?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
In August 2018, the US FDA granted accelerated approval for nivolumab in small cell lung cancer (SCLC) that has progressed after platinum-based chemotherapy and at least one other line of therapy. The objective of this study was to evaluate the cost-effectiveness of nivolumab vs. usual care as third-line (3 L) therapy for patients with recurrent SCLC (rSCLC) from the health payer perspective. Given the potential for a meaningful fraction of treated patients to achieve long-term response to nivolumab, we also assessed the impact of using mixture cure modeling (MCM) vs. parametric survival modeling on survival estimates and cost-effectiveness from the US Medicare payer perspective.
Methods
We created a partitioned survival decision model to assess the cost-effectiveness of 3 L nivolumab vs. usual care in rSCLC, based on observed US treatment patterns. Using this approach, we assessed the impact of extrapolating long-term survival from the CheckMate 032 trial, using both MCM and standard parametric curve fits. Nivolumab survival, resource use, and Grade 3/4 adverse event rates were derived from CheckMate 032. Usual care survival, resource use, and costs were derived from an analysis of patients receiving 3 L treatment for rSCLC in the SEER-Medicare registry. We applied 2020 Wholesale Acquisition Cost for drugs and 2020 CMS reimbursement for procedures. Utilities were derived from the literature. We estimated life years (LY), quality-adjusted life years (QALYs), and costs over a lifetime horizon.
Results
MCM and parametric survival model extrapolations resulted in 0.43 versus 0.38 more LYs, 0.34 versus 0.30 more QALYs, and $69,308 versus $61,336 more expenditure for nivolumab vs. usual care, respectively. The costs per QALY gained using mixture cure versus parametric survival modeling were $204,386 and $207,431, respectively.
Conclusions
Mixture cure modeling was equivalent compared to parametric modeling in estimating the cost-effectiveness of nivolumab-based therapy due to the small fraction of patients achieving a long-term response with nivolumab (12.9%).
Background
Based on the results of the CheckMate032 trial, the US Food and Drug Administration granted approval for nivolumab in recurrent small cell lung cancer (rSCLC) that has progressed after platinum-based chemotherapy and at least one other line of therapyCitation1–3. Following regulatory approval for marketing, decision makers for third party payers are interested in understanding factors that influence the cost-effectiveness of novel therapies such as nivolumab relative to usual care, specifically how to account for the novel mechanism of action and duration of response for immunotherapies in lung cancer. As has been shown for immunotherapy treatments in a similar but more prevalent NSCLCCitation4 a fraction of rSCLC patients achieve long-term response following treatment with nivolumab. Survival modeling is necessary to translate observed survival from trials into estimates of lifetime survival.
Mixture cure models (MCM) are an established method to empirically estimate long-term survival in clinical scenarios where there is potential for long-term response, but limited observed follow-up timeCitation5. If there is a substantial fraction with durable response, failure to account for heterogeneity in survival outcomes could result in biased estimates of expected survival over a lifetime horizon, as well as undervaluing the effects of immunotherapy in terms of cost per life year and/or quality-adjusted life year gainedCitation5. Because the nivolumab-treated patients in the CheckMate032 trial appeared to have a durable survival response, the use of MCM to estimate cost-effectiveness was potentially appropriate.
Accordingly, the objective of this methodology-oriented study was to understand the impact of MCM versus alternative parametric survival models on estimates of the cost-effectiveness of nivolumab versus usual care (topotecan) for rSCLC patients with progression after platinum-based chemotherapy and at least one other line of therapy. This approach was taken because there is increasing use of MCM in the cost-effectiveness literature, but relatively few studies that explore the implications of MCM versus traditional parametric survival models on cost-effectiveness outcomes. Using the results of the CheckMate 032 trialCitation2, we estimated mean lifetime survival for nivolumab- and topotecan-treated patients using MCM and extrapolations using standard parametric models. Our goal was to assess the relative impact of accounting for the fraction in the trial observed to have durable response on cost-effectiveness outcomes for the treated patient population, using the healthcare payer perspective. Our findings can contribute to ongoing discussions about optimal conditions to apply MCM survival models versus alternative survival extrapolation approaches.
