Abstract
Aim
To build upon previous outdated studies by comprehensively assessing the direct healthcare burden of autosomal dominant polycystic kidney disease (ADPKD).
Materials and methods
Patients with ≥2 diagnoses for ADPKD (ADPKD cohort) were identified in the US fee-for-use IBM Truven Health Analytics MarketScan Commercial Claims and Encounters and IBM Truven Health Analytics MarketScan Medicare Supplemental databases (01 January 2015–31 December 2017) and matched (1:3) to controls without ADPKD (non-ADPKD cohort). The index date was the last calendar date followed by 12 months continuous enrollment (study period). Patients with ADPKD were stratified into one of seven mutually exclusive groups based on chronic kidney disease (CKD) stages (I–V), end-stage renal disease requiring renal replacement therapy (ESRD-RRT), and unknown stage.
Results
During the 12-month study period, patients with ADPKD incurred significantly higher total healthcare costs than those without ADPKD (mean cost difference = $22,879 per patient per year [PPPY]; p < .001). Besides CKD stages I and II, total healthcare cost differences increased as patients progressed beyond CKD stage III, with the greatest difference observed among patients with ESRD-RRT. Total healthcare cost differences between cohorts were more pronounced in subgroups of patients with hypertension ($29,347) and with high risk of rapid progression ($39,976). Similar results were observed in the Medicare Supplemental population, with a total mean cost difference of $42,694 PPPY (p < .001); cost difference was also higher in the hypertension ($46,461 PPPY) and high risk of rapid progression ($45,708 PPPY) subgroups.
Limitations
Results may not be representative of the overall ADPKD US population; CKD stage was based on diagnosis and procedure codes; criteria used to identify ADPKD at risk of rapid progression did not rely on laboratory values; there may be billing inaccuracies and omissions in health insurance claims data.
Conclusions
This study demonstrated the substantial healthcare costs associated with ADPKD, which increased as patients progressed through more severe CKD stages.
JEL Classification Codes:
- H51 – Government Expenditures and Health
- H5 – National Government Expenditures and Related Policies
- H – Public Economics
- H50 – General
- H5 – National Government Expenditures and Related Policies
- H – Public Economics
- H00 – General
- H – Public Economics
- I15 – Health and Economic Development
- I1 – Health
- I – Health
- Education
- and Welfare
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease, with a prevalence of 4.3 per 10,000 in the United States (US)Citation1. ADPKD is characterized by the development of renal cysts that disrupt urine concentration and may cause symptoms such as hypertension, hematuria, abdominal pain, and urinary tract infectionsCitation2,Citation3. In early stages, the disease is usually asymptomatic for decades, with renal function appearing normal from compensatory mechanisms; in later stages, the disease may cause functional deteriorationCitation4. However, a significant number of patients with ADPKD can progress through chronic kidney disease (CKD) stages at a younger age and reach end-stage renal disease (ESRD) at a faster rateCitation5. In most patients, ADPKD eventually progresses to end-stage renal disease requiring renal replacement therapy (ESRD-RRT), such as long-term dialysis or kidney transplantCitation3. Accordingly, ADPKD disproportionately accounts for 7–10% of all ESRD cases in EuropeCitation4 and about 5% in the US reported by USRDS (United States Renal Data System).
Several studies have previously evaluated the economic burden of ADPKD and have identified substantial costs that increase with decline in renal functionCitation6–8. However, none of them comprehensively assessed the burden by accounting for both the progressive nature of ADPKD and the probability and speed of progression that varies by patient and CKD stage. In addition, the results from these studies are likely outdated, with data encompassing up to 2012.
Therefore, this retrospective matched cohort study built upon previously published studies and used a unique design to comprehensively assess the direct healthcare burden of a representative population of privately and nationally (Medicare) insured patients with ADPKD in the US.
Methods
Data source
Data from 1 January 2015 to 31 December 2017 from the IBM Truven Health Analytics MarketScanFootnotei Commercial Claims and Encounters and the IBM Truven Health Analytics MarketScan Medicare Supplemental databases were used to conduct the study.
