Abstract
Aims
This study compared medication use, healthcare resource utilization (HRU), and exacerbations among individuals with chronic obstructive pulmonary disease (COPD) who initiated glycopyrrolate/eFlow Closed System nebulizer 25 mcg/mL glycopyrrolate (hereafter GLY) in a real-world setting before and after treatment initiation.
Materials and methods
Retrospective claims and hospital charge master data were used to identify individuals ≥ 40 years of age diagnosed with COPD who initiated GLY between 1 April 2018 and 28 February 2019 (first prescription claim = index date). Patients were excluded if they had ≥1 asthma diagnosis in the 6-month pre-index period. The proportion of patients with COPD-related medications, other outpatient HRU, hospitalizations, and exacerbations were compared between the 6-month pre-index and 6-month follow-up periods. Among patients utilizing the service, per-person utilization rates were compared between the two periods.
Results
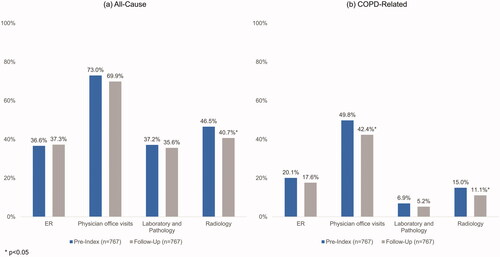
Among patients initiating GLY (n = 767), the mean age was 71.4 years, 56.1% were female, and the mean Charlson Comorbidity Index score was 2.0. The mean number of GLY claims per person was 3.8 during the follow-up period. Compared to the pre-index period, a lower proportion of patients had claims for COPD medications including oral corticosteroids (62.1% vs. 69.1%, p = .0001) and fixed-dose SAMA/SABA (26.1% vs. 33.0%, p < .0001) and a higher proportion of patients had claims for LABA (29.7% vs. 22.6%, p < .0001) during the follow-up period. Fewer patients had ≥1 COPD-related physician office visit (42.4% vs. 49.8%, p < .0001), radiology test (40.7% vs. 46.5%, p = .005), or moderate exacerbation (48.0% vs. 53.2%, p = .01) after initiating GLY. Among patients with linkage to inpatient data (n = 316), fewer were hospitalized (7.9% vs. 13.0%, p = .037) and hospital length of stay was shorter (1.9 vs. 3.6 days, p = .017) after initiating GLY/eFlow.
Conclusions
Among patients initiating GLY in a real-world setting, COPD medications, hospitalizations, other HRU, and exacerbations decreased after treatment initiation compared with the 6-month pre-index period.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive and debilitating lung disease that affects over 24 million people in the USACitation1. It has been the third leading cause of death in the USA since 2008Citation2 and can result in significant health resource utilization (HRU) and costs. In 2012, the age-adjusted hospital and ER discharge rates were 2.6 and 7.2 per 1,000 people with COPD, respectivelyCitation3. Hospitalization and ER rates are over four times higher among elderly patients compared with patients under 44Citation3 and are up to twice as high among patients with comorbidities compared with those without comorbiditiesCitation4,Citation5. The occurrence of exacerbations requiring hospitalizations is associated with an increased risk of future exacerbationsCitation6–8. Increasing exacerbation frequency is associated with a multiplicative increase in all-cause and COPD-related costsCitation9.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) report states that COPD treatment and management goals include the reduction of symptoms and the risk for future exacerbationsCitation6. Administered alone or in combination, inhaled long-acting β2-agonists (LABA) and long-acting muscarinic antagonists (LAMA) are the mainstay bronchodilators used as maintenance therapy in COPDCitation6. These medications are typically administered through a handheld inhaler device including metered-dose inhalers, dry power inhalers, or soft mist inhalers. Previous studies have shown that as many as 87% of COPD patients make at least one error when using these devicesCitation10. Patient characteristics such as older age, coordination limitations, and reduced inspiratory flow in particular have been shown to be associated with inhalation delivery errorsCitation11,Citation12. As an alternative to handheld inhalers, nebulizers produce a fine mist of medication which allows patients to breathe normally without the need for high inspiratory flow, hand–breath coordination, or breath-holding that is required for handheld devicesCitation13,Citation14. The choice of device for treatment delivery (e.g. nebulizer or handheld inhaler) should be tailored to patients’ ability to operate the device as well as their preferences in order to achieve optimal outcomes.
