dynamics of pneumococcal infections from person to person, nor were PMS or NBPP considered.
Model inputs
Target population
The target population of the study was a 2018 cohort of unvaccinated newborns in the US. The cohort size of 3.8 million newborns was based on the overall number of births in the US in 2018 according to the US Centers for Disease Control and Prevention (CDC) National Vital Statistics reportCitation24.
Epidemiologic inputs
Epidemiological data obtained from published literature and publicly available reports were used as inputs (). Age-specific IPD incidence rates and serotype distributions were taken from CDC Active Bacterial Core (ABC) surveillance dataCitation2,Citation11,Citation25,Citation26. Meningitis and bacteremia (including bacterial pneumonia) comprised 15% and 85% of IPD cases, respectively, in the pre-PCV7 era, 9% and 91% in the pre-PCV13 era, and 13% and 87% in the post-PCV13 eraCitation27,Citation28. CFRs were 10% for meningitisCitation29 and 3% for bacteremiaCitation28; these were applied across all periods to reflect current access to medical care and treatment options.
Table 1. Epidemiological inputs†.
Age-specific AOM incidence rates were obtained from a retrospective study of AOM-associated ambulatory visitsCitation12, and serotype distribution was taken from published surveillance and observational studiesCitation16,Citation30. The proportion of AOM cases associated with S. pneumoniae was 44% in the pre-PCV7 era, 47% in the pre-PCV13 era, and 21% in the post-PCV13 eraCitation31.
Cost inputs
Total costs per episode of IPD or AOM comprised direct medical costs and indirect costs (). Costs were expressed in 2018 US dollars and discounted at 3%. Direct costs were retrieved from a published report on the health and economic impact of PCV13Citation32. Indirect costs included both productivity losses in children associated with premature death from IPD, and work loss among caregivers of children with IPD or AOM, both of which were calculated using the human capital approachCitation33. Lost lifetime income due to premature death was calculated using age-specific life expectancy values obtained from life tables and age-specific average income per year. Work loss in parents caring for children with pneumococcal disease was calculated using the number of work days missed multiplied by the daily wage rate and labor force participation rate in the US in 2016Citation34. The numbers of work days missed were 7 days per episode for bacteremiaCitation35, 11 days for meningitisCitation29, and 1 day for AOM. The annual income of individuals under 20 years of age was assumed to be zero.
Table 2. Cost inputs (2018 US dollars).
Model verification and validation
The validity of the model was checked during collaboration with experts and by comparing its structure with that of a previously published modelCitation32. Several tests were built into the model for verification and to ensure internal validityCitation36. For example, the sum of the distribution of persons in each compartment at each point of time and age was verified analytically to be equal to the size of the population at that age.
Model outputs
Modeled outcomes included the number of IPD cases and deaths, number of AOM cases, and total, direct, and indirect costs attributable to IPD and AOM. Cases, deaths, and costs were estimated overall and by vaccine-type serotype.
Sensitivity analysis
One-way sensitivity analysis, conducted by varying the incidence, CFR, and cost data by ±20%, and varying the discount rate to 0% and 5%, was performed to assess the impact of uncertainties of the key model parameters and assumptions on model results in the pre-PCV7 period.
Results
Clinical events
Invasive pneumococcal disease cases by serotype
The model estimated that 18,983 IPD cases in the pre-PCV7 period, 4,697 IPD cases in the pre-PCV13 period, and 948 IPD cases in the post-PCV13 period were attributable to V114 serotypes. The majority of IPD cases (16,321 cases; 86%) were attributable to PCV7 serotypes in the pre-PCV7 period ().
Table 3. IPD cases attributable to V114 serotypes in the pre-PCV7, pre-PCV13, and post-PCV13 periods.
IPD cases associated with the six additional serotypes in PCV13 increased from 2,338 cases in the pre-PCV7 period to 4,009 cases in the pre-PCV13 period. The increase was primarily due to increases in serotype 19A from 514 (3%) to 2,081 (44%) cases, 7F from 246 (1%) to 1,400 (30%) cases, and serotype 3 from 147 (1%) to 226 (5%) cases. Serotype 6A decreased significantly between the pre-PCV7 and pre-PCV13 scenarios, from 1,056 (6%) to 27 cases (1%). Overall, there was a net increase of 1,671 IPD cases due to the six additional serotypes in PCV13 in the pre-PCV13 period compared with the pre-PCV7 period.
