Abstract
Aims
To estimate the economic impact of the introduction of gilteritinib for the treatment of relapsed/refractory (R/R) FLT3 mutation-positive (FLT3mut+) acute myeloid leukemia (AML) from a US payer’s perspective.
Methods
A budget impact model (BIM) was developed to evaluate the 3-year total budgetary impact of treating adults with R/R FLT3mut+ AML eligible for gilteritinib in a hypothetical US health plan. Total costs (drugs/administration, hospitalization, monitoring, adverse events, transfusions, subsequent hematopoietic stem cell transplantation, post-progression, and FLT3 testing) were estimated before and after gilteritinib entry. The budget impact was the total cost difference between the two scenarios. The target population size and cost inputs were based on public data or published literature, drug market share was informed by market research data, and the model included recommended treatments for R/R FLT3mut+ AML per clinical guidelines. Deterministic sensitivity analyses (DSAs) and scenario analyses varying key model inputs and assumptions were conducted to test for robustness.
Results
In a hypothetical health plan with 1 million members, 20.9 adults with R/R FLT3mut+ AML were estimated to be eligible for gilteritinib. Of these, it was assumed 30.0% would be treated with gilteritinib in Year 1 following gilteritinib entry, increasing the total plan budget by $663,795 and the per-member-per-month (PMPM) cost by $0.055. In Years 2–3, the market share of gilteritinib increased to 45.0%, increasing the total plan budget impact by $1,078,371 and $1,087,230, and the PMPM cost by $0.090 and $0.091, respectively. The model results remained robust in DSAs and scenario analyses, with the largest impact observed when the projected uptake of gilteritinib was changed.
Limitations
The results of this BIM are contingent upon the model’s assumptions and inputs.
Conclusions
Adding gilteritinib to the formulary for the treatment of adults with R/R FLT3mut+ AML had a minimal budget impact from a US payer’s perspective.
Introduction
Acute myeloid leukemia (AML) is a group of life-threatening hematopoietic neoplasms caused by genetic mutations in the hematopoietic precursor cells, and is characterized by the proliferation of myeloid precursors with limited differentiation capacityCitation1. In the US, AML accounts for approximately 80% of adult leukemia and has an incidence of approximately 3–5 cases per 100,000 individualsCitation2.
While response rates to initial chemotherapy could be as high as 80% for patients newly diagnosed with AML, most patients will ultimately relapse or develop refractory (R/R) AMLCitation3. A variety of salvage chemotherapy (SC) combination options are available for R/R AML depending on a patient’s disease stage and treatment history, and the National Comprehensive Cancer Network (NCCN) treatment guidelines recommend specific treatment regimens based on multiple prognostic factors such as age, gene mutation type, and duration of the most recent remissionCitation4. Despite these treatment options, there is no specific SC regimen that is considered to be the “standard of care”Citation3.
In addition, approximately 30% of patients with AML have one or more confirmed mutations in the FMS-like tyrosine kinase 3 gene (FLT3mut+)Citation5,Citation6, and patients with R/R FLT3mut+ AML have a particularly poor prognosisCitation6. Few effective therapy options existed for patients with R/R FLT3mut+ AML before the introduction of the targeted therapy, gilteritinibCitation3,Citation6. Specifically, conventional treatments included SC followed by hematopoietic stem cell transplantation (HSCT) if eligible, hypomethylating agents with or without sorafenib, or best supportive care (BSC)Citation7. However, the 1-year survival rate after initial relapse has been reported as just 10% among patients with R/R FLT3mut+ AML treated with conventional therapy, with a median overall survival (OS) of approximately 3 monthsCitation3,Citation8–10.
Due to the unmet need for effective therapies for this patient population, there has been a recent surge in the development of more effective therapies for AMLCitation6, although most options (all except gilteritinib) are not specifically indicated for R/R FLT3mut+ AML. Midostaurin was approved by the U.S. Food and Drug Administration (FDA) in April 2017 for the treatment of newly diagnosed AML patients with FLT3 mutations. In addition, venetoclax in combination with azacitidine, decitabine, or low-dose cytarabine (LDAC) was approved by the FDA in November 2018 for the indication of newly diagnosed patients with AML aged 75 years or older. Other therapies approved for treatment of AML include glasdegib, enasidenib, CPX-351, gemtuzumab ozogamicin, and gilteritinib. Among these novel treatments, gilteritinib was the first and only targeted therapy to receive approval from the FDA (in November 2018) with an indication for the treatment of R/R FLT3mut+ AML.
