Abstract
Aims
To assess the real-world healthcare resource utilization (HRU) and costs associated with different proteasome inhibitors (PIs) for the treatment of patients with relapsed and/or refractory multiple myeloma (RRMM) in Germany.
Methods
We conducted a retrospective medical chart review of treatment patterns, outcomes, and HRU for patients with RRMM treated with bortezomib, carfilzomib, or ixazomib in second- or third-line (2L or 3L) therapy in Germany. Data were collected between 1 January 2017 and 30 June 2017. Costs were calculated based on drug prices and unit costs in Germany.
Results
Physicians provided data on 302 patients. Mean monthly total direct costs per patient receiving PI-based therapy were €7,925 and €10,693 for 2L and 3L, respectively, of which approximately 90% was anti-myeloma drug costs. Overall, the highest costs were associated with patients receiving 3L therapy. Regardless of treatment line, costs were higher for patients who had received a stem cell transplant (SCT) in a previous treatment line than for those who had not; the data suggest that this reflects the use of triplet regimens following a SCT. Patients with a complete response (CR) experienced no unplanned hospitalizations during the study period, whereas patients with progressive disease experienced the highest number of unplanned and planned hospitalizations. In 2L therapy, the highest proportion of patients with a CR was observed in those receiving carfilzomib (12% carfilzomib; 4% bortezomib; 0% ixazomib).
Limitations
Patients with missing or incomplete follow-up data were included in the study and were accounted for using monthly cost estimates.
Conclusions
Anti-myeloma drugs were the main contributor to total HRU costs associated with RRMM in Germany. Improved treatment response was associated with lower costs and reduced hospitalizations.
Introduction
Multiple myeloma (MM) is a malignant disease characterized by the proliferation of clonal plasma cells in the bone marrowCitation1. There are over 150,000 new cases of MM and 110,000 related deaths each year worldwideCitation2; of these, approximately 7,700 incident cases and 4,500 deaths from MM occur in GermanyCitation3. Three-quarters of patients with MM are over 65 years of ageCitation2,Citation4,Citation5 and MM incidence is expected to rise as the population agesCitation6.
The treatment of MM has evolved significantly over the past 20 years, with the introduction of novel agents resulting in deep and durable treatment responses, improved disease control, and longer survivalCitation7,Citation8. In Germany, five-year survival rates increased from 47% in 2002–2004 to 54% in 2008–2010Citation5. Despite this, MM remains an incurable disease and almost all patients who respond to initial therapy eventually relapse and require further treatmentCitation9.
The first-generation proteasome inhibitor (PI) bortezomib was approved for use in patients with relapsed and/or refractory MM (RRMM) in Europe in 2004, and showed improved survival compared with single-agent thalidomide and high-dose dexamethasone (DEX)Citation10–13. The introduction of second-generation PIs, carfilzomib and ixazomib, has further improved outcomes. Although head-to-head comparisons are limited, data from randomized phase 3 trials suggest that second-generation PIs may have superior efficacy and toxicity profiles compared with bortezomibCitation10,Citation14–17. Furthermore, triplet combination therapies consisting of a PI, an immunomodulatory drug, and DEX have demonstrated improvements in patient outcomes when compared with doublet therapiesCitation18,Citation19.
Treatment regimens incorporating carfilzomib or ixazomib were approved in Europe for use in RRMM in December 2015 and September 2016, respectivelyCitation15,Citation16. Therefore, all three approved PIs have been available in Germany since 2017. Bortezomib and carfilzomib can be used in doublet regimens with DEX (Vd and Kd, respectively)Citation20. Bortezomib can be used in a triplet regimen with DEX and daratumumab (DVd), whilst carfilzomib and ixazomib can be used in triplet regimens with lenalidomide and dexamethasone (KRd and IRd, respectively)Citation14,Citation15,Citation20,Citation21. With the availability of multi-drug combinations for the treatment of RRMM, the associated improvement in survival rate is expected to prolong treatment exposure, increasing the costs of RRMM therapy. Currently, limited data are available on the real-world use of PIs in patients with RRMM in Germany.
To better understand the costs associated with current PI-based regimens for RRMM in Germany, a retrospective chart review was conducted among hospital- and office-based onco‐hematologists treating patients with RRMM with a PI-based regimen in 2017. Characteristics and treatment experiences of patients with RRMM who received PI-based second-line (2L) therapy in Germany have been published recentlyCitation22,Citation23. Real-world healthcare resource utilization (HRU) and costs associated with the use of different PIs in the relapse setting are reported here.
Methods
Objectives
The primary objective of the study was to describe the demographic and clinical characteristics of PI‐treated patients at diagnosis and at PI initiation: these data are reported elsewhereCitation22,Citation23. This manuscript reports HRU data that were collected to estimate the costs associated with RRMM management in PI-treated patients in 2L or third-line (3L) therapy, as an exploratory analysis.