The number of cases associated with serotypes 22F and 33F combined was 324 (2%) in the pre-PCV7 period, 545 (12%) in the pre-PCV13 period, and 298 (31%) in the post-PCV13 period.
Mortality due to invasive pneumococcal disease
The number of estimated deaths associated with V114 serotypes was 769 in the pre-PCV7 period, 172 in the pre-PCV13 period, and 37 in the post-PCV13 period (). Most of these deaths (657; 85%) were attributable to PCV7 serotypes in the pre-PCV7 period. The numbers of deaths attributable to the six additional serotypes in PCV13 were 147 (85%) in the pre-PCV13 period and 17 (45%) in the post-PCV13 period.
Table 4. IPD deaths attributable to V114 serotypes in the pre-PCV7, pre-PCV13, and post-PCV13 periods.
Acute otitis media cases by serotype
V114 serotypes were associated with 5.4 million AOM cases in the pre-PCV7 period, 3.0 million AOM cases in the pre-PCV13 period, and 217,461 AOM cases in the post-PCV13 period (). The majority of AOM cases (4.6 million; 85%) were attributable to PCV7 serotypes in the pre-PCV7 period.
Table 5. AOM cases attributable to V114 serotypes in the pre-PCV7, pre-PCV13, and post-PCV13 periods.
The number of AOM cases associated with the six additional serotypes in PCV13 increased from 0.8 million (15%) in the pre-PCV7 period to 2.8 million (96%) in the pre-PCV13 period. The increase was primarily due to an increase in serotype 19A from 226,546 (4%) to 2,192,250 (74%) cases, and serotype 3 from 164,761 (3%) to 274,034 (9%) cases. Serotype 6A decreased between the pre- and post-PCV periods, from 391,306 (7%) cases to 383,647 cases (13%). Overall, there was a net increase of 2.1 million AOM cases due to these six serotypes in the pre-PCV13 period compared with the pre-PCV7 period.
The proportion of cases attributable to serotypes 22F and 33F together was 0 cases (0%) in the pre-PCV7 period, 0 cases in the pre-PCV13 period, and 66,911 cases (31%) in the post-PCV13 period.
Economic impact by serotype
Total discounted costs (direct medical costs and indirect costs; US dollars) of IPD and AOM for all V114 serotypes were estimated to be $1.7 billion in the pre-PCV7 period, $730 million in the pre-PCV13 period, and $75 million in the post-PCV13 period (). IPD and AOM associated with PCV7 serotypes accounted for the majority of costs in the pre-PCV7 period ($1.4 billion; 86%).
Table 6. Discounted direct and indirect costs due to IPD and AOM in the pre-PCV7, pre-PCV13, and post-PCV13 periods (2018 US dollars).
The six additional serotypes in PCV13, but not in PCV7, increased from $229 million (14%) in the pre-PCV7 period to $690 million (95%) in the pre-PCV13 period. This increase was primarily due to increases in serotype 19A from $62 million (4%) to $508 million (70%), serotype 3 from $39 million (2%) to $63 million (9%), and serotype 7F from $7 million (0.4%) to $33 million (5%). Overall, there was a net increase of $462 million due to these six serotypes in the pre-PCV13 period compared with the pre-PCV7 period.
Total costs associated with serotype 22F and 33F together were $9 million (1%) in the pre-PCV7 period, $13 million (2%) in the pre-PCV13 period, and $23 million (31%) in the post-PCV13 period.
Sensitivity analysis
The one-way sensitivity analysis indicated that the discounted total cost was sensitive to uncertainties around all key parameters. It was most affected by the discount rate, whereby total IPD and AOM costs increased by 64–189% and decreased by 18–40% for 0% and 5% discount rates, respectively (). When the incidence of IPD and AOM varied by 20%, total costs increased and decreased by 20% accordingly. When the CFR for meningitis and bacteremia varied by 20%, the total cost varied by 4–15%. When the direct medical cost of an IPD and AOM episode varied by 20%, total costs varied by 5–16%.
Table 7. One-way sensitivity analyses results (2018 US dollars).
Discussion
In this modeling analysis, the 15 pneumococcal serotypes included in V114 contributed to a substantial number of IPD and AOM cases and deaths during the pre-PCV7, pre-PCV13, and post-PCV13 periods in a hypothetical unvaccinated US birth cohort over 20 years. V114 serotypes were associated with high total discounted costs during all three time periods. Although the overall number of cases and costs reduced over time, the proportion attributable to the six additional serotypes in PCV13 increased in the pre-PCV13 period compared with the pre-PCV7 period, primarily driven by serotypes 19A, 7F, and 3. Similarly, the proportion of cases and costs attributed to the two unique serotypes in V114 increased in the pre-PCV13 and post-PCV13 periods compared with the pre-PCV7 period.