Methods
Model overview
We developed a partitioned survival framework in Microsoft Excel to estimate long-term clinical and economic outcomes in patients receiving third-line treatment for rSCLC. Patients were tracked in pre-progression and progression health states estimated from the progression-free survival (PFS) curve and overall survival (OS) curves (). The model evaluates cohorts with advanced (Stage IIIB/IV) rSCLC who have progressed or failed two previous lines of treatment and have a mean age of 63 years in monthly cycles over a lifetime horizon (20 years) from a U.S. payer perspective. Life year, quality-adjusted life year (QALY), and direct medical expenditure outcomes were discounted at 3% per yearCitation6.
Figure 1. Simplified schematic of the cost-effectiveness analysis model framework. The model uses an integrated decision tree and Markov state-transition model to track lifetime small cell lung cancer clinical and economic outcomes.
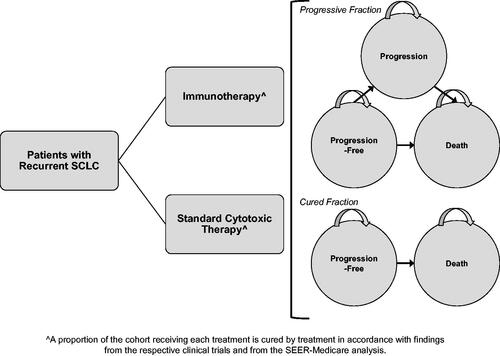
The comparators for the model were nivolumab versus usual care. Because no clinical trials have directly compared the efficacy of nivolumab with usual care, and usual care in the third line setting is poorly defined, we considered two different options as comparators to nivolumab: (1) observed treatments from a large payer database (termed “usual care”), and (2) oral or IV topotecan from a large payer database. Topotecan is a recommended option, has supporting evidence, and is widely used in this settingCitation7,Citation8. Accordingly, we utilized information from clinical trial publications and retrospective databases to inform outcomes for patients treated with topotecan.
SEER-Medicare analysis to identify outcomes and costs for comparator regimens
To understand patterns of third-line treatment in SCLC, we conducted a retrospective, observational cohort study using data from the United States National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) cancer registry linked with United States Medicare enrollment and claims dataCitation8 for persons ages 65 and older. Administrative claims for hospital, physician, outpatient, home health, and hospice bills from the Medicare program were linked to the tumor and demographic characteristics recorded in SEER for patients newly diagnosed with SCLC diagnosed between 2007 and 2011. Medicare claims for these patients were available for calendar years 2006‒2013 to identify comorbid conditions, treatments, and outcomes.
Patients were included if they were age ≥66 years at time of SCLC diagnosis to allow for at least a one-year “look-back” period to identify comorbid conditions. Patients were also required to have 12 continuous months of Medicare Part A and B with no managed care plan prior to diagnosis. Patients must have had microscopically confirmed SCLC as the first, primary diagnosis and limited or extensive disease at initial diagnosis. All patients must have received outpatient chemotherapy within 90 days of diagnosis and a platinum-based chemotherapy regimen prior to initiating third-line therapy (cisplatin or carboplatin in combination with another agent).
Patients were excluded for the following reasons: diagnosis by death certificate or autopsy, death in the month of diagnosis, or receipt of chemotherapy prior to the month of SEER diagnosis. To align better with CheckMate 032 clinical trial exclusion criteria, patients receiving third-line therapy were excluded if they had evidence of any of the following conditions at the time of initiating third-line therapy (see Supplemental material for additional details): rheumatoid arthritis: psoriatic arthritis, multiple sclerosis, lupus, Addison’s disease, hepatitis B, hepatitis C: HIV or AIDS, Crohn’s disease, ulcerative colitis, brain or spine metastases, or other nervous system metastasesCitation2.