In addition, subgroup analyses were performed for HRU and costs; these were assessed by PI-based therapy received, line of treatment, response to treatment, and whether the patient had received a stem cell transplant (SCT) during first-line (1L) therapy. HRU and costs were not assessed by treatment response in 3L therapy analyses owing to small sample sizes.
Study design
This national retrospective chart review study was conducted among physicians involved in the management of patients with RRMM in Germany. Quota sampling was used to select a sample of representative physicians across the country in terms of region and type of care (i.e. hospital- vs office-based).
Inclusion and exclusion criteria
Physicians were eligible to participate if they were directly responsible for treatment initiation in patients with symptomatic MM, used PIs in daily practice in accordance with approved indications, managed at least four patients who met the eligibility criteria, and had at least 3 years of clinical experience at the time of study initiation. Patients were eligible for inclusion if they had symptomatic RRMM, were an adult (aged ≥18 years) at the time of MM diagnosis, and received at least one dose of bortezomib, carfilzomib, or ixazomib for the treatment of RRMM between 1 January 2017 and 30 June 2017. Patients with monoclonal gammopathy of undetermined significance, smoldering myeloma, and those who received a PI within a clinical trial were excluded.
Data collection
A case report form (CRF) was used to capture data on patient characteristics, disease progression, level of response, and HRU. Data were extracted by study physicians from the medical records of all consecutive eligible patients until 1 April 2018 or death, whichever occurred first. The CRFs were designed to ensure that all utilized resources were accurately attributed to the corresponding regimen, line of therapy, and complete time period covered while on the index PI regimen (i.e. the PI regimen associated with study inclusion). A line of therapy was defined as the period during which a patient was treated with a specific anti-myeloma regimen until they experienced either intolerable toxicity or relapse and required the active treatment to be changed; a change in line of therapy occurred when the patient started a new anti-myeloma drug regimen. Dose changes were not considered to be a new therapy line, and re-treatment with the same regimen was considered to be a new line only if it followed disease progression.
Data quality control process
Owing to the retrospective nature of the study, missing or incomplete data were expected in some of the medical records. Data quality control checks were conducted at two different time points: 1) at study completion when CRFs were collected from participating physicians and 2) during data analysis when the results were aggregated into a single data file and logic controls were applied to the total population. This data quality control check was conducted initially when 10% of the total number of patients had been recorded. At study completion, a checklist was used to identify data inconsistencies, and these were addressed by the participating physician in the presence of the research assistant. No concerns were raised and therefore all patients were included in the analysis.
Healthcare resource utilization and costs data analyses
Cost data were calculated based on information in the CRF. A distinction was made between HRU that was planned (i.e. expected within routine treatment practice) or unplanned (i.e. unexpected events requiring medical resources that were not considered in normal monitoring or treatment). Total planned costs were defined as the sum of the costs associated with the following healthcare resources: anti-myeloma drug treatment, concomitant medications, planned hospitalizations, routine scans, radiotherapy, and other procedures. Total costs were calculated as the price per resource multiplied by the number of units or procedures utilized. Costs of each PI accounted for the number of cycles, the number of days of drug administration per cycle, the dose, price, and volume per unit of each treatment, and the body weight/body surface area of the patient. Additionally, the costs of the multi-drug regimens were accounted for when a PI was administered in combination with novel therapies. For the remaining non-PI anti-myeloma drugs administered in combination with the PI, costs were based on the number of administrations, a reference dose, and the price per unit. Systemic anti-cancer therapy costs were calculated based on anti-cancer therapy drug price and did not include costs from anti-cancer therapy administration or treatment-related adverse events (AEs). Costs for other concomitant medications were based on the number of administrations, a reference dose, and the price per unit. Drug prices were obtained from the German price database (Lauer-Taxe)Citation24. Costs included only those incurred during 2L and 3L therapy; although the SCT status of each patient was recorded, the cost of a SCT occurring in 1L therapy was not included in this analysis.
Hospitalization costs were calculated using diagnosis-related group (DRG) valuesCitation25. Additional costs per day of hospitalization were added for any hospitalization over 30 days. Costs for scans and procedures were calculated based on the number received in the index PI line of treatment. These costs and those from supportive care were based on the uniform assessment standard (Einheitlicher Bewertungsmaßstab, EBM) when in an ambulatory settingCitation23. A similar approach was taken for unplanned resource use (i.e. hospitalizations, scans and procedures, other concomitant medications). No comparisons were made between the HRU and costs between hospital- and office-based physicians or settings.
Mean monthly costs were calculated based on the duration of observation time in each line of treatment in which the PI was received. All costs pertain to the treatment period only; treatment‐free periods were not accounted for in the analyses. Costs related to HRU in RRMM were calculated by multiplying the amount of each type of resource used with a corresponding unit cost. All costs were based on 2018 Euro values and all cost analyses were conducted using a health system perspective; indirect costs were not included.