Lonhala Magnair [glycopyrrolate (GLY)/eFlow Closed System nebulizer; Sunovion Pharmaceuticals Inc., Marlborough, MA, USA] inhalation solution (hereafter GLY) is a LAMA/handheld vibrating membrane nebulizer approved in 2017 for the long-term maintenance treatment of airflow obstruction in patients with COPDCitation15. The licensed recommended dose is 25 mcg/mL GLY administered twice daily with the eFlow Closed System (Magnair, PARI Pharma GmbH, Starnberg, Germany)Citation15. The small, light-weight device is virtually silent and delivers nebulized GLY in 2–3 min with proper assembly and cleaningCitation15. With this nebulizer, patients can inhale with regular breathing that does not require hand–breath coordinationCitation13. In a previous study, patients reported high satisfaction with the eFlow device attributesCitation16.
While nebulized GLY has been studied in randomized controlled trialsCitation17,Citation18, little is known about the outcomes associated with the use of nebulized GLY in a real-world setting. The objectives of this study were to compare the hospitalizations, occurrence of exacerbations, medication use, and other outpatient HRU among individuals with COPD who initiated nebulized GLY in a real-world setting prior to and after treatment initiation.
Methods
Study design and data sources
This retrospective cohort study used data from the IQVIA patient-centric data warehouse from October 2017 to August 2019. The warehouse extracts de-identified data from all healthcare channels in the USA, including hospitals, providers, and pharmacies. The following data sources from the warehouse were used in this study: Professional Fee Claims (including diagnoses and procedures based on International Classification of Diseases 9th Revision and 10th Revision, Clinical Modification; ICD-9-CM and ICD-10-CM, respectively), Prescription Claims (including retail or mail-order claims), and Hospital Charge Data Master (CDM; including inpatient and outpatient hospital encounters). Approximately 1.7 billion professional fee claims per year are included in the Professional Fee Claims database, which represents 82% of physician activity in the USA. The Prescription Claims database represents 90% of pharmacies in the USA. CDM includes over 7 million annual inpatient stays and 60 million annual outpatient visits from over 400 hospitals in the USA. These data capture insured patients across all payer types (e.g. commercial, Medicare, Medicaid).
Study population
Selection criteria
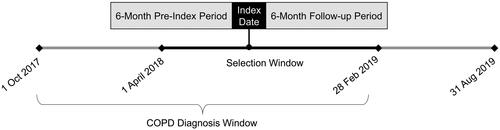
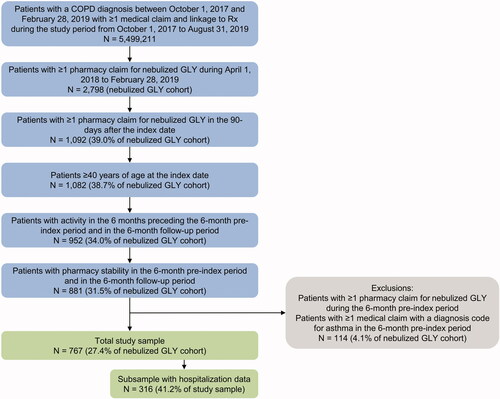
Individuals ≥ 40 years of age with a COPD diagnosis (ICD-9-CM codes 491.xx, 492.xx, 496.xx or ICD-10-CM codes J41.x, J42.x, J43.x, J44.x in any position) between 1 October 2017 and 28 February 2019 were identified from the Professional Fee Claims database (“COPD window”; ). Of these patients, the subset with at least one claim for nebulized GLY (NDC: 63402030101 or 63402020100) between 1 April 2018 and 28 February 2019 (“GLY selection window”) in the Prescription Claims database were identified. The date of the first claim was termed the “index date.” Patients were excluded from the study if they had any pharmacy claim for nebulized GLY in the 6-month pre-index period.