Gilteritinib is a novel, highly selective oral type I inhibitor of FLT3, with activity against both FLT3–internal tandem duplication and FLT3–tyrosine kinase domain mutationsCitation6,Citation11,Citation12. It also inhibits the tyrosine kinase AXL that is commonly overexpressed in AML and has been implicated in resistance to FLT3 inhibitorsCitation11–13. The efficacy of gilteritinib was established in the phase 3 randomized ADMIRAL trial comparing treatment with gilteritinib to SCCitation14–16. The trial reported that gilteritinib significantly improved patient survival outcomes, with 1-year survival rates of 37% vs 17% for SCCitation16. The median OS was significantly higher for patients treated with gilteritinib than with SC (9.3 months vs 5.6 months; hazard ratio for death = 0.637; p < 0.001), and a higher proportion of patients treated with gilteritinib achieved complete remission than those treated with SC (21.1% vs 10.5%). The higher response rates and longer duration of response associated with gilteritinib increase the likelihood for patients to proceed to HSCT, which could provide a long-term cureCitation16. As an effective oral therapy that can be administered in an outpatient setting, gilteritinib also reduced hospitalization days during the active treatment period as well as patients’ dependency on blood and platelet transfusionsCitation16,Citation17. Patients treated with gilteritinib reported improvement in quality of life, possibly related to outpatient administration, reduced hospitalization, and improved clinical outcomesCitation18–20. Consequently, the 2018 NCCN guidelines recommended gilteritinib as a category 1 treatment option for patients with R/R FLT3mut+ AML based on uniform consensus from the NCCN regarding its efficacyCitation21.
The demonstrated efficacy findings indicate the potential for gilteritinib to provide enhanced clinical benefits in treating R/R FLT3mut+ AML and address an unmet need for this patient population. However, given limited healthcare resources, the expected budgetary impact of new treatments is often requested in order to guide decision-making about coverage and formulary placement. Thus, the objective of this analysis was to estimate the budgetary impact of the introduction of gilteritinib for the treatment of R/R FLT3mut+ AML from the perspective of a US healthcare plan payer.
Methods
Model overview
This budget impact model (BIM) was developed for a hypothetical healthcare plan of 1 million covered lives over a 3-year period (). In addition to gilteritinib monotherapy, 11 other treatment regimens for R/R FLT3mut+ AML were included in the model. These regimens represent common treatment options in the US that are also recommended by the NCCN treatment guidelines for R/R FLT3mut+ AMLCitation21. Targeted treatments included midostaurin monotherapy, sorafenib and azacitidine combination therapy, and venetoclax combination therapy (i.e. venetoclax in combination with azacitadine, decitabine, or LDAC). Intensive chemotherapy regimens included fludarabine, cytarabine, granulocyte-colony stimulating factor, and idarubicin (FLAG-IDA); mitoxantrone, etoposide, and cytarabine (MEC); high-dose cytarabine (HiDAC); and cytarabine and anthracycline (7 + 3 regimen). Less intensive chemotherapy regimens included azacitidine monotherapy, decitabine monotherapy, and LDAC. Finally, the model also included BSC (i.e. no active leukemia-directed therapy).
Figure 1. Model framework. Abbreviations. AML, acute myeloid leukemia; FLT3mut+, FMS-like tyrosine kinase 3 mutation-positive.
Total healthcare costs were estimated under two scenarios, before and after the entry of gilteritinib, and consisted of the following components: costs of treatment and its administration, hospitalization, monitoring, adverse events (AEs), blood and platelet transfusions, subsequent HSCT, post-progression medical care, and FLT3 testing. The budgetary impact was estimated as the difference in total healthcare costs among adult patients with R/R FLT3mut+ AML between the two scenarios for each year over a 3-year time horizon.
Key model assumptions
The key model assumptions are as follows. First, the size of the target population, adult patients with R/R FLT3mut+ AML, was assumed to remain constant before and after gilteritinib entry. Second, the drug, administration, and hospitalization costs for all treatments were calculated using the fee-for-service approach. The costs were estimated separately using individual cost components. Third, patients were assumed to be fully adherent to the prescribed regimen until treatment discontinuation; the treatment duration for different comparators was estimated based on the median or mean treatment durations reported in their respective clinical trial publications. Fourth, as BSC does not include any active antileukemic treatment and patients are unlikely to achieve a response or proceed to subsequent HSCT, the costs related to drug acquisition and administration, hospitalization and monitoring prior to disease progression, AEs, and subsequent HSCT were assumed to be zero. Finally, in order to maintain consistency across the patient and target populations, all patients were assumed to have received one FLT3 test, regardless of the treatment regimen.
Model inputs
Target population
The size of the target population in a hypothetical health plan with 1 million enrollees was estimated using inputs from public databases, published literature, and assumptions. Of 1 million enrollees, 77.4% were assumed to be adults (aged ≥18 years) based on the US Census estimatesCitation12. The proportion of adults diagnosed with AML was 0.018%, obtained from the prevalence estimates from the Surveillance, Epidemiology, and End Results Program databasesCitation22. Based on internal market research data, 90% of the adult patients with AML were assumed to have received FLT3 testing (data on file, 2019; Astellas Pharma Corporation). Among the tested population, 27.4% were assumed to have confirmed FLT3 gene mutations (derived from Nagel et al.Citation23) and 61.9% of the patients with FLT3 mutations were assumed to have R/R AML (based on Iqbal et al.Citation24 and Dombret et al.Citation25). The size of the target population was assumed to remain constant before and after gilteritinib entry.