Cost of adverse events
Costs related to grade 3 or 4 AE profiles for each regimen were calculated using the AE rates reported in the CRFs. AEs considered were those occurring in at least 5% of patients of any regimen, as reported in the prescribing information or clinical trial data of each drug. AEs that occurred in a hospital setting were calculated using DRG values.
Data analysis
All analyses were descriptive and exploratory, and were conducted using Stata (v 15.0) and COSI (v 4.7) packagesCitation26. Categorical data were summarized by number and percentage of patients. Continuous data were summarized by mean and standard deviation. No formal statistical tests comparing results across PI regimens or lines of therapy were conducted. Exploratory statistical analyses of direct costs by response were conducted using an independent t-test. The study was not powered to enable formal analyses of these variables and as such the results of significance testing should be considered hypothesis-generating only.
Results
Patient characteristics
Overall, 44 physicians across Germany participated in the study and provided data on 302 patients with RRMM who received at least one dose of a PI (219 in 2L and 83 in 3L). Patient characteristics are detailed in . The mean age at diagnosis was 67 years and 70% of patients were male. Overall, 47% of patients had stage III disease based on the MM International Staging System (ISS) and 34% of patients tested had high-risk cytogenetics at diagnosis. The mean time from diagnosis to initiation of the index PI treatment was 29 months. In total, 37% of patients had at least one SCT in a prior treatment line, 90% of which were in 1L therapy. These patients were more likely to be younger and to have fewer comorbidities, an Eastern Cooperative Oncology Group (ECOG) performance status of 1, or an ISS stage of I at diagnosis than those who had not undergone a SCT; patients who underwent a SCT were more than three times as likely to have high-risk disease at diagnosis (data not shown).
Table 1. Characteristics of patients with RRMM receiving a PI during 2L or 3L treatment.
The mean follow-up time of the study cohort from initiation of the index PI regimen to either end of treatment or end of study, whichever occurred first, was 9 months (range 1–18 months). At the study end, PI therapy was ongoing in 67% and 71% of patients in 2L and 3L therapy, respectively. The median duration of PI treatment was 11 months in 2L (range 9–50 months) and 10 months in 3L (range 9–18 months). Of those who discontinued treatment during the observation period, the main reasons for discontinuation were treatment completion (2L: 41%; 3L: 27%) and disease progression (2L: 34%; 3L: 50%).
Proteasome inhibitor treatment patterns
In 2L therapy, 42% (n = 92), 48% (n = 106), and 10% (n = 21) of patients received bortezomib, carfilzomib, and ixazomib, respectively. In 3L therapy, 15% (n = 12), 61% (n = 51), and 24% (n = 20) received bortezomib, carfilzomib, and ixazomib, respectively (). Patients with a 2L bortezomib-based treatment were more likely to have received a lenalidomide-based regimen than a PI-based regimen in 1L. In contrast, those who were prescribed carfilzomib or ixazomib as 2L index treatment were more likely to have received a bortezomib-based regimen at 1L (). Patients with a 3L PI-based treatment were more likely to have received a lenalidomide-based regimen in 2L than a PI-based one ().
Figure 1. Treatment patterns for patients with RRMM receiving a PI in second or third line. (A) Treatments administered during first and second line for patients receiving a PI in second line (n = 219). (B) Treatments administered during first, second and third line for patients receiving a PI in third line (n = 83). PI-based regimens administered for the treatment of RRMM in real-world clinical practice in Germany are in accordance with national and international treatment guidelines. Abbreviations. BTZ, bortezomib; CFZ, carfilzomib; IXA, ixazomib; LEN, lenalidomide; PI, proteasome inhibitor; RRMM, relapsed and/or refractory multiple myeloma.
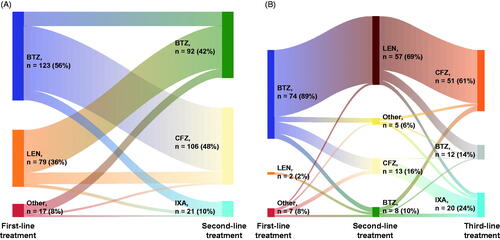
At 2L, bortezomib was most frequently given in combination with DEX in 2L (Vd: for 77% of all 2L uses of bortezomib; 3L: 8%). At 3L, bortezomib was most frequently given with DEX and daratumumab (DVd: 2L: 3%; 3L: 58%). Among those who received a carfilzomib-based regimen, carfilzomib was frequently combined with DEX (Kd: 2L: 41%; 3L: 73%) or with lenalidomide and DEX (KRd: 2L: 55%; 3L: 20%). Ixazomib was mainly administered in combination with lenalidomide and DEX (IRd: 2L and 3L data combined owing to small sample sizes, 76%). These combinations reflected the approved EU indications.