Eligible patients were required to have a second pharmacy claim for nebulized GLY in the 90-day period after the index date. Proxies for continuous enrollment (due to the open source nature of the data assets) were implemented based on patient activity and pharmacy reporting to capture individuals’ HRU within these databases during the study period. Specifically, patients were required to have had (a) at least one office visit and prescription during the 6 months preceding the 6-month pre-index period and during the 6 months after the index date (“follow-up period”), and (b) pharmacy stability in the 6-month pre-index and follow-up periods, defined as consistent reporting of data by the pharmacy associated with the index date. Patients were excluded from the study if they had at least one medical claim with a diagnosis for asthma (ICD-9-CM: 493.x; ICD-10-CM: J45.x) in the 6-month pre-index period.
For analyses pertaining to hospitalizations and hospital stays, patients must have had linkage to the CDM database (i.e. be present in the database) to identify the subset with inpatient data.
Baseline characteristics
Patient demographic characteristics were summarized, including age, gender, geographic region, and payer type at index date. In addition, patient clinical characteristics were summarized using available data in the 6-month pre-index period including comorbidities and the Charlson Comorbidity Index (CCI)Citation19,Citation20. Patient long-term oral corticosteroid (OCS) use, defined as having at least 180 days’ supply of OCS during the 6-month pre-index period, was also summarized.
Study outcomes
All HRU outcomes were calculated for the 6-month pre-index and follow-up periods. The following outpatient HRU outcomes were captured: prescription medications, ER visits, physician office visits, laboratory and pathology tests, and radiology tests. Among the subset of patients present in the CDM database, the following inpatient HRU outcomes were captured: hospital admission and 30-day readmission, along with length of stay for each.
Both all-cause and COPD-specific HRU were calculated, where COPD-specific HRU was defined as medical claims with a diagnosis code for COPD (primary position for inpatient hospitalizations and any position for outpatient claims) or, for prescription medications, a COPD-related therapy [short-acting β2-agonists (SABAs), LABAs, short-acting muscarinic antagonists (SAMAs), LAMAs, methylxanthines, and anti-inflammatory therapies including antibiotics inhaled corticosteroids (ICS), OCS, phosphodiesterase-4 (PDE-4) inhibitors, and fixed-dose combination bronchodilator therapies (i.e. LABA/ICS, LAMA/LABA, LAMA/LABA/ICS, SAMA/SABA)].
Moderate and severe COPD exacerbations were calculated for the 6-month pre- and follow-up periods, defined based upon the peer-reviewed literatureCitation21. Moderate exacerbations were defined as either an ER visit with a COPD diagnosis in the primary position or an office visit with a COPD diagnosis code in any position plus a pharmacy claim for OCS or antibiotic within 7 days of the office visit. Severe exacerbations were defined as an inpatient admission with a COPD diagnosis code in the primary position and, hence, were only calculated for the subset of patients present in CDM. Exacerbations occurring within 14 days of each other were considered a single exacerbation episode and classified according to the higher severity event.
Statistical analysis
Demographic and clinical characteristics were summarized as mean and standard deviation (SD) for continuous measures and patient counts and percentages for categorical measures. For the study outcomes, patient counts and percentages for COPD-related therapies, other HRU, and exacerbations were calculated. Among individuals with HRU in a service category and those experiencing exacerbations in either the 6-month pre-index or follow-up periods, the per-person rate of events and SD were also calculated. Comparisons between the 6-month pre-index and follow-up periods were conducted using paired t-test for continuous variables and using McNemar’s test for categorical variables. For outcomes among patients with at least one service or exacerbation, outcomes were reported among patients with the event either in the pre-index or follow-up period; dependent comparisons were made. A p value of .05 was pre-specified as the threshold for statistical significance. All analyses were conducted using SAS Release 9.4 (SAS, Cary, NC).
Results
Study population
A total of 2,798 individuals with COPD had a claim for nebulized GLY from April 2018 to February 2019. Seven hundred and sixty-seven patients met all inclusion and exclusion criteria and were included in the study (). The number of patients initiating GLY each month ranged from 36 patients (December 2018) to 94 patients (June 2019). No seasonality pattern of treatment initiation was observed (see Supplementary Appendix). Of the 767 patients, 316 (41%) had linkage to the CDM database and were included in the sub-analysis of hospitalizations and hospital stays.