The commercial database includes medical and pharmacy claims for employees and their spouses and dependents from all US census regions who are covered by employer-sponsored private health insurance. The Medicare Supplemental database includes medical and pharmacy claims for retirees with Medicare Supplemental insurance paid by employers. Both databases include data on prescription drugs claims, inpatient (IP) and outpatient (OP) services, and other medical care, with service and provider types reported for medical claims. Cost data include the portions paid by the private payer and by Medicare (Medicare Supplemental database).
Data were de-identified and complied with the requirements of the Health Insurance Portability and Accountability Act (HIPAA). Therefore, no institutional review board approval was needed.
Study design and population
The analyses were conducted using a retrospective matched cohort design (shown in ). Commercially insured and Medicare Supplemental individuals were classified into two mutually exclusive cohorts: the ADPKD cohort and the non-ADPKD cohort. Unlike previous studies that assessed the healthcare costs associated with ADPKDCitation6–8, the index date in this study was defined as the last calendar date that was followed by 12 months of continuous healthcare plan enrollment (prevalence-based approach). The baseline period spanned the 6-month period prior to the index date, while the study period spanned the 12-month period following the index date. The observation period was defined as the period spanning from the start of data until the end of data.
Sample selection
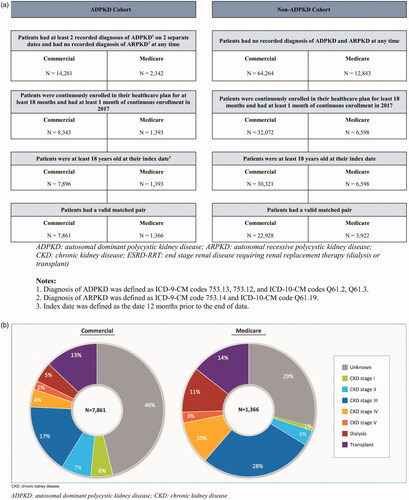
Patients were included in the ADPKD cohort if they met the following criteria (shown in ): (1) had ≥2 diagnoses for autosomal polycystic kidney disease (International Classification of Diseases, Ninth revision, Clinical Modification [ICD-9-CM] codes: 753.13, 753.12; International Classification of Diseases, Tenth revision, Clinical Modification [ICD-10-CM] codes: Q61.2, Q61.3) recorded on two separate dates at any time; (2) did not have any recorded diagnoses of autosomal recessive polycystic kidney disease (ARPKD; ICD-9-CM code: 753.14; ICD-10-CM code: Q61.11, Q61.19) at any time; (3) were continuously enrolled in their healthcare plan for ≥18 months and had ≥1 month of continuous enrollment in 2017; and (4) were ≥18 years old at their index date.
Individuals were included in the non-ADPKD cohort if they met the following criteria: (1) had no recorded diagnosis for ADPKD or ARPKD at any time; (2) were continuously enrolled in their healthcare plan for ≥18 months and had ≥1 month of continuous enrollment in 2017; and (3) were ≥18 years old at their index date.
Each patient in the ADPKD cohort was exactly matched to up to three individuals in the non-ADPKD cohort based on age, gender, region of residence, type of health insurance plan, and calendar year at the index date.
Index CKD stage
Patients in the ADPKD cohort were further stratified into one of seven mutually exclusive groups based on the five CKD stages (I–V) without ESRD, dialysis, or renal transplant; ESRD-RRT stage, including patients receiving dialysis or renal transplant; and unknown CKD stage. CKD stage, dialysis, and renal transplant status were identified based on diagnosis and procedure codes obtained from the claims data. The index stage of a patient was based on the patient’s latest stage indicator before the index date. If no stage indicator was observed before the index date, the first observed stage after the index date was defined as the patient’s index stage. Patients without any recorded diagnosis or procedure of CKD stage, dialysis, or renal transplant at any time during the observation period were categorized into the unknown CKD stage group. Higher CKD stages represent more advanced disease, and ESRD-RRT was considered as the highest stage level in this analysis. Consistent with the approach used by Blanchette et al.Citation5, this analysis only considered upward transitions through CKD stages; therefore, indicators of a lower-level CKD stage observed after an indicator of a higher-level CKD stage, and indicators of CKD stages observed after dialysis or renal transplant were not considered.