Healthcare resource utilization and associated costs
Monthly total direct costs for patients with RRMM on a PI regimen were €7,925 in 2L (€4,821 with bortezomib; €9,884 with carfilzomib; €11,638 with ixazomib) and €10,693 in 3L (€12,299 with bortezomib; €8,799 with carfilzomib; €14,559 with ixazomib) (). Anti-myeloma drug costs were the main cost drivers, accounting for approximately 90% of total direct costs (). In 2L, mean monthly drug costs for bortezomib, carfilzomib, and ixazomib were €3,099, €5,532, and €5,868, respectively, which represented 84%, 60%, and 55% of the monthly total cost of anti-myeloma drugs for each of these PI-based regimens. Similarly, bortezomib, carfilzomib, and ixazomib accounted for 27% (€3,103), 84% (€6,659), and 46% (€6,405) of total monthly drug costs associated with each PI-based 3L regimen, respectively.
Table 2. Mean monthly direct costs (in Euros [€]) associated with PI-based regimens in patients with RRMM.
Variability in monthly total drug cost across treatment lines and PI-based regimens was mainly owing to the different multi-drug combinations offered within each PI-based treatment line. The mean drug costs for bortezomib in combination with DEX were €3,200 in 2L and €3,538 in 3L, compared with €14,417 in 2L and €16,518 in 3L for a bortezomib-based regimen including both DEX and daratumumab. Carfilzomib and ixazomib were often given in combination with lenalidomide, which increased their total drug costs; the mean costs of carfilzomib therapy with DEX and lenalidomide were €10,748 in 2L and €9,996 in 3L, compared with €7,423 in 2L and €7,606 in 3L for carfilzomib therapy with DEX only.
After excluding the cost of anti-myeloma drugs from the total direct cost of treating RRMM, the mean monthly costs were €903 and €797 in 2L and 3L, respectively (€1,115, €685, and €1,071 in 2L and €850, €842, and €651 in 3L for bortezomib, carfilzomib, and ixazomib, respectively). Irrespective of line of treatment, 43% of these costs were attributed to planned hospitalizations, 26% to unplanned hospitalizations, 24% to planned concomitant medications, and 7% to planned scans, laboratory tests, and other procedures. In total, 28 of 219 patients (13%) in 2L and 12 of 83 patients (15%) in 3L had at least one planned hospitalization lasting more than one day during the study period.
The monthly total direct costs of unplanned hospitalizations per patient were 2% (€242) and 3% (€190) of the mean monthly total direct costs in 2L and 3L, respectively. Overall, the leading causes of unplanned hospitalizations were infection (17%), neutropenia (13%), anemia (11%), fatigue (9%), and thrombocytopenia (6%).
Healthcare resource utilization and associated costs in patients who had received a previous stem cell transplant
Patients who had received a SCT in a previous treatment line incurred higher monthly drug costs than those who had not. Total monthly direct costs were €10,549 in 2L and €11,440 in 3L for patients who had received a SCT compared with €6,640 in 2L and €9,997 in 3L for those who had not (Supplementary Table S1). In both 2L and 3L, costs associated with planned hospitalizations appeared to be higher in patients who had received a previous SCT than those who had not (Supplementary Table S1), regardless of the PI regimen administered (Supplementary Table S2). This was mainly owing to PI agents being administered in combination with additional anti-myeloma drugs and at a higher dosing intensity in patients who had received a previous SCT than in those who had not. The data suggest that patients who had undergone a SCT were younger and fitter than those who had not received a SCT.
Costs by best response to treatment achieved
Of the patients receiving 2L treatment, 36% achieved either a physician-reported complete response (CR) (6%) or a very good partial response (VGPR) (30%), 45% achieved a partial response (PR), and 4% had either stable disease (SD) or progressive disease (PD). The remaining 16% of patients were not evaluated at the time of data collection. Overall, age, comorbidities, ECOG performance status, and ISS stage at diagnosis were more favorable in patients who achieved a CR, and progressively deteriorated with decreasing depth of treatment response, with the least favorable values among those with SD or PD (data not shown).
Overall, in 2L, the mean monthly total cost associated with patients who achieved a CR, VGPR, or PR was €8,237 compared with €8,649 for patients with SD or PD. Expectedly, the lowest mean monthly total cost was observed in patients with a CR (€7,952). In 2L, the PI-based regimens with the highest proportion of patients achieving either a CR or a VGPR were carfilzomib combined with DEX and lenalidomide (CR: 12%; VGPR: 49%), followed by ixazomib- (CR: 0%; VGPR: 48%) and bortezomib-based regimens (CR: 4%; VGPR: 19%).
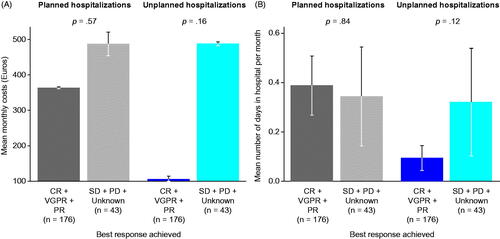
Mean monthly costs of planned and unplanned hospitalizations and the mean monthly number of unplanned hospital days were lower for patients with a CR, VGPR, or PR than for those with SD or PD (). Patients with physician-reported CR had no unplanned hospitalizations during the study period, whereas patients with PD experienced the highest number of both planned and unplanned hospitalizations (18 planned and 25 unplanned hospitalizations per 100 patients).