Our findings are consistent with the literature. Prior to the introduction of PCVs, the majority of IPD and AOM cases were caused by serotypes in PCV7Citation27,Citation37,Citation38. Based on US ABC surveillance reports, PCV7 serotypes were responsible for >80% of all IPD cases in children aged <5 years prior to the introduction of PCV7Citation27. Two-thirds of AOM cases have been attributed to PCV7 serotypes in the US prior to PCV7 introductionCitation30. Following the introduction of PCV7, there was a significant increase in the incidence of IPD and AOM associated with non-PCV7 serotypes due to serotype replacement, in particular serotypes 3, 7F, and 19ACitation16,Citation39–42. Several studies have also highlighted the persistence of select vaccine serotypes after PCV13 introduction, particularly serotype 3Citation17,Citation18.
The next generation of pediatric pneumococcal vaccines continues to maintain serotypes that were highly prevalent and invasive prior to the introduction of earlier-generation PCVs, while extending coverage to non-vaccine serotypes currently causing disease. This modeling analysis demonstrates the importance of this strategy by estimating cases and deaths attributable to licensed and non-vaccine-type serotypes (22F and 33F) in a hypothetical cohort of US children followed from birth to 20 years of age.
Substantial clinical and economic burden continues to be prevented by serotypes contained in previously licensed vaccines, particularly those included in PCV7. In the pre-PCV7 period, more than 16,000 PCV7-type IPD cases and nearly 4.6 million PCV7-type AOM cases were predicted to occur in a single birth cohort, with a total cost of $1.4 billion. More than 2,000 IPD cases, and nearly 0.8 million AOM cases, were attributable to the six additional serotypes in PCV13, with a total cost of $229 million. The disease burden associated with these six additional serotypes further increased in the pre-PCV13 period. Excluding these serotypes from next-generation pediatric pneumococcal vaccines could have severe morbidity and mortality consequences, should these serotypes return. In addition, the near elimination of these serotypes from nasopharyngeal carriage through vaccination has prevented transmission and disease in older unvaccinated age groupsCitation43,Citation44.
Evidence of serotype persistence suggests a need for vaccines with greater effectiveness against select serotypesCitation45,Citation46. In this simulation, serotype 3 still caused 202 IPD cases and 66,911 AOM cases in the post-PCV13 period. Finally, despite the substantial reduction of disease following the introduction of PCVs, the residual disease caused by non-vaccine serotypes remains. In the US, non-PCV13 serotypes accounted for 75.7% of IPD for children <5 years of age in 2017Citation21. In this simulation, in a single birth cohort, serotypes 22F and 33F were associated with 298 IPD cases and 66,911 AOM cases over 20 years in the post-PCV13 period.
The value of next-generation pediatric pneumococcal vaccines should therefore not be limited to disease prevented by the non-vaccine serotypes only. This analysis demonstrates that the full value of next-generation vaccines should account for both those serotypes in the previously licensed vaccines, as well as the additional non-vaccine type serotypes.
A limitation of this analysis was that PMS and the prevention of NBPP were not considered. The availability of data for the pre-PCV7 period was limited, particularly for AOM. Furthermore, the analysis did not include direct non-medical costs borne by families or caregivers, such as transportation and lodging. Indirect costs associated with productivity loss were estimated using conservative values for earnings; for caregivers, only absenteeism, not presenteeism, was included in the productivity loss calculation. For children, the average income for those under 20 years of age was assumed to be zero. Consequently, overall estimates of health and economic burden of IPD and AOM associated with V114 serotypes were conservative.
Conclusions
This modeling analysis showed that PCV7 serotypes contributed to a high amount of morbidity and costs during both pre-PCV7 and pre-PCV13 eras in a hypothetical US birth cohort followed for 20 years. Three of the additional serotypes in PCV13 (3, 7F, and 19A) were associated with substantial IPD-related morbidity after the introduction of PCV7; serotype 19A was also associated with substantial AOM-related morbidity and overall costs pre-PCV13. Unique V114 serotypes 22F and 33F were associated with additional morbidity and costs during all three periods. Investigational PCVs for pediatric populations must continue to retain and provide protection against serotypes in currently licensed vaccines to maintain disease reduction while expanding coverage to non-vaccine serotypes.