Market share
Market shares prior to the entry of gilteritinib were obtained from the internal market research data based on physician survey results () (data on file, 2019; Astellas Pharma Corporation). In the scenario prior to the entry of gilteritinib, 10 commonly used treatment regimens (sorafenib and azacitidine, midostaurin, all chemotherapy regimens, and BSC) were considered available treatment options for patients with R/R FLT3mut+ AML. In the scenario after gilteritinib entry, gilteritinib monotherapy was assumed to replace the market shares of existing treatments. The replacement rate was informed by physician inputs. In general, the market shares of existing treatment options that had higher usage prior to gilteritinib entry were assumed to decline more compared to those with low usage. The market share of BSC was assumed to remain relatively stable because the share of AML patients who are unfit for any active treatments will likely not be affected by the entry of novel therapies. Furthermore, the market uptake of venetoclax combination therapy, which received approval for AML around the same time as gilteritinib, was also considered during the time periods after gilteritinib entry. Although venetoclax combination therapy was not indicated for the target population, it was also considered in the list of included regimens to be more comprehensive of possible novel treatment options in the constantly evolving treatment landscape. The market share of gilteritinib was assumed to be 30% in the first year following its entry, and increased to 45% in Years 2 and 3.
Table 1. Market share by treatment regimen, before and after gilteritinib entry.
Treatment and medical costs
Treatment and medical cost inputs were mainly derived from publicly available data sources, respective clinical trial publications, and published literature. A summary of the key model inputs and sources is provided in . All cost inputs in the model were reported in, or inflated to, 2019 US dollars, with the exception of drug costs, which reflect 2020 costs. Specifically, the model considered the cost components discussed below.
Table 2. Key treatment and medical cost inputs.
Drug acquisition, administration, and hospitalization costs
Drug acquisition costs were calculated based on the dosing schedules (i.e. the frequency and dosing) specified in the US prescribing information (USPI) or the clinical trial publications for the entire treatment durationCitation16,Citation26–30. For gilteritinib, the dosing schedule (120 mg daily) and treatment duration (median duration of five 28-day cycles) were based on the ADMIRAL trial resultsCitation15. For therapies dosed based on body surface area (BSA), the model assumed a BSA of 1.80 m2 based on the ADMIRAL trial dataCitation16. Drug unit costs were based on average wholesale acquisition costs from IBM Micromedex RED BOOKCitation31. Vial sharing was allowed when estimating the drug costs.
The unit costs of drug administrations were based on the Centers for Medicare & Medicaid Services (CMS) Physician Fee ScheduleCitation15, and were calculated for the entire treatment duration. Oral therapies, including gilteritinib and midostaurin monotherapy, were assumed to have zero administration costs. For combination therapies, it was assumed that treatments were administered consecutively and that the total administration time was the sum of time for all individual treatments. This model separately estimated the hospitalization costs associated with initial treatments, as most treatments considered in the model (particularly intensive chemotherapy regimens) are often administered in an inpatient setting due to associated AEsCitation32. The costs of hospitalization associated with initial treatment were calculated for the event-free survival (EFS) period. The model separately considered post-progression costs, which included hospitalization costs and other medical costs incurred after disease progression. The monthly hospitalization costs were estimated based on the unit costs of stays in intensive care unit (ICU) and non-ICU settings, and the length of stay (LOS) in each setting. Unit cost inputs of ICU and non-ICU inpatient stays were derived from Medeiros et al.Citation33 and Dasta et al.Citation34. LOS inputs were based on the ADMIRAL trial data and published literatureCitation16,Citation35.
AE costs
AE costs were estimated based on grade 3/4 AE rates from clinical trial publications, ADMIRAL trial data (data on file, 2018; Astellas Pharma Corporation), or the USPICitation7,Citation16,Citation26,Citation28,Citation36–41. For gilteritinib, the AE rates were obtained from ADMIRAL trial dataCitation16. The ADMIRAL trial results reported overall AE rates for all SC regimens combined. Thus, the AE rates of chemotherapy regimens were sourced from their respective clinical trial publications to better reflect their individual safety profiles. The AE reporting standard varied across publications. For example, only a few AE categories were reported for midostaurin monotherapy based on Fischer et al.Citation26 In contrast, the ADMIRAL trial publication comprehensively captured all AEs that occurred in ≥10% of patients in any treatment armCitation16. Therefore, the total AE costs for midostaurtin monotherapy were underestimated in the current model. The unit costs of individual AEs came from the literature or public data sourcesCitation36,Citation42–44.
Monitoring, testing, and subsequent procedure costs
Monitoring, procedures, and frequencies were informed by USPI or the NCCN guidelinesCitation17,Citation21,Citation36,Citation45, and unit costs were based on the CMS Clinical Laboratory Fee ScheduleCitation46. The proportions of patients receiving blood and platelet transfusions, the average number of transfusions received per year, and the unit cost per transfusion were based on USPI or published literatureCitation17,Citation35,Citation47,Citation48. The proportion of patients with subsequent HSCT after initial treatment was based on the ADMIRAL trial and published literatureCitation16,Citation28,Citation49,Citation50. The cost of HSCT was based on Medeiros et al.Citation33. Post-progression costs included those associated with outpatient visits, emergency room visits, inpatient stays, diagnostic procedures, and lab tests, and were estimated for the post-EFS period following disease progressionCitation15,Citation33,Citation34,Citation51. Since the target population was R/R FLT3mut+ AML, all patients were assumed to incur the cost of one FLT3 testing of $249 regardless of the treatment receivedCitation46.