Figure 2. Planned and unplanned hospitalizations in patients with RRMM receiving a PI by best response achieved. (A) Mean monthly costs (Euros). (B) Mean number of days in hospital per month. Cost expenditures were much decreased in responders vs non-responders. Data are shown from a total of 219 patients in second line. Of those, one-third of patients had a planned hospitalization (n = 64) and 5% an unplanned hospitalization (n = 11). Error bars indicate standard errors. Abbreviations. CR, complete response; PD, progressive disease; PI, proteasome inhibitor; PR, partial response; RRMM, relapsed and/or refractory multiple myeloma; SD, stable disease; VGPR, very good partial response.
Discussion
This cohort study is, to the best of our knowledge, the first real-world evaluation of HRU and costs for patients with RRMM treated with PI-based regimens in the German healthcare system. Expectedly, our results show that the costs associated with treating patients with RRMM at 2L and 3L are primarily driven by the cost of the anti-myeloma drugs, rather than by the costs of comedications, diagnostics, and/or complication management.
Baseline characteristics were consistent with previous analyses of patients with RRMM in GermanyCitation27,Citation28. Treatment pattern data show that the use of PI-based regimens in real-world clinical practice in Germany align with recommendations in national and international MM treatment guidelinesCitation29–32. In this study conducted in 2017, carfilzomib-based regimens were the most commonly prescribed PI treatment for both 2L and 3L. This is consistent with data from a recently published European patient chart audit and may reflect an increased use of bortezomib at 1LCitation33.
Our finding that total direct costs of treating RRMM were mainly driven by anti-myeloma drugs is consistent with results from other single-country and European studiesCitation9,Citation34–37. Within each line of treatment, anti-myeloma drug costs differed substantially across PI regimens, with ixazomib-based regimens being consistently more costly than bortezomib- or carfilzomib-based regimens. The total direct costs associated with treating patients with RRMM were lower for patients in 2L (€7,925) than those in 3L (€10,693). This is in line with previously reported European cohorts and likely reflects an increased use of novel agents and multi-drug combinations at 3L compared with 2LCitation34,Citation36. Indeed, bortezomib was frequently administered as part of a doublet regimen in 2L and a triplet regimen in 3L, comprising 84% and 27% of anti-myeloma drug costs at 2L and 3L, respectively.
Our results verified that hospitalization costs represent a small fraction of total direct costs for patients with RRMM, although indirect costs may be substantial. Along with increasing costs, more frequent and prolonged hospital stays are likely to reduce the quality of life (QoL) of patients and caregivers. The use of more effective therapies may help to reduce the burden of hospitalizations; our data show that patients with better treatment response had lower mean monthly rates of planned and unplanned hospitalizations, the latter issue becoming an increasingly important factor in treatment decisions for patients with RRMM. Furthermore, recent real-world data in patients with MM from Germany show that patient QoL decreases with increasing treatment lineCitation38. Improvements in both treatment response (leading to longer treatment-free periods) and toxicity profiles with anti-myeloma therapies have been associated with superior QoL in patients with RRMMCitation14,Citation20,Citation39–41. It has been suggested that QoL will become an increasingly important factor in treatment decisions for RRMMCitation40,Citation42–45.
Improvements in overall survival and progression-free survival associated with second-generation PI therapies may result in higher HRU and costs owing to patients receiving treatment for longer. However, there is a trade-off associated with newer therapies: their improved efficacy and reduced toxicity not only provide substantial benefits for the patient, but also lead to reduced direct and indirect costs as a result of fewer hospitalizations, scans, treatment-related AEs, radiotherapy, and other procedures. Furthermore, deep and durable responses achieved with these agents may lead to a longer treatment-free intervalCitation4; notably, our study assessed costs during the treatment period only and may have underestimated this cost benefit.
The lower mean monthly costs observed in patients who achieved a CR versus patients who achieved a SD or PD (€7,952 vs €8,649, respectively) also correlated with fewer unplanned hospitalizations in patients with a CR. Patients who received carfilzomib combined with DEX and lenalidomide were more likely to achieve a CR or VGPR than those receiving ixazomib- or bortezomib-based regimens. This is consistent with clinical trial data showing improved CR rates for carfilzomib compared with recent standards of careCitation14–16. Notably, the proportion of patients with a CR in this study (6%) was lower than the proportions reported in recent clinical trials of PI-based regimens (12–18%)Citation14,Citation15. This likely reflects the different diagnostic intensity used to select patients for inclusion in randomized clinical trials versus real-world treatment practices, and highlights the importance of understanding the effectiveness and value of treatment in real-world clinical practice.