Baseline demographic and clinical characteristics of the full study population are provided in . The mean (SD) age was 71 (9.8) years, with 13.7% of the sample aged 85 years or older. More than half of the study population was female (56.1%). The mean (SD) CCI score was 2.0 (1.5), with 29.3% of patients having a CCI score of at least 3. Across the study population, the most common comorbidities were hypertension (39.9%), chronic respiratory failure (21.0%), diabetes (21.0%), and hypercholesterolemia (20.1%). Influenza/respiratory infection was also common (26.7%).
Table 1. Patient characteristics.
Outpatient HRU
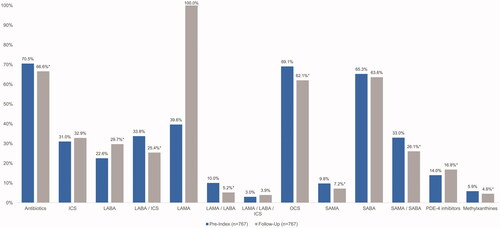
COPD medication use during the 6-month pre-index and follow-up periods is provided in . All patients (100%) had at least one claim for nebulized GLY (a LAMA) during the follow-up period, whereas 39.6% of patients had a claim for any LAMA during the pre-index period. Patients had a mean (SD) of 3.8 (1.8) claims for nebulized GLY during the follow-up period including the index claim. The proportion of patients with claims for other COPD-related medications was generally lower during the follow-up period compared with the pre-index period, including for antibiotics (66.6% vs. 70.5%, p = .045), OCS (62.1% vs. 69.1%, p = .0001), SAMA (7.2% vs. 9.8%, p = .0153), and the fixed-dose combination therapies SAMA/SABA (26.1% vs. 33.0%, p < .0001), LABA/ICS (25.4% vs. 33.8%, p < .0001), and LAMA/LABA (5.2% vs. 10.0%, p < .0001). A higher proportion of patients had claims for LABA during the follow-up period compared with the pre-index period (29.7% vs. 22.6%, p < .0001).
Figure 3. Medication utilization. *p < .05. Abbreviations. ICS, inhaled corticosteroids; LABA, long-acting beta-agonist; LAMA, long-acting muscarinic antagonist; OCS, oral corticosteroids; PDE-4, phosphodiesterase type 4; SABA, short-acting beta-agonist; SAMA, short-acting muscarinic antagonist.
The proportion of patients with other outpatient HRU also declined during the follow-up period compared to the pre-index period (). provides the per-person rate of utilization among patients experiencing each event.
Table 2. Per-patient rates of outpatient healthcare resource utilization.
Hospitalizations
Among the 316 patients with linkage to CDM, a lower proportion of patients were admitted to the hospital during the 6-month follow-up period versus the 6-month pre-index period (7.9% vs. 13.0%, p = .037). Among those with a hospitalization, the length of stay was shorter for those admitted during the follow-up period compared to the pre-index period (mean: 1.9 vs 3.6 days, p = .017). The proportion of patients with readmissions within 30 days was not statistically significant between the pre-index period (n = 3, 0.9%) and follow-up period (n = 2, 0.6%).
Exacerbations
Fewer patients experienced a moderate exacerbation in the follow-up period versus the pre-index period (48.0% vs. 53.2%, p = .01) (). Among the 509 patients who experienced a moderate exacerbation in either the pre-index or follow-up periods, the number of exacerbations per patient was lower during the follow-up period (1.3 vs 1.5, p = .005). Among the 316 patients with hospitalization data, 19 experienced a severe exacerbation during either period, with fewer patients experiencing a severe exacerbation during the post-index period (2.2% vs. 4.4%), although this result was not statistically significant (p = .0896) ().
Table 3. Exacerbations.
Discussion
This study of the real-world use of nebulized GLY showed that in the six months after initiating treatment, fewer patients experienced COPD exacerbations, utilized COPD-related medications, or utilized other health services including hospitalizations and COPD-related physician visits compared to the pre-index period.