SEER-Medicare analyses were conducted using R (version 3.4.4). Additional details about the SEER-Medicare analysis are provided in Supplementary Material.
Third-line treatments considered
Three SCLC interventions were assessed in the model. First, we projected outcomes for treatment with nivolumab given at 3 mg/kg once per 14-day cycle until progression using CheckMate 0322 data. Second, we projected outcomes for “usual care” – a blended average of all third-line treatments observed in a SEER-Medicare linked database analysis of rSCLC. Lastly, we projected outcomes for treatment with topotecan (oral) – the most prevalent treatment among SCLC observed in SEER-Medicare. Oral topotecan was given at 2.3 mg/m2 per day on days 1–5 of 21-day cycle until disease progressionCitation9, and survival outcomes were derived from the sub-group receiving topotecan in the SEER-Medicare analysis noted above.
Modeling survival inputs
Data for nivolumab PFS and OS curves were derived from the CheckMate 032 trialCitation2. For topotecan and usual care, we used survival estimates from the SEER-Medicare analysis described above. Survival data did not distinguish between patients treated with oral or IV topotecan, as effectiveness is expected to be the same with each formulationCitation7. Usual care survival data reflected all chemotherapy regimens administered in the third-line treatment of advanced SCLC, and the most commonly administered regimens were topotecan (28.4%), paclitaxel (19.7%), and platinum + irinotecan (7.6%).
The CheckMate 032 trial did not ascertain long-term survival for all patients enrolled, so we extrapolated survival beyond the trial time horizon in order to evaluate potential cost-effectiveness over a lifetime horizon. Using patient-level data from CheckMate 032, we fit four models to the in-trial survival data – 2 parametric (Exponential & Weibull) and 2 mixture cure models (MCM)Citation10 (MCM with Exponential & MCM with Weibull) (). For the MCMs, the mean OS of the cured proportion is the mean of the background mortality (SB), whereas the mean OS for the non-cured patients is a function of both the background mortality (SB) and the disease-related mortality (SE). The mean of a random variable with survival function S(t) is equal to and so the mean OS for cured patients is equal to
and the mean OS for non-cured patients is equal to
Background mortality for the MCMs was derived from age- and gender-matched mortality data from U.S. life tablesCitation11. We assessed curve fit of each of the 4 fits (Exponential, Weibull, MCM Exponential, and MCM Weibull) using Akaike information criterion (AIC) and Bayesian Information Criterion (BIC). The best fitting curve (MCM Exponential) was selected based on minimal AIC and BIC among the group and clinical plausibilityCitation11. Mean and median life years (LY) were calculated over a lifetime horizon with this extrapolation approach.
Treatment adverse events
Rates for grade 3 and 4 adverse events (AEs) were based on clinical trial dataCitation2,Citation7. In the base case, the costs of managing grade 3/4 AEs was assumed to be comprised of 50% of the cohort receiving inpatient treatment and 50% receiving outpatient treatment. Inpatient costs were estimated using the mean cost to treat corresponding ICD-9/10 codes from the AHRQ healthcare cost and utilization project (HCUP)Citation12. AE-related outpatient costs were based on a single office visit ().
Table 1. Model inputs.
Health-related quality of life inputs
Health-related quality of life was incorporated into the model using mean EQ-5D utility values derived from the CheckMate 032 trial in the base caseCitation2,Citation3. Disutilities associated with incident adverse events were assumed to be captured by pre-progression (on treatment) utilities and were therefore not explicitly modeled.
Due to a lack of utilities specific to the comparator regimens, we conservatively assumed comparable pre- and post-progression values to nivolumab in the base case analysis. We also conducted a scenario analysis assuming a lower pre-progression utility for patients receiving usual care (0.700) to explore the potential implications of greater Grade 3/4 AE rates associated with usual care relative to nivolumab.