This study has limitations: patients with incomplete follow-up data were not excluded. However, monthly cost estimates were used to reduce the potential impact of these missing dataCitation34,Citation43. This cost-averaging approach assumed that treatment costs associated with a PI regimen occurred with equal distribution across the duration of therapy; however, it has been reported that total patient monthly costs for bortezomib regimens decline steadily over the treatment duration within each treatment lineCitation46. As the cost-averaging approach was skewed towards the early stages of each treatment line, it is likely that the benefit of deep responses to treatment and a subsequent treatment-free period may have been underestimated. There are also some limitations associated with calculating costs based on DRG values, which provide a fixed price that may not accurately reflect actual costs in each hospital. Additionally, costs for drug administration were not included, but were minimal within the German administrative costs and therefore were not drivers of substantial cost differences between the three PIs. Moreover, all cost estimates were based on data from 2018, meaning that in some cases costs may have changed. Furthermore, licensing and reimbursement approval of ixazomib regimens were granted in Germany recent to the start of this study, resulting in limited real-world data on this therapy; caution is therefore necessary when interpreting data on ixazomib regimens. Similarly, the study was not designed to detect statistically significant differences across subgroups; therefore, differences across subgroups should be interpreted with caution. Only direct costs have been evaluated in this study; information on both direct and indirect costs is required to understand the overall impact of RRMM treatment on societies and healthcare systems. Additionally, costs for AEs considered only those occurring in at least 5% of patients for each regimen, as reported in the prescribing information or clinical trial data for each drug, which may exclude some rare but serious and costly AEs. Finally, owing to the real-world nature of the study, response assessments were based on each physician’s expertise rather than on strictly defined criteria (as is the case in clinical trials), and sample sizes for cost analysis by treatment response were limited. Therefore, the results of this analysis should be interpreted with caution because the physician’s response assessment may or may not reflect the conventionally defined criteria used in clinical trials.
Published studies on HRU and costs associated with RRMM have generally used different study designs, treatments, and patient populations (Supplementary Table S3)Citation34,Citation47–53. Furthermore, there are limited real-world data on the use of PIs in patients with RRMM in Europe. Although these factors limit the ability to conduct cross-study comparisons, results suggest that improved treatment responses are associated with lower mean costs.
Conclusions
This real-world study of HRU and costs associated with patients with RRMM treated with PIs demonstrated that anti-myeloma drug costs are the main driver of total direct HRU costs in Germany. Regimens with second-generation PIs, such as carfilzomib, have the potential to increase response rates and prolong survival times, which may result in increased HRU and costs in the future. This, however, must be balanced with the potential savings associated with reduced hospitalizations and reduced treatment of AEs. PI agents play a key role in alleviating the collective burden associated with RRMM and are likely to remain a mainstay in the treatment paradigm.
Transparency
Declaration of funding
This study was funded by Amgen.
Declaration of financial/other relationships
T. H. Steinmetz has received research funding from Amgen, Celgene, Novartis, and Vifor, is a member of the board of directors/speaker’s bureau/advisory committee for Amgen, ARIAD, BMS, Celgene, Hexal-Sandoz, Janssen-Cilag, Medice, Novartis, Pfizer, Pharmacosmos, and Vifor, and received travel grants from Alexion, Amgen, Bayer, Celgene, Janssen-Cilag, and Novartis. M. Singh is an employee of Amgen Ltd. A. Lebioda is an employee of Amgen GmbH and holds Amgen stock and options. L. Fink is an employee of Kantar Health and received funding from Amgen GmbH to conduct this research. M. Schoehl is an employee of Amgen GmbH and holds Amgen stock and options. A. Rieth is an employee of Amgen GmbH and holds Amgen stock and options. S. Gonzalez-McQuire is an employee of Amgen (Europe) GmbH and holds Amgen stock. M. Engelhardt has received educational and trial support from Amgen, BMS, Celgene, Janssen, Karyopharm, Novartis, and Takeda, and has received honoraria and consultancy fees from Amgen, BMS, Celgene, Janssen, Novartis, and Takeda.
A peer reviewer on this manuscript has disclosed that they have received research funding from Amgen, Janssen, Roche, AbbVie and Novartis. The peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
T. H. Steinmetz was involved in the conception and design, data collection, analysis and interpretation of the data as well as in revising the paper critically for intellectual content. M. Singh, A. Lebioda, A. Rieth, S. Gonzalez-McQuire, and M. Schoehl were involved in the conception and design, analysis and interpretation of the data as well as in drafting and revising the paper critically for intellectual content. L. Fink was involved in the conception and design, data collection, analysis of the data as well as in revising the paper critically for intellectual content. M. Engelhardt was involved in the analysis and interpretation of the data as well as in revising the paper critically for intellectual content.
All authors have approved the final version to be published and agree to be accountable for all aspects of the work.
Previous presentations
The work described here was previously presented as an abstract at the American Society of Hematology Annual Meeting in 2018.