Model outputs
The budget impact among adult patients with R/R FLT3mut+ AML was estimated as the difference in costs between the scenarios with and without gilteritinib for the total plan and per-member-per-month (PMPM) costs for each year over a 3-year time horizon. The target population size and the market share data were used to estimate the number of patients receiving each treatment in each scenario. The total per-patient treatment costs were calculated as the sum of all cost inputs by treatment regimen, which were then multiplied by the number of patients receiving each treatment to derive the per-regimen costs. These per-regimen costs were summed to derive the total healthcare costs across all regimens considered in each scenario.
The budget impact at the plan level was the difference in total plan costs between the two analyzed scenarios. The PMPM budget impact results were calculated by dividing the total plan budget impact results by the target population size and further dividing by 12 months.
Sensitivity analyses
To evaluate the uncertainties in model parameter inputs and assumptions, deterministic (one-way) sensitivity analyses (DSAs) were performed to test the robustness of the results. Specifically, the DSAs included the market uptake rate of gilteritinib, target population size, proportion of patients receiving FLT3 testing, and alternative AE rates and cost inputs. Key parameters were varied from their default values by ±15% while holding other inputs at the base-case values. In addition, a scenario analysis was added where only gilteritinib, instead of gilteritinib and venetoclax, was considered for increased market share uptake in the R/R FLT3mut+ population following gilteritinib entry.
Model validation
The model was validated by academic experts from the University of Utah. Based on the guidelines from the International Society for Pharmacoeconomics and Outcomes ResearchCitation52, the academic experts reviewed and validated the model structure, model inputs, and model calculation. Suggestions for revision were addressed prior to finalizing the model.
Results
Base-case analysis
In a hypothetical health plan of 1 million members, 20.9 adults were estimated to have R/R FLT3mut+ AML. In the scenario after gilteritinib entry, gilteritinib monotherapy and venetoclax combination therapy replaced the market shares of treatments available prior to their entries. The market share of gilteritinib was assumed to increase from 30% (6.3 patients) in Year 1 to 45% (9.4) in Year 3 ().
Costs per patient
The total per-patient costs for each regimen and the detailed breakdown by cost component are presented in Supplementary Table S1. Total per-patient costs (from treatment initiation to death) were $323,360 for gilteritinib, $177,349–$232,258 for other targeted therapies, $239,572–$262,804 for intensive chemotherapy regimens, $176,259–$212,523 for less intensive chemotherapy regimens, and $93,784 for BSC. Because the treatment duration varied greatly across treatments (range: 1–5 cycles), the average costs per treatment cycle were also calculated to offer an alternative view of costs by treatment. The average costs per treatment cycle were calculated by dividing the total costs per patient by the number of treatment cycles reported for each treatment. After adjusting for the differences in treatment cycles, average costs per treatment cycle were $64,672 for gilteritinib, $77,419–$99,316 for other targeted therapies, $118,033–$230,628 for intensive chemotherapy regimens, and $176,259–$212,523 for less intensive chemotherapy regimens.
Total budget impact
The yearly total plan costs before the entry of gilteritinib were $4,129,473, compared with $4,793,268 in Year 1, $5,207,844 in Year 2, and $5,216,703 in Year 3 after the entry of gilteritinib. On a total plan level, the model estimated a budget impact of $663,795 in Year 1, $1,078,371 in Year 2, and $1,087,230 in Year 3 (). The corresponding PMPM costs were $0.055 in Year 1, $0.090 in Year 2, and $0.091 in Year 3 ().
Figure 2. Budget impact results. (a) Incremental total plan costs. (b) PMPM costs. Abbreviations: AE, adverse event; FLT3, FMS-like tyrosine kinase 3; HSCT, hematopoietic stem cell transplantation; PMPM, per-member per-month.
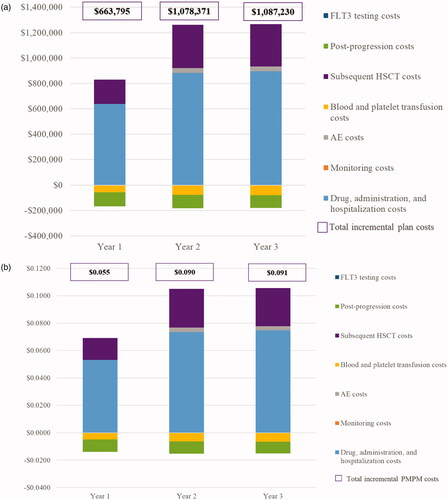
Overall, the total drug, administration, and hospitalization costs for the entire plan increased from $637,194 (Year 1) to $896,400 (Year 3), total AE costs from $1,651 (Year 1) to $35,900 (Year 3), and total subsequent HSCT costs from $191,874 (Year 1) to $335,589 (Year 3), following the entry of gilteritinib. The increase in drug and administration costs was mainly driven by the increased treatment exposure of gilteritinib vs alternative treatments. These budget increases were partially offset by savings in the total plan blood and platelet transfusion costs of $56,828 (Year 1) to $77,320 (Year 3), post-progression costs of $109,318 (Year 1) to $101,983 (Year 3), and monitoring costs of $778 (Year 1) to $1,357 (Year 3).