Cost inputs
We included costs related to drug treatment, adverse events, and disease progression. Weekly treatment and monitoring costs were calculated using dosing schedules, and unit costs for the drugs based on the wholesale acquisition cost for nivolumab, IV topotecan, and oral topotecan and expenditures from the SEER-Medicare cohort. Unit cost estimates can be found in . The model calculates the total cost of treatment assuming that patients are treated until progression or death.
To calculate the cost of treatment for usual care, we calculated the per-day treatment cost for the SEER-Medicare sample (third-line treatment expenditure divided by number of days from initial receipt of third line treatment to final receipt of that same regimen) and multiplied that value by the mean days to progression in the usual care group in the SEER-Medicare sample. This yielded mean treatment durations that were longer (e.g. 3.9 months for nivolumab vs. 1.3 months observed for usual care) and third line treatment expenditure that was greater (e.g. $16,702 for nivolumab vs. $7,936 observed for usual care) than what was actually observed among patients in the SEER-Medicare analysis. This approach was taken reflect the younger and healthier cohort in Checkmate 032, and that a true counterfactual comparator cohort receiving usual care would likely tolerate treatment better than that observed among older and sicker patients in SEER-Medicare. For example, the mean age of patients in the SEER-Medicare usual care cohort was 73 years, whereas mean age in the nivolumab arm of Checkmate 032 was 63Citation2. Younger, healthier patients would be expected to tolerate cytotoxic regimens better and complete a greater proportion of their planned treatment course.
The monthly cost during progression is based on a study by Mariotto et al., which estimated the cost of treating of lung cancer in the initial, continuing, and terminal care (last 12 months of life) phasesCitation13. All costs were adjusted to 2020 USD using the medical care component of the U.S. Consumer Price Index.
Model outcomes
Utility-adjusted time spent in each health state was summed to provide estimates of quality-adjusted life years (QALYs) for each intervention. Model outcomes of interest include: Total health care costs, Life-years, QALYs, and Incremental cost-effectiveness (ICER) ratios (cost/life-year and cost/QALY)
Sensitivity and scenario analyses
Uncertainty was evaluated using one-way and probabilistic sensitivity analysis (PSA). Input ranges were based on 95% confidence intervals or plausible ranges and distributional assumptions were based on recommended guidelinesCitation14. Sensitivity analyses did not include uncertainty related to PFS and OS duration as the model utilizes static curve fits. Accordingly, a greater degree of variability in results would be expected in “real world” cost-effectiveness outcomes.
A scenario analysis was conducted to evaluate a lower pre-progression health state utility for patients receiving usual care versus nivolumab (0.700) to explore the potential implications of greater Grade 3/4 adverse event rates associated with usual care relative to nivolumab. In this scenario, we assumed health state utility of 0.700 in order to approximate the potential health-related quality of life decrement associated with the more toxic treatments given as “usual care” (0.091 decrement).
Results
SEER-medicare analysis
The SEER-Medicare analysis identified 472 patients who received platinum-based chemotherapy and at least one other line of therapy. The mean age of the cohort was 73 years with 50% female and 89% White. In this cohort, 73% were initially diagnosed with extensive disease and 93% received platinum plus etoposide as first-line therapy. For second-line therapy, 35% received topotecan monotherapy, 28% received platinum etoposide, 11% received platinum irinotecan, 10% received paclitaxel alone or with platinum, and 12% received another regimen. There were 43 patients who received fourth-line therapy during the observation period.
In the overall cohort, approximately 39% survived for 6 months, 14% survived for 12 months, and 4% survived for 24 months. The proportion of patients who had not progressed (ended third-line therapy) or died was 36% at 3 months, 11% at 6 months, and 1% at 12 months. Mean and median duration of therapy varied by treatment from 30 and 22 days for cyclophosphamide doxorubicin vincristine (n = 22) to 113 and 83 days for platinum irinotecan (n = 36). The mean cost (2020 dollars) of outpatient therapy ranged from $2,443 for paclitaxel to $12,395 for cyclophosphamide + doxorubicin + vincristine.