Patient characteristics and study outcomes
Patient characteristics were described for the ADPKD cohort before matching and included age, gender, region of residence, health insurance plan type at the index date, Charlson Comorbidity Index (CCI) score, mental and physical comorbidities, co-medications during the baseline period, and presence of indicators of hypertension and risk of rapid progression (i.e. hypertension before the age of 35 years; hematuria before the age of 30 years; albuminuria at any time; advanced-stage kidney disease according to age [stage II by 30 years, stage III by 50 years, and stage IV/V, ESRD or transplantation by 55 years); defined by Blanchette et al.Citation5) at any time prior to the index date. Medical visits by provider type and renal imaging were also described during the baseline period and study period, although information on specialist encounters tends to be underestimated in claims data.
Total all-cause healthcare costs were measured from the payers’ perspective during the study period and reported per-patient-per-year (PPPY). Healthcare costs included pharmacy and medical components.
Statistical analysis
Patient characteristics were summarized using means, medians, and standard deviations (SDs) for continuous variables, and frequencies and proportions for categorical variables.
Healthcare costs were adjusted for inflation and reported in 2017 USD using the Medical Care Component of the Consumer Price Index (CPI)Citation9. Healthcare costs were compared between the matched ADPKD and non-ADPKD cohorts with cost differences estimated using a generalized linear model (GLM) with a log link and a gamma distribution. Results were reported as cost differences with their p-values.
All analyses were conducted using the Statistical Analysis System (SAS) Enterprise Guide, Version 7.1 (SAS, Cary, North Carolina, USA) and Stata, Version 14.1 (StataCorp LLC, College Station, Texas, USA).
Subgroup analyses
The cost analyses were replicated among subgroups of patients in the ADPKD cohort with hypertension and those at high risk of rapid progressionCitation5, as measured prior to the index date, and their matched controls from the non-ADPKD cohort.
Results
Patient characteristics
Of the 7,896 patients with ADPKD in the commercially insured population, 7,861 were exactly matched to 22,928 individuals in the non-ADPKD cohort (shown in ). Of the 1,393 patients with ADPKD in the Medicare Supplemental population, 1,366 were exactly matched to 3,922 individuals in the non-ADPKD cohort (shown in ). By definition, matched patients in both cohorts had the same characteristics as controls in terms of age, gender, region of residence, type of health insurance plan, and calendar year at the index date; hence the subsequent sections focus on the characteristics of patients in the ADPKD cohort prior to matching.
Characteristics are summarized for patients with CKD stage I, ESRD-RRT, and unknown CKD stage; full results by each stage are presented in the corresponding tables.
Commercially insured population
On average, commercially insured patients with CKD stage I and an unknown CKD stage were younger than those with ESRD-RRT (41.3, 43.6, and 54.5 years, respectively; shown in ).
Table 1. Patient characteristics before matching (commercially insured population).
The majority of the patients had hypertension, comprising 61.4% among patients with CKD stage I, 94.0% among those with ESRD-RRT, and 51.9% among those with an unknown CKD stage (shown in ). Additionally, the proportions of patients at high risk of rapid progression were lower, at 28.0% among patients with CKD stage I, 61.9% among those with ESRD-RRT, and 15.0% among those with an unknown CKD stage. On average, patients with CKD stage I and unknown CKD stage had a more favorable comorbidity profile compared to those with ESRD-RRT (0.7 and 0.3 compared to 1.6, respectively).
During the baseline and study period, a lower proportion of patients with CKD stage I consulted a kidney specialist (74.8%; 72.7% with nephrology and 8.7% with urology) compared to those with ESRD-RRT (85.9%; 83.8% with nephrology and 24.2% with urology); the proportion was particularly low among those with an unknown CKD stage (36.8%; 26.3% with nephrology and 14.2% with urology; shown in ). Renal imaging was done among approximately half of patients with CKD stage I, ESRD-RRT, and unknown CKD stage (49.2%, 57.9%, and 48.3%, respectively).