Chronic disease management can be complicated by the presence of multiple comorbidities, resulting in a poorer prognosisCitation6,Citation22,Citation23. The baseline data in this study suggests that this study population represents a COPD population that can be difficult to manage, as evidenced by the high prevalence of comorbidities and COPD medication use at baseline. Prior to initiating nebulized GLY, one in five patients had chronic respiratory failure, one in four patients had influenza or respiratory infection, and nearly one in three patients had a CCI score of at least 3. Compared to other COPD studies using U.S. claims data, higher proportions of patients in this study used ICS (31% versus 3% to 21.6%Citation24–28) OCS (69% versus 14% to 49%Citation25,Citation27,Citation29,Citation30) SABA (65% versus 20% to 54%Citation25–27,Citation29–31) or SAMA (10% versus 1% to 8%Citation25–27,Citation29) during the pre-index period. As corticosteroids (ICS or OCS), short-acting agents (SAMA or SABA), and antibiotics are treatment options for the treatment of acute exacerbationsCitation6, the high use of these therapies during the pre-index period suggests a population with a history of exacerbations, which is a risk factor for future exacerbationsCitation7.
Utilization of short-acting agents and OCS declined after initiation of nebulized GLY despite the high prevalence of comorbidities and utilization of COPD medications in this study population during the pre-index period. This finding may suggest better symptom control in the post-index period compared to the pre-index period. The decline in the proportion of patients using OCS observed in this study (69% to 62%) aligns with the decline in number of patients experiencing moderate exacerbations (53% to 48%). As prolonged OCS therapy has been shown to be associated with pneumonia and mortalityCitation6,Citation32 and short courses of OCS are also associated with an increased risk of adverse eventsCitation33, the decline in the proportion of patients using OCS in this study suggests that nebulized GLY may contribute to lower OCS-related morbidity in this elderly COPD population.
Utilization of combination therapy of LAMA/LABA decreased after initiation of nebulized GLY (from 10.0% to 5.2%). However, there was a corresponding increase in the utilization of LABA (from 22.6% to 29.7%). This may indicate that patients requiring both LAMA and LABA continued to receive LABA in a separate nebulizer or inhaler once nebulized GLY had been initiated. Patients initiating nebulized GLY had an average of 3.8 fills during the 6-month follow-up period, indicating that full adherence was sub-optimal. Future efforts to increase adherence to medication may further improve outcomes for patients.
An important finding of this study is that fewer patients were admitted to the hospital or experienced exacerbations during the 6-month period after initiating nebulized GLY although nebulized GLY has not been studied nor indicated for prevention or treatment of exacerbations in randomized clinical studies. Among patients with COPD, frequent exacerbations negatively impact lung function and increase hospitalizations and readmissionsCitation7,Citation8,Citation34. As a history of exacerbations is one of the strongest predictors of future exacerbationsCitation7, treatments that control COPD symptoms are important for reducing the risk of future exacerbationsCitation6. Physician office visits and radiology tests, which are often used to assess exacerbations, declined during the follow-up period which may indicate that patients have better control of COPD after initiating nebulized GLY. Together, the results of this study suggest that real-world use of nebulized GLY may reduce risk of future exacerbations and hospitalizations, ultimately improving the management of COPD.
Despite the expected challenges of managing COPD in an elderly and comorbid patient population, improved outcomes including decreased use of short-acting agents and OCS, as well as fewer hospitalizations and exacerbations were observed in this patient cohort that received nebulized GLY. LAMA is a mainstay of COPD therapy, and less than 40% of patients in this study used a LAMA during the pre-index period. In a post-hoc subgroup analysis of patients with a prior LAMA claim, a similar decrease in exacerbations and hospitalizations were observed (see Supplementary Appendix). These results suggest that the observed effects of nebulized GLY are not due to LAMA use alone. One potential factor that contributed to improved outcomes is the eFlow inhalation device used to deliver GLY. In real-world settings, successful treatment with COPD medications depends in part on the inhalation device, and the choice of device should be tailored to a patient’s abilities and preferencesCitation6. Some patients with COPD, such as those who are elderly, have suboptimal inspiratory flow and may face cognitive or coordination challenges when using handheld inhalersCitation6,Citation35,Citation36. Meta-analyses suggest device errors as high as 87% for patients using metered-dose inhalersCitation10, 59% for soft mist inhalersCitation37, and 50% for dry power inhalersCitation38. Device mishandling is associated with worse outcomes and increased exacerbations, hospitalizations, and HRUCitation6,Citation39,Citation40. Furthermore, patients prefer small inhaler devices that are portable, durable, easy to use, and fast in medication administrationCitation41, along with medications that are fast in onset of actionCitation42, and device satisfaction is associated with better outcomesCitation43–45. The eFlow nebulizer in this study delivers a fine mist through natural breathing, which allows patients to breathe normally without the need for hand–breath coordinationCitation13. It is also relatively lightweight, small, portable, and designed to administer the medication in 2–3 min (with proper assembly and cleaning), and patients have reported high satisfaction with these device attributesCitation16. As observed in this study, nebulized GLY and the unique features of the eFlow device may contribute to better symptom control and fewer hospitalizations for elderly, comorbid patients with COPD.