As an additional set of analyses, the results for the entire cohort were repeated for the subgroup who received third-line topotecan (n = 134). In this subgroup, the mean age was 73 years, 88% were White, and 79% had extensive disease at diagnosis.
Base case cost-effectiveness results
The MCM with exponential fit used to model base case nivolumab survival in the base case estimated that 12.9% of patients would experience long-term survival (“cure”)Citation6. In the base case, treatment with nivolumab versus usual care showed an increase of 0.26 LYs in the PF state, an additional 0.43 LYs overall, an additional 0.34 QALYs, and an increase of $69,307. This yielded a mean cost per LY of $161,263 and cost per QALY of $204,386 ().
Table 2. Deterministic analysis results: nivolumab (MCM) vs. alternative regimens.
Table 3. Deterministic analysis results for nivolumab, mixture cure model vs. parametric curve fit.
Treatment with nivolumab versus IV topotecan showed an increase of 0.31 LYs in the PF state, an additional 0.56 LYs overall, an additional 0.44 QALYs, and a cost increase of $91,786. This yielded a mean cost per LY of $164,917 and cost per QALY of $209,079 ().
Treatment with nivolumab versus oral topotecan resulted in an increase of 0.31 LYs in the PF state, an additional 0.56 LYs overall, an additional 0.44 QALYs, and an increase of $47,575. This yielded a mean cost per LY of $85,480 and cost per QALY of $108,370 ().
Cost-effectiveness results with alternative nivolumab survival curve fits
Results with a nivolumab MCM using a Weibull fit, a Weibull parametric fit, and an exponential parametric fit were very similar to the base case scenario that used a MCM with an exponential fit (). An MCM with Weibull fit estimated that 13.5% of patients would have long-term survival (“cure”) and resulted in 1.15 life years (0.11 more vs. base case) on nivolumab, 0.90 QALYs (0.08 more than base case), $144,180 in total cost ($8,776 more than base case), a cost per life year gained of $146,435 ($14,828 less than base case) vs. usual care, and a cost per QALY gained of $185,870 ($18,516 less than base case) vs. usual care.
A Weibull parametric fit resulted in 1.04 life years (equal to base case) on nivolumab, 0.82 QALYs (equal to base case), $132,297 in total cost ($3,107 less than base case), a cost per life year gained of $157,138 ($4,125 less than base case) vs. usual care, and a cost per QALY gained of $199,315 ($5,071 less than base case) vs. usual care.
An exponential parametric fit resulted in 0.99 life years (0.05 less than base case) on nivolumab, 0.78 QALYs (0.04 less than base case), $127,432 in total cost ($7,972 less than base case), a cost per life year gained of $163,609 ($2,346 more than base case) vs. usual care, and a cost per QALY gained of $207,431 ($3,045 more than base case) vs. usual care.
Scenario analysis
Lowering pre-progression health state utility among patients receiving usual care (0.700), treatment with nivolumab vs. usual care resulted in an increase of 0.26 LYs in the PF state, an additional 0.43 LYs overall, an additional 0.37 QALYs, and an increase of $69,307 yielding an incremental cost per LY of $161,263 and cost per QALY of $188,007.
Uncertainty analyses
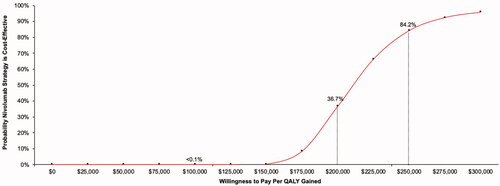
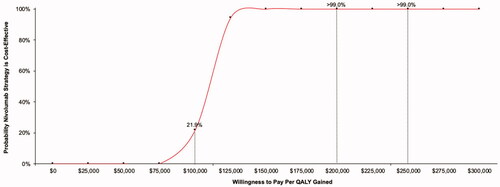
Probabilistic sensitivity analyses were performed with 5,000 simulation runs for each of the base-case comparators. All analyses yielded >99% of results in the upper-right quadrant of the cost-effectiveness acceptability plane – indicating that nivolumab is expected to increase direct medical expenditure and increase quality-adjusted survival vs. all comparators. The resulting cost-effectiveness acceptability curve for the nivolumab vs. usual care comparison is shown in . In comparisons of nivolumab vs. comparators, the probability of cost-effectiveness of $100,000 $200,000 and $300,000 per QALY gained were: <0.1%, 36.7%, and 95.8% vs. usual care (); 49.1%, >99.9%, and >99.9% vs. oral topotecan (); and <0.1%, 15.1%, and 99.7% vs. IV topotecan, respectively.