Sensitivity analyses
The model results remained robust in sensitivity analyses, which varied inputs and assumptions as described in Supplemental Figure S1. The model was most sensitive to changes in the projected uptake of gilteritinib, followed by the total treatment costs of gilteritinib, size of the target population, and subsequent HSCT costs. In Year 1, when the projected uptake rate of gilteritinib was decreased or increased by 15%, the PMPM budget impact ranged from $0.030 to $0.081. When the total treatment costs for gilteritinib were decreased or increased by 15%, the PMPM budget impact ranged from $0.046 to $0.065 in the first year. DSA results were similar in Years 2 and 3.
The base-case analysis considered the contribution of the increased market shares of gilteritinib and venetoclax combination therapy to the total budget impact. In the alternative scenario, which considered market uptake of gilteritinib alone (i.e. the market share of venetoclax combination therapy was set to 0 both before and after gilteritinib entry), the budget impact on the total plan costs decreased to $598,173 in Year 1, $1,041,542 in Year 2, and $1,048,296 in Year 3. The corresponding PMPM costs were $0.050, $0.087, and $0.087 in Years 1, 2, and 3, respectively.
Discussion
As conventional treatments for patients with R/R FLT3mut+ AML have been limited to SC regimens with low response rates, short remission duration, and poor tolerabilityCitation3,Citation6, there is a substantial unmet need for innovative treatments for this patient population. Gilteritinib was the first targeted therapy approved for R/R FLT3mut+ AML in the US, and it demonstrated clinical efficacy in the phase 3 ADMIRAL trial with significantly prolonged OS (9.3 vs 5.6 months) and EFS (2.8 vs 0.7 months) in comparison with SCCitation16,Citation18–20. However, gilteritinib has higher drug acquisition costs compared with conventional SC regimens, and, thus, the economic impact of including it as a new therapy is important to comprehensively assess. Budget impact analysis has become an increasingly important tool for payers to evaluate the economic impact of the inclusion of new pharmaceutical products on annual healthcare expenditures. It is not only crucial for resource planning, but can also provide valuable insight into service provisions within healthcare systemsCitation52.
The present study is the first to estimate the budget impact of adding gilteritinib to a health plan formulary for the treatment of adult patients with R/R FLT3mut+ AML from a US payer’s perspective. The results of the model indicated that adding gilteritinib to a hypothetical US payer with 1 million members resulted in a limited budget impact ($0.055–$0.091, PMPM). As this is the first study to assess the budget impact in R/R FLT3mut+ AML, there are no other studies to compare the present results to. For other indications related to AML, Jensen et al.Citation53 estimated the budget impact of using daunorubicin-cytarabine liposome in patients with newly diagnosed therapy-related AML, and reported PMPM in the range of $0.0025–$0.0045. This estimate is not directly comparable to the current study as it focused on a smaller patient segment within AML, and considered fewer cost components than the current model. Within a broader context that included other oncology conditions, recent budget impact analyses conducted from the US payer’s perspective reported PMPMs ranging from $0.015 to $0.528. Comparatively, the findings from the present study are at the lower end of the previous estimatesCitation54–60.
The entry of gilteritinib modestly increased the drug, administration, and hospitalization costs ($0.053–$0.075, PMPM), AE costs (<$0.000–$0.003, PMPM), and subsequent HSCT costs ($0.016–$0.028, PMPM). The cost increases were partly driven by the prolonged survival and increased treatment exposure of patients receiving gilteritinib versus alternative treatment options. When total costs per treatment cycle were compared across different regimens, gilteritinib-treated patients incurred the lowest costs per treatment cycle compared with all alternative treatment options. In addition, these cost increases were partially offset by decreases in post-progression costs (−$0.0085 to −$0.0091, PMPM); and blood and platelet transfusion costs (−$0.006 to −$0.005, PMPM). Medical cost reductions mainly resulted from the improved efficacy of gilteritinib in delaying disease progression relative to other treatments, and from the benefit of reducing dependence on transfusion among the target patient population. Sensitivity analyses were consistent with the base-case model and confirmed the robustness of the main findings.