Limitations
Claims data used in this analysis were generated for the purposes of provider reimbursement and not for research. Claims data do not provide as much clinical detail as medical records as they are primarily collected for the purposes of payment. As with any analysis of claims data, diagnosis and procedure codes are subject to data coding variation and data entry errors so that misclassification may be present. Unlike administrative health plan claims data, it is not possible to apply continuous enrollment requirements to our data. Although proxies for continuous enrollment were implemented, it is likely that some patient healthcare activity within these databases was not captured during the study period. The databases are open source, meaning a patient could visit a healthcare provider that does not contribute to the data, and this activity would not be captured in this database. In order to capture healthcare resource utilization and exacerbations during both the pre-index and follow-up periods, patients who died during the study period were excluded from analysis and our sample may be biased towards a healthier population. On the other hand, the requirement for a physician visit and prescription claim in both the 6-month pre-index and follow-up periods (a proxy for continuous enrollment) potentially limited our sample to a less healthy population. This study was of COPD patients aged > =40 years without asthma who initiated GLY/eFlow in a real-world setting. 767 (27%) of patients with a claim for nebulized GLY met all selection criteria with 61% of sample dropped by the requirement for a second claim for nebulized GLY in the follow-up period. This second claim was required to evaluate the impact of treatment per the study objectives. Results may not be generalizable to other patient populations such as those aged less than 40 years, diagnosed with asthma, or outside the USA.
Clinical characteristics of patients were measured during the 6-month pre-index period. As such, the full history of patients is unknown, and comorbidities are likely underreported (e.g. tobacco use, asthma). For example, in a cross-sectional study of U.S. patients with COPD in 1999–2008, 69% of the study population reported having ever smoked and 60% were femaleCitation22. In this study, a similar percentage of the population was female (56%) and only 19% reported tobacco use (current or former smokers). This lower percentage of tobacco use may be due to the short history of claims prior to the index period. Without spirometry assessment, we were not able to assess the severity of the COPD in this study population and instead relied upon the presence of comorbidities and medication utilization as proxies. Treatment received during an inpatient hospital or a residential treatment facility stay, or over-the-counter medication use, was not reported in this dataset, so medication utilization is likely underreported. Patients initiating any new therapy for COPD may receive detailed instruction and education on device use, which can lead to improvements in inhalation techniqueCitation11,Citation46, but these data are not available in this dataset. Other clinically important characteristics including the type of physician seen, pulmonary rehabilitation use, non-pharmaceutical interventions such as smoking cessation, COPD diagnosis confirmation, and vaccinations for influenza and pneumococcal, which may have an impact on outcomes, were not reported in this dataset or were not the focus of this research. Future research may be warranted in these areas. Finally, hospitalization data were available for only 40% of the study sample.
Conclusion
Among 767 patients with COPD initiating nebulized GLY in a real-world setting, COPD medications and moderate exacerbations decreased compared with the 6-month pre-index period. A significant reduction in hospitalizations was also found among 316 patients with linkage to CDM. These findings suggest that nebulized GLY may reduce the burden of COPD management among those experiencing difficulties with COPD treatment delivery.
Transparency
Declaration of funding
This study was funded by Sunovion Pharmaceuticals Inc.
Declaration of financial/other relationships
MD, VD and VRA are employees of IQVIA, which received a consulting fee for conducting this study. CD, SS and XN are employees of Sunovion.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
XN, VD, MD, VRA, and CD made substantial contributions to the conception and design of this study. Data acquisition and analysis was conducted by VD, MD, and VRA. All authors contributed to the interpretation of data, took part in the drafting and revising of the manuscript, and gave final approval of the manuscript to be published. All authors agree to be accountable for all aspects of this work.