Within the ADPKD cohort, nearly half had an unknown CKD stage (46.2%). Additionally, 5.4% of the patients had CKD stage I, 7.3% had CKD stage II, 17.0% had CKD stage III, 4.3% had CKD stage IV, 2.1% had CKD stage V, 4.9% had dialysis, and 12.9% had a kidney transplant (shown in ).
Medicare supplemental population
On average, Medicare Supplemental patients with CKD stage I and ESRD-RRT were younger than those with an unknown CKD stage (72.2, 73.8 years, and 75.6 years, respectively; shown in ).
Table 2. Patient characteristics before matching (Medicare insured population).
The majority of the patients had hypertension, comprising 100.0% among patients with CKD stage I, 98.0% among those with ESRD-RRT, and 85.9% among those with an unknown CKD stage (shown in ). The proportions of patients at high risk of rapid progression were lower, at 30.8% among patients with CKD stage I, 10.3% among those with ESRD-RRT, and 2.5% among those with an unknown CKD stage. On average, patients with CKD stage I and unknown CKD stage had a less severe comorbidity profile compared to those with ESRD-RRT (1.3 and 1.0 compared to 2.5, respectively).
During the baseline and study periods, a lower proportion of patients with CKD stage I (46.2%; 38.5% with nephrology and 23.1% with urology) and unknown CKD stage (41.7%; 8.7% with nephrology and 34.7% with urology) consulted a kidney specialist compared to those with ESRD-RRT (66.8%; 61.2% with nephrology and 29.3% with urology; shown in ). Renal imaging was done in more than half of patients with CKD stage I, ESRD-RRT, and unknown CKD stage (76.9%, 64.8%, and 66.7%, respectively).
Within the ADPKD cohort, 29.1% had an unknown CKD stage. Additionally, 1.0% of patients had CKD stage I, 3.7% had CKD stage II, 27.6% had CKD stage III, 10.5% had CKD stage IV, 2.6% had CKD stage V, 11.1% had dialysis, and 14.4% had a kidney transplant (shown in ).
Healthcare costs, overall and stratified by CKD stage
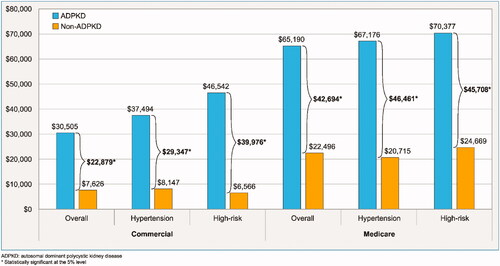
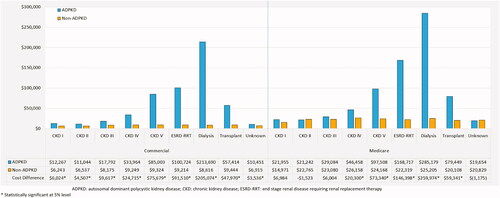
During the 12-month study period, commercially insured patients in the ADPKD cohort incurred significantly higher total healthcare costs than the non-ADPKD cohort (mean cost difference = $22,879 PPPY; p < .001; shown in ). The higher costs within the ADPKD cohort remained significant even when patients were stratified by CKD stage (p < .01 for all comparisons at each stage; shown in ). More specifically, patients with an unknown CKD stage had the lowest total healthcare cost difference between the ADPKD and non-ADPKD cohorts (mean cost difference = $3,536 PPPY; p < .001). Aside from CKD stage I and stage II, total healthcare cost differences increased as patients progressed beyond CKD stage III. Indeed, patients with ESRD-RRT in the ADPKD cohort incurred the highest costs and cost difference compared to patients in the non-ADPKD cohort (mean cost difference = $91,510 PPPY; p < .001), mainly driven by the subset of patients receiving dialysis (mean cost difference = $205,073 PPPY; p < .001).