Corresponding to the rapidly evolving treatment landscape for R/R FLT3mut+ AML, this study considered a comprehensive set of regimen options. These included new and existing treatments, either FDA-approved or used off-label, to more realistically reflect clinical practices. Consequently, the base-case budget impact estimates reflected budget increases associated with both gilteritinib and venetoclax, although the impact was lower if only gilteritinib entry and its increase in market share were considered. In this scenario, the budget impact on the PMPM cost ranged from $0.050 to $0.087. Gilteritinib monotherapy is a promising treatment option that has the potential to bring substantial clinical benefits and improvements in quality of life to adult patients with R/R FLT3mut+ AMLCitation16. Our results suggest that while adding gilteritinib to a formulary is an affordable option for payers given its low budget impact, further studies are needed to assess the cost-effectiveness of gilteritinib. The cost-effectiveness analysis will assess not only costs, but also the benefits of gilteritinib vs the standard of care, which could provide an alternative perspective to understand the economic value of gilteritinib. Indeed, the International Society for Pharmacoeconomics and Outcomes Research recommended both budget impact and cost-effectiveness analyses to provide a comprehensive economic evaluation to inform formulary or reimbursement decisionsCitation52,Citation61.
Limitations
As with all economic models, the results of this BIM are contingent upon the model’s assumptions and inputs. First, some model inputs were based on evidence from clinical trials, which may not reflect real-world practices. For example, the dosing information and treatment duration could differ in the real world due to dose modification or reduction. However, the model inputs were based on the best evidence available for the specific population from public sources. Second, costs for lower grade (<3) AEs were not included in the model. However, these costs are expected to be low and are likely to have limited impact on the budget. Third, despite best efforts to select the most accurate model inputs from existing studies, different sources were used to inform model inputs. Nevertheless, the results of the DSA indicated that the model was generally robust to input variations. Lastly, due to the rapidly evolving treatment landscape, the model results could vary over time due to changes in the target population size and the entry of other novel therapies. Therefore, further studies should be conducted to re-evaluate the budget impact results as new information becomes available.
Conclusions
This is the first study to assess the budgetary impact of the introduction of gilteritinib for the treatment of adult patients with R/R FLT3mut+ AML from the perspective of a US payer. Based on the results from the BIM, adding gilteritinib to the formulary for this indication had a minimal budget impact on a US payer, while providing a robust alternative to address the unmet needs in this patient population. These results may help healthcare decision-makers in integrating this novel treatment option into an existing formulary and in more efficiently allocating the limited resources within healthcare systems.
Transparency
Declaration of funding
This study was sponsored by Astellas Pharma, Inc.
Declaration of financial or other interests
BJP and MVS are employees of Astellas Pharma Global Development, Inc., and hold stock/stock options. HY and CZQ are employees of Analysis Group, Inc., which received funding from Astellas for the conduct of this research. CS has nothing to disclose.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Portions of this research were presented in poster format at the Academy of Managed Care Pharmacy Nexus during October 29–November 1, 2019 at National Harbor, MD.
Supplemental Material
Download MS Word (68.3 KB)Acknowledgements
Medical writing assistance was provided by Richard Xie, PhD, Muhan Yuan, and Shelley Batts, PhD, employees of Analysis Group, Inc. Editorial assistance was provided by Elizabeth Hermans, PhD, of OPEN Health Medical Communications (Chicago, IL) and funded by the study sponsor. The authors would like to thank Dr. Diana Brixner, Dr. Gary M. Oderda, and Dr. Joseph Biskupiak from the University of Utah for validating the model and providing technical insights.
References
- Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136–1152.
- Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood. 2012;119(1):34–43.
- Ramos N, Mo C, Karp J, et al. Current approaches in the treatment of relapsed and refractory acute myeloid leukemia. J Clin Med. 2015;4(4):665–695.
- O'Donnell MR, Tallman MS, Abboud CN, et al. Acute myeloid leukemia. NCCN Clinical Practice Guidelines in Oncology (version 3.2017). J Natl Compr Canc Netw. 2017;15(7):926–957.
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738–1752.
- Daver N, Schlenk RF, Russell NH, et al. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312.
- Song Y, Magenau J, Li Y, et al. FLT3 mutational status is an independent risk factor for adverse outcomes after allogeneic transplantation in AML. Bone Marrow Transplant. 2016;51(4):511–520.
- Warren M, Luthra R, Yin CC, et al. Clinical impact of change of FLT3 mutation status in acute myeloid leukemia patients. Mod Pathol. 2012;25(10):1405–1412.
- Mangan JK, Luger SM. Salvage therapy for relapsed or refractory acute myeloid leukemia. Ther Adv Hematol. 2011;2(2):73–82.
- Xu J, Lv T-T, Zhou X-F, et al. Efficacy of common salvage chemotherapy regimens in patients with refractory or relapsed acute myeloid leukemia: a retrospective cohort study. Medicine. 2018;97(39):e12102
- Lee LY, Hernandez D, Rajkhowa T, et al. Preclinical studies of gilteritinib, a next-generation FLT3 inhibitor. Blood. 2017;129(2):257–260.
- Mori M, Kaneko N, Ueno Y, et al. Gilteritinib, a FLT3/AXL inhibitor, shows antileukemic activity in mouse models of FLT3 mutated acute myeloid leukemia. Invest New Drugs. 2017;35(5):556–565.