Supplemental Material
Download MS Word (58.8 KB)Acknowledgements
The authors kindly acknowledge Alexandra Ellis from Stratevi, LLC for her medical writing contributions and logistical support in preparing this manuscript.
Data availability statement
Original de-identified data used in this analysis were obtained from and are the property of IQVIA. IQVIA has restrictions prohibiting the authors from making the minimal data set publicly available. Interested researchers may contact IQVIA to apply to gain access to the study’s data in the same way the authors obtained the data (https://www.iqvia.com/contact/sf).
References
- American Lung Association. Trends in COPD (chronic bronchitis and emphysema): morbidity and mortality. 2013 [cited 2020 Jun 23]. Available from: https://www.lung.org/getmedia/4f74781e-481f-4f10-9255-8dfc9dc56974/copd-trend-report.pdf.pdf
- Centers for Disease Control and Prevention. Leading causes of death. 2017 [cited 2020 Jun 26]. Available from: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm
- Ford ES. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults: findings from the nationwide inpatient sample 2001-2012 and Nationwide Emergency Department Sample 2006-2011. Chest. 2015;147:989–998.
- Kumbhare SD, Beiko T, Wilcox SR, et al. Characteristics of COPD patients using United States emergency care or hospitalization. J Copd F. 2016;3:539–548.
- Singh JA, Yu S. Utilization due to chronic obstructive pulmonary disease and its predictors: a study using the U.S. National Emergency Department Sample (NEDS). Respir Res. 2016;17:1.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2020. p. 1–125.
- Hurst JR, Vestbo J, Anzueto A, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138.
- Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;1370:786–796.
- Dhamane AD, Moretz C, Zhou Y, et al. COPD exacerbation frequency and its association with health care resource utilization and costs. Int J Chron Obstruct Pulmon Dis. 2015;10:2609–2618.
- Cho-Reyes S, Celli BR, Dembek C, et al. Inhalation technique errors with metered-dose inhalers among patients with obstructive lung diseases: a systematic review and meta-analysis of U.S. Chronic Obstr Pulm Dis. 2019;6:267–280.
- Rootmensen GN, van Keimpema AR, Jansen HM, et al. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23:323–328.
- Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195:1333–1343.
- Dhand R, Dolovich M, Chipps B, et al. The role of nebulized therapy in the management of COPD: evidence and recommendations. COPD. 2012;9:58–72.
- Yawn BP, Colice GL, Hodder R. Practical aspects of inhaler use in the management of chronic obstructive pulmonary disease in the primary care setting. Int J Chron Obstruct Pulmon Dis. 2012;7:495–502.
- Prescribing information: LONHALA MAGNAIR 2019 [cited 2020 June 26]. Available from: https://www.lonhalamagnair.com/LonhalaMagnair-Prescribing-Information.pdf
- Stephenson JJ, Dembek C, Caldwell-Tarr A, et al. Observational real-world study to assess clinical characteristics and device satisfaction in patients with COPD treated with glycopyrrolate/eFlow® CS. Int J Chron Obstruct Pulmon Dis. 2020;15:1713–1727.
- Ferguson GT, Goodin T, Tosiello R, et al. Long-term safety of glycopyrrolate/eFlow® CS in moderate-to-very-severe COPD: results from the Glycopyrrolate for Obstructive Lung Disease via Electronic Nebulizer (GOLDEN) 5 randomized study. Respir Med. 2017;132:251–260.
- Kerwin E, Donohue JF, Goodin T, et al. Efficacy and safety of glycopyrrolate/eFlow((R)) CS (nebulized glycopyrrolate) in moderate-to-very-severe COPD: results from the glycopyrrolate for obstructive lung disease via electronic nebulizer (GOLDEN) 3 and 4 randomized controlled trials. Respir Med. 2017;132:238–250.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139.
- Wallace AE, Kaila S, Bayer V, et al. Health care resource utilization and exacerbation rates in patients with COPD stratified by disease severity in a commercially insured population. JMCP. 2019;25:205–217.
- Schnell K, Weiss CO, Lee T, et al. The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999-2008. BMC Pulm Med. 2012;12:26. Jul 9
- Sullivan J, Pravosud V, Mannino DM, et al. National and state estimates of COPD morbidity and mortality - United States, 2014-2015. Chronic Obstr Pulm Dis. 2018;5:324–333.