Transparency
Declaration of funding
This analysis was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Declaration of financial/other interests
All authors are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, who may own stock and/or hold stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
TP, TH, TW, and KOE designed the study; TP and TH analyzed the study data; and TP, TH, and TW interpreted the study data. All authors critically reviewed the manuscript and approved the final version for submission.
Previous presentations
An earlier iteration of this model was presented as a poster at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) annual conference, 18–22 May 2019, New Orleans, LA, USA.
Acknowledgements
The authors would like to thank Elamin Elbasha for technical advice on the pediatric Markov model. Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments was provided by Rachel Wright, PhD, and Gauri Saal, MA Economics, and editorial support, was provided by Annabel Ola and Ian Norton, all of Scion, London, supported by Merck & Co. Inc., Kenilworth, NJ, USA, according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288). The Sponsor was involved in the collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
References
- Centers for Disease Control and Prevention [Internet]. The Pink Book: epidemiology and prevention of vaccine-preventable diseases. Chapter 17: pneumococcal disease. Atlanta (GA): CDC; 2018 [2019 September 18]. Available from: https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html
- Centers for Disease Control and Prevention [Internet]. Active bacterial core surveillance (ABCs) report: Streptococcus pneumoniae. Atlanta (GA): CDC; 2017 [2020 April 15]. Available from: https://www.cdc.gov/abcs/reports-findings/survreports/spneu17.html
- Stockmann C, Ampofo K, Byington CL, et al. Pneumococcal meningitis in children: epidemiology, serotypes, and outcomes from 1997-2010 in Utah. Pediatrics. 2013;132(3):421–428.
- Said MA, Johnson HL, Nonyane BA, et al. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8(4):e60273.
- Huang SS, Johnson KM, Ray GT, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–3412.
- Wahl B, O’Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000-15. Lancet Glob Health. 2018;6(7):e744–e757.
- Suaya JA, Gessner BD, Fung S, et al. Acute otitis media, antimicrobial prescriptions, and medical expenses among children in the United States during 2011-2016. Vaccine. 2018;36(49):7479–7486.
- Tong S, Amand C, Kieffer A, et al. Trends in healthcare utilization and costs associated with acute otitis media in the United States during 2008-2014. BMC Health Serv Res. 2018;18(1):318.
- Vergison A, Dagan R, Arguedas A, et al. Otitis media and its consequences: beyond the earache. Lancet Infect Dis. 2010;10(3):195–203.
- Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49:1–35.
- Centers for Disease Control and Prevention [Internet]. Pneumococcal disease: surveillance and reporting. Atlanta (GA): CDC; 2017 [2019 September 18]. Available from: https://www.cdc.gov/pneumococcal/surveillance.html
- Kawai K, Adil EA, Barrett D, et al. Ambulatory visits for otitis media before and after the introduction of pneumococcal conjugate vaccination. J Pediatr. 2018;201:122–127.
- Feikin DR, Kagucia EW, Loo JD, et al. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 2013;10(9):e1001517.
- Centers for Disease Control and Prevention. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children – Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258–261.
- Balsells E, Guillot L, Nair H, et al. Serotype distribution of Streptococcus pneumoniae causing invasive disease in children in the post-PCV era: a systematic review and meta-analysis. PLoS One. 2017;12(5):e0177113.
- Pichichero M, Kaur R, Scott DA, et al. Effectiveness of 13-valent pneumococcal conjugate vaccination for protection against acute otitis media caused by Streptococcus pneumoniae in healthy young children: a prospective observational study. Lancet Child Adolesc Health. 2018;2(8):561–568.
- Beall B, Chochua S, Gertz RE, Jr., et al. A population-based descriptive atlas of invasive pneumococcal strains recovered within the U.S. during 2015-2016. Front Microbiol. 2018;9:2670.
- Kandasamy R, Voysey M, Collins S, et al. Persistent circulation of vaccine serotypes and serotype replacement after 5 years of infant immunization with 13-valent pneumococcal conjugate vaccine in the United Kingdom. J Infect Dis. 2020;221:1361–1370.
- Rupp R, Hurley D, Grayson S, et al. A dose ranging study of 2 different formulations of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Hum Vaccin Immunother. 2019;15(3):549–559.
- Greenberg D, Hoover PA, Vesikari T, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV15) in healthy infants. Vaccine. 2018;36(45):6883–6891.