In one-way sensitivity analysis, the most influential inputs impacting the cost per QALY gained were the price of nivolumab, health state utility for progressive disease, and health state utility for pre-progression, and cost of post-progression care across all comparators.
Discussion
Using simulation modeling and data from pivotal trials and retrospective claims databases, we evaluated the impact of different approaches to survival modeling on estimates of cost-effectiveness of nivolumab versus a mix of systemic therapies observed from the SEER-Medicare database as well as topotecan – a commonly used regimen in third-line treatment of rSCLC. To understand the impact of modeling that accounts for long term survivors, we used survival extrapolations based on typical parametric curve fits to in-trial data (Exponential & Weibull), as well as using MCM (MCM with Exponential & MCM with Weibull). The overall cost per QALY gained results in the base case using mixture cure versus parametric survival modeling were $204,386 and $207,431 (a $3,045 decrease with MCM), respectively. Overall, MCM vs. standard parametric fits had limited impact on survival, cost, and ICER estimates in this analysis because the nivolumab cure fraction was small (12.9%) limiting potential bias by not accounting for cure fractions.
In one-way sensitivity analysis, results were largely driven by differences in third-line treatment cost. In probabilistic analysis, the likelihood that nivolumab could be cost-effective at the upper bounds of willingness-to-pay per QALY gained thresholds was <1% vs. usual care and IV topotecan. Nivolumab has higher relative price per month of third-line treatment vs. usual care and IV topotecan and is used until progression, resulting in a substantially higher lifetime cost vs. the comparators.
Our findings are similar to those of a prior analysis of the cost-effectiveness of third-line nivolumab vs. oral and IV topotecan conducted by Smare et al. that also took a U.S. payer perspectiveCitation15. In that study, the authors used a partitioned survival model framework with a standard parametric survival extrapolation to calculate long-term nivolumab OS (generalized gamma distribution). They estimated lifetime direct medical expenditure for nivolumab, IV topotecan, and oral topotecan of $187,728 (vs. $135,404 in this model), $55,329 (vs. $43,617 in this model), and $81,565 (vs. $87,829 in this model), respectively. Furthermore, they estimated lifetime horizon QALYs for nivolumab, IV topotecan, and oral topotecan of 1.23 (vs. 0.82 in this model), 0.37 (vs. 0.38 in this model), and 0.37 (vs. 0.38 in this model), respectively. Using these outcomes, Smare et al. estimated ICERs for IV topotecan and oral topotecan vs. nivolumab of $152,312 (vs. $209,079 in this model) and $123,003 (vs. $108,370 in this model), respectively.
Several limitations of this analysis should be noted. First, survival results for CheckMate 032 were not mature at the time of this analysis, thus increasing the uncertainty of our estimates of short and long term (lifetime) survival. Second, usual care was modeled in two ways, based on observed treatments for Medicare-eligible patients and for topotecan, a commonly used 3rd line therapy. Treatment mixes for younger patients are not available, thus raising uncertainty regarding the applicability of our results to younger persons with rSCLC. Third, matching criteria were limited to variables available in SEER-Medicare, which do not include information about performance status or biomarkers that might impact treatment choice or survival. Long term survival for the “cured” population was based on age-adjusted population estimates, and may be optimistic for a lung cancer cohort with other comorbidities. Finally, we caution that our study is focused on the relative impact of MCM versus parametric modeling, and is not intended to be used for clinical decisions or resource allocation. The CheckMate 331 study – evaluating nivolumab versus topotecan or amrubicin in patients with small cell lung cancer (SCLC) who relapsed following platinum-based chemotherapy–did not meet its primary endpoint of overall survival. In immunotherapy naïve patients, nivolumab or nivolumab plus ipilimumab may have a role in the third-line settingCitation2.