Supplemental Material
Download MS Word (53.4 KB)Acknowledgements
Medical writing was provided by Angeles Brooks of Kantar Health and Kelly Soady, PhD, of PharmaGenesis London, London, UK who received funding from Amgen. Editorial support was provided by Carine Thual of Amgen (Europe) GmbH and Valentina Ponn of Amgen GmbH.
References
- Swerdlow SC, Harris NL, Jaffe ES, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. International Agency for Research on Cancer publications, World Health Organization Press. Revised 4th ed.; Vol. 2. 2008.
- (IHME) Institute for Health Metrics and Evaluation. GBD Compare. Seattle (WA): IHME, University of Washington 2015 [accessed February 2019]. Available from: http://vizhub.healthdata.org/gbd-compare
- Krebs in Deutschland für 2013/2014. 11. Ausgabe. Robert Koch-Institut (Hrsg) und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (Hrsg). Berlin; 2017.
- Yong K, Delforge M, Driessen C, et al. Multiple myeloma: patient outcomes in real-world practice. Br J Haematol. 2016;175:252–264.
- Pulte D, Jansen L, Castro FA, et al. GEKID Cancer Survival Working Group. Trends in survival of multiple myeloma patients in Germany and the United States in the first decade of the 21st century. Br J Haematol. 2015;171:189–196.
- Becker N. Epidemiology of multiple myeloma. Recent Results Cancer Res. 2011;183:25–35.
- Landgren O, Iskander K. Modern multiple myeloma therapy: deep, sustained treatment response and good clinical outcomes. J Intern Med. 2017;281:365–382.
- Gerecke C, Fuhrmann S, Strifler S, et al. The diagnosis and treatment of multiple myeloma. Dtsch Arztebl Int. 2016;113:470–476.
- Fragoulakis V, Kastritis E, Psaltopoulou T, et al. Economic evaluation of therapies for patients suffering from relapsed-refractory multiple myeloma in Greece. Cancer Manag Res. 2013;5:37–48.
- Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487–2498.
- Jagannath S, Barlogie B, Berenson JR, et al. Updated survival analyses after prolonged follow-up of the phase 2, multicenter CREST study of bortezomib in relapsed or refractory multiple myeloma. Br J Haematol. 2008;143:537–540.
- Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617.
- Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571.
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152.
- Moreau P, Masszi T, Grzasko N, et al. TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–1634.
- Dimopoulos MA, Goldschmidt H, Niesvizky R, et al. Carfilzomib or bortezomib in relapsed or refractory multiple myeloma (ENDEAVOR): an interim overall survival analysis of an open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:1327–1337.
- Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901.
- Cook G, Zweegman S, Mateos MV, et al. A question of class: treatment options for patients with relapsed and/or refractory multiple myeloma. Crit Rev Oncol Hematol. 2018;121:74–89.
- Durer C, Durer S, Lee S, et al. Treatment of relapsed multiple myeloma: evidence-based recommendations. Blood Rev. 2020; 39:100616.
- Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38.
- Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–766.
- Steinmetz HT, Singh M, Lebioda A, et al. Management of patients with relapsed/refractory multiple myeloma (RRMM) treated with proteasome inhibitor based therapy at first relapse in routine clinical practice in Germany. Blood. 2018;132:1953.
- Steinmetz HT, Singh M, Lebioda A, et al. Patient characteristics and outcomes of relapsed/refractory multiple myeloma in patients treated with proteasome inhibitors in Germany. Oncol Res Treat. 2020;43:449–459.
- LAUER-FISCHER GmbH. Pharmaceutical information for all drugs and contracts registered in Germany. [accessed February 2018]. Available from: https://www.cgm.com/lauer-fischer/loesungen_lf/lauer_taxe_lf/lauertaxe_online_4_0/online_en.de.jsp
- InEK GmbH. Institute for the remuneration system in the hospital, 2007–2019. Germany. [accessed February 2018]. Available from: https://www.g-drg.de/G-DRG-System_2018/Grouper_Zertifizierung/Grouper_Zertifizierung_2018
- StataCorp. 2017. Stata statistical software: release 15. College Station TSL.
- Merz M, Kellermann L, Poenisch W, et al. Diagnosis and treatment of multiple myeloma in Germany: analysis of a nationwide multi-institutional survey. Ann Hematol. 2017;96:987–993.
- Gengenbach L, Reinhardt H, Ihorst G, et al. Navigating the changing multiple myeloma treatment landscape: clinical practice patterns of MM patients treated in- and outside German DSMM study group trials. Leuk Lymphoma. 2018;59:2692–2699.
- National Comprehensive Cancer Network. Multiple myeloma. Version 4. 2020 [accessed 2020 May 15]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf
- Rajkumar SV, Kumar S. Multiple myeloma: diagnosis and treatment. Mayo Clin Proc. 2016;91:101–119.
- Sonneveld P, Broijl A. Treatment of relapsed and refractory multiple myeloma. Haematologica. 2016;101:396–406.