- Varghese J, Chochua S, Tran T, et al. Multistate population and whole genome sequence-based strain surveillance of invasive pneumococci recovered in the USA during 2017. Clin Microbiol Infect. 2020;26:512.e511.
- Balsells E, Dagan R, Yildirim I, et al. The relative invasive disease potential of Streptococcus pneumoniae among children after PCV introduction: a systematic review and meta-analysis. J Infect. 2018;77(5):368–378.
- Adam HJ, Golden AR, Karlowsky JA, et al. Analysis of multidrug resistance in the predominant Streptococcus pneumoniae serotypes in Canada: the SAVE study, 2011-15. J Antimicrob Chemother. 2018;73(7):vii12–vii19.
- Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2018. Natl Vital Stat Rep. 2019;68(13):1–47.
- Centers for Disease Control and Prevention [Internet]. Active bacterial core surveillance (ABCs): surveillance report. Atlanta (GA): CDC; 1999 [updated 1999]. Available from: https://www.cdc.gov/abcs/reports-findings/survreports/spneu99.html
- Centers For Disease Control and Prevention [Internet]. Active bacterial surveillance (ABCs): surveillance report. Atlanta (GA): CDC; 2009. Available from: https://www.cdc.gov/abcs/reports-findings/survreports/spneu09.html
- Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201(1):32–41.
- Moore MR, Link-Gelles R, Schaffner W, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–309.
- Olarte L, Barson WJ, Barson RM, et al. Impact of the 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis in US children. Clin Infect Dis. 2015;61(5):767–775.
- McEllistrem MC, Adams JM, Patel K, et al. Acute otitis media due to penicillin-nonsusceptible Streptococcus pneumoniae before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2005;40(12):1738–1744.
- Kaur R, Morris M, Pichichero ME. Epidemiology of acute otitis media in the postpneumococcal conjugate vaccine era. Pediatrics. 2017;140(3):e20170181.
- Rubin JL, McGarry LJ, Strutton DR, et al. Public health and economic impact of the 13-valent pneumococcal conjugate vaccine (PCV13) in the United States. Vaccine. 2010;28(48):7634–7643.
- Nyman JA. Cost recommendations in the second edition of cost-effectiveness in health and medicine: a review. MDM Policy Pract. 2018;3(1):2381468318765162.
- United States Department of Labor [Internet]. Bureau of labor statistics: databases, tables & calculators by subject. Washington (DC): US Dept. of Labor; 2017 [cited 2018 July 06]. Available from: https://data.bls.gov/timeseries/CUUR0000SAM?output_view=pct_12mths
- Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736–740.
- Dasbach EJ, Elbasha EH. Verification of decision-analytic models for health economic evaluations: an overview. Pharmacoeconomics. 2017;35(7):673–683.
- Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7(10):e1000348.
- Joloba ML, Windau A, Bajaksouzian S, et al. Pneumococcal conjugate vaccine serotypes of Streptococcus pneumoniae isolates and the antimicrobial susceptibility of such isolates in children with otitis media. Clin Infect Dis. 2001;33(9):1489–1494.
- Centers for Disease Control and Prevention [Internet]. Active bacterial core surveillance (ABCs): surveillance reports. Atlanta (GA): CDC; 2019 [2019 September 18]. Available from: https://www.cdc.gov/abcs/index.html
- Moore MR, Gertz RE, Jr., Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197(7):1016–1027.
- Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196(9):1346–1354.
- Kaplan SL, Barson WJ, Lin PL, et al. Serotype 19A Is the most common serotype causing invasive pneumococcal infections in children. Pediatrics. 2010;125(3):429–436.
- Klugman KP. Herd protection induced by pneumococcal conjugate vaccine. Lancet Glob Health. 2014;2(7):e365–e366.
- Hammitt LL, Akech DO, Morpeth SC, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health. 2014;2(7):e397–e405.
- van der Linden M, Falkenhorst G, Perniciaro S, et al. Effectiveness of pneumococcal conjugate vaccines (PCV7 and PCV13) against invasive pneumococcal disease among children under two years of age in Germany. PLoS One. 2016;11(8):e0161257.
- Andrews N, Kent A, Amin-Chowdhury Z, et al. Effectiveness of the seven-valent and thirteen-valent pneumococcal conjugate vaccines in England: the indirect cohort design, 2006-2018. Vaccine. 2019;37(32):4491–4498.
- Delgleize E, Leeuwenkamp O, Theodorou E, et al. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model. BMJ Open. 2016;6(11):e010776.