Our study suggests that use of a MCM had a small impact on the survival estimate versus standard survival modeling. This may reflect the fact that the survival data were not fully mature, or that there is not a large enough fraction of long term survivors to warrant a MCM-type analysis. Further study is recommended, particularly as survival experience matures for rSCLC patients treated with nivolumab to refine the estimates provided here.
Transparency
Declaration of funding
This study was supported by funding from Bristol-Myers Squibb.
Declaration of financial/other relationships
JR, MO, MD, and SR are consultants for Bristol-Myers Squibb.
YY, SW, and JP are employees of Bristol-Myers Squibb.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors have contributed to the manuscript in accordance with Journal of Medical Economics authorship guidelines.
Supplemental Material
Download MS Word (63.6 KB)Acknowledgements
None reported.
References
- Research C for DE and FDA grants nivolumab accelerated approval for third-line treatment of metastatic small cell lung cancer. FDA. [Published online 2019 Feb 9; cited 2020 May 5]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-nivolumab-accelerated-approval-third-line-treatment-metastatic-small-cell-lung-cancer
- Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895.
- Ready N, Farago AF, de Braud F, et al. Third-line nivolumab monotherapy in recurrent SCLC: CheckMate 032. J Thorac Oncol. 2019;14(2):237–244.
- Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20(10):1395–1408.
- Estimating the long-term outcomes associated with immuno-oncology therapies: challenges and approaches for overall survival extrapolations. ISPOR | International Society For Pharmacoeconomics and Outcomes Research. [cited 2020 May 5]. https://www.ispor.org/publications/journals/value-outcomes-spotlight/abstract/january-february-2018/estimating-the-long-term-outcomes-associated-with-immuno-oncology-therapies-challenges-and-approaches-for-overall-survival-extrapolations.
- Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103.
- Eckardt JR, von Pawel J, Pujol J-L, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25(15):2086–2092.
- SEER Cancer Statistics Review, 1975-2017. SEER. [cited 2020 May 5]. https://seer.cancer.gov/csr/1975_2017/index.html
- National Comprehensive Cancer Network. Clinical practice guidelines in non-small cell lung cancer, Version 1.2020. [cited 2019 Dec 10]. Available from: https://www.Nccn.Org/Professionals/Physician_gls/Pdf/Nscl.Pdf.
- Othus M, Bansal A, Koepl L, et al. Accounting for cured patients in cost-effectiveness analysis. Value Health. 2017;20(4):705–709.
- Ramsey SD, Othus M, Roth JA, et al. PRM126 – A COMPARISON OF MIXTURE CURE AND STANDARD PARAMETRIC MODELING TO ESTIMATE LONG-TERM SURVIVAL FOR SMALL-CELL LUNG CANCER IN PATIENTS TREATED WITH NIVOLUMAB AFTER PROGRESSION. Value Health. 2018;21:S377.
- Healthcare Cost and Utilization Project (HCUP). [cited 2020 May 5]. http://www.ahrq.gov/data/hcup/index.html.
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128.
- Decision Modelling for Health Economic Evaluation – Health Economics Research Centre (HERC). [cited 2020 May 5]. https://www.herc.ox.ac.uk/downloads/decision-modelling-for-health-economic-evaluation.
- Smare C, Dave K, Juarez-Garcia A, et al. PCN227 A PARTITIONED SURVIVAL MODEL TO DETERMINE COST-EFFECTIVENESS OF THIRD-LINE NIVOLUMAB MONOTHERAPY FOR SMALL CELL LUNG CANCER. Value in Health. 2019;22:S480.