- Ding B, Kallenbach L, Slipski L, et al. Patient characteristics and healthcare resource utilization among patients with COPD new to LAMA/LABA fixed-dose combination treatment in US-based real-world practice. Int J Chron Obstruct Pulmon Dis. 2020;15:775–786.
- Davis JR, Kern DM, Williams SA, et al. Health care utilization and costs after initiating budesonide/formoterol combination or fluticasone/salmeterol combination among COPD patients new to ICS/LABA treatment. JMCP. 2016;22:293–304.
- Mapel D, Laliberte F, Roberts MH, et al. A retrospective study to assess clinical characteristics and time to initiation of open-triple therapy among patients with chronic obstructive pulmonary disease, newly established on long-acting mono- or combination therapy. COPD. 2017;12:1825–1836.
- Bogart M, Stanford RH, Reinsch T, et al. Clinical characteristics and medication patterns in patients with COPD prior to initiation of triple therapy with ICS/LAMA/LABA: a retrospective study. Respir Med. 2018;142:73–80.
- Diette GB, Dalal AA, D'Souza AO, et al. Treatment patterns of chronic obstructive pulmonary disease in employed adults in the United States. Int J Chron Obstruct Pulmon Dis. 2015;10:415–422.
- Moretz C, Sharpsten L, Bengtson LG, et al. Real-world effectiveness of umeclidinium/vilanterol versus fluticasone propionate/salmeterol as initial maintenance therapy for chronic obstructive pulmonary disease (COPD): a retrospective cohort study. COPD. 2019;14:1721–1737.
- Palli SR, Frazer M, DuCharme M, et al. Differences in real-world health and economic outcomes among patients with COPD treated with combination tiotropium/olodaterol versus triple therapy. J Manag Care Spec Pharm. 2020;26:1363–1374.
- Ganapathy V, Stensland MD. Health resource utilization for inpatients with COPD treated with nebulized arformoterol or nebulized formoterol. Int J Chron Obstruct Pulmon Dis. 2017;12:1793–1801.
- Sivapalan P, Ingebrigtsen TS, Rasmussen DB, et al. COPD exacerbations: the impact of long versus short courses of oral corticosteroids on mortality and pneumonia: nationwide data on 67 000 patients with COPD followed for 12 months. BMJ Open Resp Res. 2019;6:e000407.
- Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415.
- Donaldson GC, Wedzicha JA. COPD exacerbations .1: epidemiology. Thorax. 2006;61:164–168.
- Jarad N. Chronic obstructive pulmonary disease (COPD) and old age? Chron Respir Dis. 2011;8:143–151.
- Taffet GE, Donohue JF, Altman PR. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30.
- Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234.
- Sanchis J, Gich I, Pedersen S, Aerosol Drug Management Improvement Team (ADMIT). Systematic review of errors in inhaler use: has patient technique improved over time? Chest. 2016;150:394–406.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49:1601794.
- Navaie M, Dembek C, Cho-Reyes S, et al. Inhaler device feature preferences among patients with obstructive lung diseases: a systematic review and meta-analysis. Medicine (Baltimore)). 2020;99:e20718.
- Tervonen T, Hawken N, Hanania NA, et al. Maintenance inhaler therapy preferences of patients with asthma or chronic obstructive pulmonary disease: a discrete choice experiment. Thorax. 2020;75:735–743.
- Chorao P, Pereira AM, Fonseca JA. Inhaler devices in asthma and COPD–an assessment of inhaler technique and patient preferences. Respir Med. 2014;108:968–975.
- Hanania NA, Braman S, Adams SG, et al. The role of inhalation delivery devices in COPD: perspectives of patients and health care providers. Chronic Obstr Pulm Dis. 2018;5:111–123.
- Molimard M, Colthorpe P. Inhaler devices for chronic obstructive pulmonary disease: insights from patients and healthcare practitioners. J Aerosol Med Pulm Drug Deliv. 2015;28:219–228.
- Dantic DE. A critical review of the effectiveness of ‘teach-back’technique in teaching COPD patients self-management using respiratory inhalers. Health Educ J. 2014;73:41–50.