- Park IK, Mishra A, Chandler J, et al. Inhibition of the receptor tyrosine kinase Axl impedes activation of the FLT3 internal tandem duplication in human acute myeloid leukemia: implications for Axl as a potential therapeutic target. Blood. 2013;121(11):2064–2073.
- Gorcea CM, Burthem J, Tholouli E. ASP2215 in the treatment of relapsed/refractory acute myeloid leukemia with FLT3 mutation: background and design of the ADMIRAL trial. Future Oncol. 2018;14(20):1995–2004.
- Perl AE, Martinelli G, Cortes JE, et al. Abstract CT184: Gilteritinib significantly prolongs overall survival in patients with FLT3-mutated (FLT3mut+) relapsed/refractory (R/R) acute myeloid leukemia (AML): results from the Phase III ADMIRAL trial. Cancer Res. 2019;79(13 Supplement):CT184.
- Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381(18):1728–1740.
- United States Food and Drug Administration (FDA). Highlights of prescribing information: XOSPATA (gilteritinib). 2019 [cited 2019 Nov 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211349s000lbl.pdf.
- Ritchie E, Cella D, Fabbiano F, et al. The relationship between transfusion status and patient-reported outcomes in patients with FLT3-mutated relapsed/refractory acute myeloid leukemia: results from the phase 3 ADMIRAL study. 5th International Conference Acute Myeloid Leukemia “Molecular and Translational”: Advances in Biology and Treatment (European School of Hematology); October 24–26, 2019. Estoril, Portugal.
- Cella D, Ritchie E, Fabbiano F, et al. The relationship between transplant status and patient-reported outcomes in patients with FLT3-mutated relapsed/refractory (R/R) acute myeloid leukemia (AML): results from the Phase 3 ADMIRAL study. ASH Annual Meeting; December 7–9, 2019. Orlando, FL, US.
- Ritchie E, Cella D, Fabbiano F, et al. The relationship between hospitalization and patient-reported outcomes (PROs) in patients with FLT3-mutated (FLT3mut+) relapsed/refractory (R/R) acute myeloid leukemia (AML): results from the Phase 3 ADMIRAL study. ASH Annual Meeting; December 7–9, 2019. Orlando, FL, US.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Acute Myeloid Leukemia. Version 3. 2018. [cited 2019 Nov 15]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program databases (data cut January 1, 2015). 2019. [2019 Feb 11]. Available from: https://seer.cancer.gov/.
- Nagel G, Weber D, Fromm E, et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol. 2017;96(12):1993–2003.
- Iqbal S, Near A, Dhopeshwarkar N, et al. Abstract: real-world study of comorbidities and complications in relapsed/refractory acute myeloid leukemia patients identified using a claims-based algorithm. Pharmacoepidem Dr S. 2018;27(3):308–308.
- Dombret H, Seymour J, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–299.
- Fischer T, Stone RM, Deangelo DJ, et al. Phase IIB trial of oral Midostaurin (PKC412), the FMS-like tyrosine kinase 3 receptor (FLT3) and multi-targeted kinase inhibitor, in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome with either wild-type or mutated FLT3. J Clin Oncol. 2010;28(28):4339–4345.
- Karanes C, Kopecky K, Head D, et al. A phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia Southwest Oncology Group Study. Leuk Res. 1999;23(9):787–794.
- Ravandi F, Alattar M, Grunwald M, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood. 2013;121(23):4655–4662.
- Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54(9):2003–2007.
- Wiernik PH, Banks PL, Case DC, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79(2):313–319.
- IBM Micromedex. RED BOOK Online. 2019. [cited 2019 Feb 11]. Available from: https://www.ibm.com/us-en/marketplace/micromedex-red-book.
- Walter RB, Taylor LR, Gardner KM, et al. Outpatient management following intensive induction or salvage chemotherapy for acute myeloid leukemia. Clin Adv Hematol Oncol. 2013;11(9):571–577.
- Medeiros BC, Pandya BJ, Chen C-C, et al. Economic burden of treatment episodes in acute myeloid leukemia (AML) patients in the US: a retrospective analysis of a commercial payer database. Blood. 2017;130(Suppl_1):4694.
- Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271.
- DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133(1):7–17.
- United States Food and Drug Administration (FDA). Highlights of prescribing information: VIDAZA (azacitidine). 2008. [cited 2019 Nov 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/050794s011lbl.pdf.
- United States Food and Drug Administration (FDA). Highlights of prescribing information: DACOGEN (decitabine). 2010. [cited 2019 Nov 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021790s006lbl.pdf.
- Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(13):1249–1259.
- Lee J-H, Choi S-J, Lee J-H, et al. Continuous infusion intermediate-dose cytarabine, mitoxantrone, plus etoposide for refractory or early relapsed acute myelogenous leukemia. Leuk Res. 2006;30(2):204–210.
- Pastore D, Specchia G, Carluccio P, et al. FLAG-IDA in the treatment of refractory/relapsed acute myeloid leukemia: single-center experience. Ann Hematol. 2003;82(4):231–235.