- Woermann B, Driessen C, Einsele H, et al. Mutiple myeloma. 2018 [accessed 2020 Jun 2]. Available from: https://www.onkopedia.com/de/onkopedia/guidelines/multiples-myelom/@@guideline/html/index.html
- Raab MS, Fink L, Schoen P, et al. Evolution of multiple myeloma treatment practices in Europe from 2014 to 2016. Br J Haematol. 2019;185:981–984.
- Gonzalez-McQuire S, Yong K, Leleu H, et al. Healthcare resource utilization among patients with relapsed multiple myeloma in the UK, France, and Italy. J Med Econ. 2018;21:450–467.
- Blommestein HM, Verelst SG, de Groot S, et al. A cost-effectiveness analysis of real-world treatment for elderly patients with multiple myeloma using a full disease model. Eur J Haematol. 2016;96:198–208.
- Gaultney JG, Franken MG, Tan SS, et al. Real-world health care costs of relapsed/refractory multiple myeloma during the era of novel cancer agents. J Clin Pharm Ther. 2013;38:41–47.
- Petrucci MT, Calabrese E, Levi A, et al. Cost of illness in patients with multiple myeloma in Italy: the CoMiM study. Tumori. 2013;99:e193–e202.
- Engelhardt M, Ihorst G, Singh M, et al. Real-world evaluation of health-related quality of life in patients with multiple myeloma in Germany. Clin Lymphoma Myeloma Leuk. 2020. DOI:https://doi.org/10.1016/j.clml.2020.10.002
- Sonneveld P, Verelst SG, Lewis P, et al. Review of health-related quality of life data in multiple myeloma patients treated with novel agents. Leukemia. 2013;27:1959–1969.
- Ludwig H, Delforge M, Facon T, et al. Prevention and management of adverse events of novel agents in multiple myeloma: a consensus of the European Myeloma Network. Leukemia. 2018;32:1542–1560.
- Ludwig H, Moreau P, Dimopoulos MA, et al. Health-related quality of life in the ENDEAVOR study: carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed/refractory multiple myeloma. Blood Cancer J. 2019;9:23.
- Hagiwara M, Delea T, Panjabi S, et al. Burden of disease progression in patients with multiple myeloma who have received at least one line of therapy in the US. Blood. 2018;132:4754.
- Despiegel N, Touboul C, Flinois A, et al. Health-related quality of life of patients with multiple myeloma treated in routine clinical practice in France. Clin Lymphoma Myeloma Leuk. 2019;19:e13–e28.
- Sekine L, Ziegelmann PK, Manica D, et al. Upfront treatment for newly diagnosed transplant-ineligible multiple myeloma patients: a systematic review and network meta-analysis of 14,533 patients over 29 randomized clinical trials. Crit Rev Oncol Hematol. 2019;143:102–116.
- Zweegman S, Engelhardt M, Larocca A, EHA SWG on ‘Aging and Hematology’. Elderly patients with multiple myeloma: towards a frailty approach? Curr Opin Oncol. 2017;29:315–321.
- Arikian SR, Milentijevic D, Binder G, et al. Patterns of total cost and economic consequences of progression for patients with newly diagnosed multiple myeloma. Curr Med Res Opin. 2015;31:1105–1115.
- Zhou X, Xia J, Mao J, et al. Real-world outcome and healthcare costs of relapsed or refractory multiple myeloma: a retrospective analysis from the Chinese experience. Hematology. 2016;21:280–286.
- Roy A, Kish JK, Bloudek L, et al. Estimating the costs of therapy in patients with relapsed and/or refractory multiple myeloma: a model framework. Am Health Drug Benefits. 2015;8:204–215.
- Armoiry X, Fagnani F, Benboubker L, et al. Management of relapsed or refractory multiple myeloma in French hospitals and estimation of associated direct costs: a multi-centre retrospective cohort study. J Clin Pharm Ther. 2011;36:19–26.
- Chen CC, Parikh K, Abouzaid S, et al. Real-world treatment patterns, time to next treatment, and economic outcomes in relapsed or refractory multiple myeloma patients treated with pomalidomide or carfilzomib. J Manag Care Spec Pharm. 2017;23:236–246.
- Gooding S, Lau IJ, Sheikh M, et al. Double relapsed and/or refractory multiple myeloma: clinical outcomes and real world healthcare costs. PLoS One. 2015;10:e0136207.
- Hagiwara M, Panjabi S, Sharma A, et al. Healthcare utilization and costs among relapsed or refractory multiple myeloma patients on carfilzomib or pomalidomide as monotherapy or in combination with dexamethasone. J Med Econ. 2019;22:818–829.
- Lin HM, Davis KL, Kaye JA, et al. Real-world treatment patterns, outcomes, and healthcare resource utilization in relapsed or refractory multiple myeloma: evidence from a medical record review in France. Adv Hematol. 2019;2019:4625787.