- Schaich M, Parmentier S, Kramer M, et al. High-dose cytarabine consolidation with or without additional amsacrine and mitoxantrone in acute myeloid leukemia: results of the prospective randomized AML2003 trial. J Clin Oncol. 2013;31(17):2094–2101.
- Kawatkar AA, Farias AJ, Chao C, et al. Hospitalizations, outcomes, and management costs of febrile neutropenia in patients from a managed care population. Support Care Cancer. 2017;25(9):2787–2795.
- Eber MR, Laxminarayan R, Perencevich EN, et al. Clinical and economic outcomes attributable to health care–associated sepsis and pneumonia. Arch Intern Med. 2010;170(4):347–353.
- Paoli CJ, Reynolds MA, Sinha M, et al. Epidemiology and costs of sepsis in the United States-an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889–1897.
- United States Food and Drug Administration (FDA). Highlights of prescribing information: VENCLEXTA (venetoclax tablets). 2016. [cited 2019 Nov 21]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208573s009lbl.pdf.
- Centers for Medicare & Medicaid Services (CMS). Clinical Diagnostic Laboratory Fee Schedule. 2018. [cited 2019 Feb 3]. Available from: https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html.
- Zhang Q, Xie L, Baser O, et al. Evaluating treatment patterns of relapsed acute myeloid leukemia (AML) among the elderly in the United States. J Clin Oncol. 2017;35(15_suppl):e18161–e18161.
- Shander A, Ozawa S, Hofmann A. Activity-based costs of plasma transfusions in medical and surgical inpatients at a US hospital. Vox Sang. 2016;111(1):55–61.
- Giles F, Vey N, DeAngelo D, et al. Phase 3 randomized, placebo-controlled, double-blind study of high-dose continuous infusion cytarabine alone or with laromustine (VNP40101M) in patients with acute myeloid leukemia in first relapse. Blood. 2009;114(19):4027–4033.
- DiNardo CD, Pollyea DA, Jonas BA, et al. Updated safety and efficacy of venetoclax with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2017;130(Suppl 1):2628.
- Griffin JD, Yang H, Song Y, et al. Treatment patterns and healthcare resource utilization in patients with FLT3-mutated and wild-type acute myeloid leukemia: a medical chart study. Eur J Haematol. 2019;102(4):341–350.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
- Jensen IS, Wu E, Sacks NC, et al. Budget impact analysis of using daunorubicin-cytarabine liposome in patients with newly diagnosed therapy-related AML or AML and myelodysplasia-related changes. Am Health Drug Benefits. 2018;11(7):380–386.
- Stellato D, Gerbasi ME, Ndife B, et al. Budget impact of dabrafenib and trametinib in combination as adjuvant treatment of BRAF V600E/K mutation-positive melanoma from a US commercial payer perspective. J Manag Care Spec Pharm. 2019;25(11):1227–1237.
- Flannery K, Drea E, Hudspeth L, et al. Budgetary impact of cabazitaxel use after docetaxel treatment for metastatic castration-resistant prostate cancer. J Manag Care Spec Pharm. 2017;23(4):416–426.
- Rose DB, Nellesen D, Neary MP, et al. Budget impact of everolimus for the treatment of progressive, well-differentiated, non-functional neuroendocrine tumors of gastrointestinal or lung origin that are advanced or metastatic. J Med Econ. 2017;20(4):395–404.
- Xie J, Diener M, De G, et al. Budget impact analysis of everolimus for the treatment of hormone receptor positive, human epidermal growth factor receptor-2 negative (HER2-) advanced breast cancer in the United States. J Med Econ. 2013;16(2):278–288.
- Wu L, Zhong L. Budget impact analysis of niraparib and olaparib for maintenance treatment of platinum-sensitive, recurrent ovarian cancer in the US. J Med Econ. 2019;22(2):187–195.
- Bly CA, Molife C, Brown J, et al. The budget impact of including necitumumab on the formulary for first-line treatment of metastatic squamous non-small cell lung cancer: US commercial payer and Medicare perspectives. J Manag Care Spec Pharm. 2018;24(6):534–543.
- Appukkuttan S, Duchesneau E, Zichlin ML, et al. A budget impact analysis of the introduction of copanlisib for treatment of relapsed follicular lymphoma in the United States. J Manag Care Spec Pharm. 2019;25(4):437–446.
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices-budget impact analysis. Value Health. 2007;10(5):336–347.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases. 2019. [cited 2019 Nov 21]. Available from: https://www.hcup-us.ahrq.gov/.
- Tikhonova IH, Snowsill TM, Cooper C, et al. Azacitidine for treating acute myeloid leukaemia with more than 30 % bone marrow blasts: an evidence review group perspective of a national institute for health and care excellence single technology appraisal. Pharmacoeconomics. 2017;35(3):363–373.
- Shahswar R, Hamwi I, Lueck C, et al. Registry for the off-label use of venetoclax in patients with relapsed or refractory acute myeloid leukemia. EHA Library. 2018;216245:PB1735.