In the Medicare Supplemental population, similar general trends were observed, but at a different magnitude. During the 12-month study period, patients in the ADPKD cohort incurred significantly higher total healthcare costs than the non-ADPKD cohort (mean cost difference = $42,694 PPPY; p < .001; shown in ). When stratified by CKD stage, the higher costs within the ADPKD cohort remained significant among patients with CKD stages III, IV, V, and ESRD-RRT (p < .05 for all comparisons; shown in ). Of note, the sample sizes of the CKD stage I and II groups were limited and thus may not have been sufficient to detect statistical differences. Total healthcare cost differences increased as patients progressed beyond CKD stage III. Indeed, patients with ESRD-RRT in the ADPKD cohort incurred the highest costs and cost difference compared to patients in the non-ADPKD cohort (mean cost difference = $146,398 PPPY; p < .001), mainly driven by the subset of patients receiving dialysis (mean cost difference = $259,974 PPPY; p < .001).
With regards to healthcare resource utilization, patients in the ADPKD cohort had significantly more inpatient admissions, inpatient days, emergency room visits, and outpatient visits compared to those in the non-ADPKD cohort in both populations (see Supplementary Tables S1 and S2 for details on healthcare resource utilization results).
Healthcare costs stratified by subgroup
Among the commercially insured population with ADPKD, patients with hypertension ($37,494) and at high risk of rapid progression ($46,542) had higher total healthcare costs than the overall cohort ($30,505; shown in ). Similarly, the total healthcare cost differences between the ADPKD and non-ADPKD cohorts were higher in the hypertension ($29,347) and high risk of rapid progression ($39,976) subgroups than the overall ADPKD cohort ($22,879; shown in ).
Among the Medicare-insured population with ADPKD, patients with hypertension ($67,176) and at high risk of rapid progression ($70,377) had higher total healthcare costs than the overall cohort ($65,190; shown in ). Similarly, the total healthcare cost differences between the ADPKD and non-ADPKD cohorts were higher in the hypertension ($46,461) and high risk of rapid progression ($45,708) subgroups than the overall ADPKD cohort ($42,694; shown in ).
Discussion
This retrospective matched cohort study identified considerable economic burden associated with ADPKD in both commercially insured and Medicare Supplemental populations. Commercially insured patients with ADPKD incurred $22,879 PPPY higher healthcare costs compared to patients without ADPKD. The cost differences tended to increase with severity of CKD, with the exception of stages I and II. The largest incremental costs were observed in the ESRD-RRT stage and were driven by the cost of dialysis. Similarly, Medicare Supplemental patients with ADPKD incurred significantly higher healthcare costs compared to patients without ADPKD, but at a higher magnitude, with a difference of $42,694 PPPY. Considering the progressive nature of the disease, this difference compared to the commercially insured population may be explained by the larger proportion of more advanced CKD stages among this older patient population. Lastly, subgroups of patients with hypertension and at high risk of rapid progression in both the commercially insured and Medicare Supplemental populations incurred substantially higher incremental costs.
The results of the present study are generally consistent with the few studies that previously evaluated the economic burden of ADPKD, which showed significantly higher costs among patients with ADPKD versus matched controlsCitation7 and higher costs associated with CKD stage V and ESRD-RRT versus earlier stagesCitation6–8. Consistently, Blanchette et al. and Lentine et al.Citation6,Citation8, also observed a decrease in costs from CKD stage I to stage II, likely representing additional costs associated with diagnostic workup and physician visits at the earlier stage of the disease.
The results of the current study build upon those of previous studies with many different considerations related to the study designCitation6–8. First, because of the use of a prevalence-based approach (i.e. the index date was not anchored to a diagnosis of ADPKD), the study population reflects a mix of disease duration, severity, and stages. Thus, patients with ADPKD included in the present study may be representative of the overall ADPKD population in a given year. Anchoring the index date to a diagnosis of ADPKD may result in front-loading costs associated with the use of medical services that lead to the index claim, which may partially explain why previous studies reported higher costs than the current study for patients with earlier CKD stagesCitation7,Citation8.
Second, prior studies estimated the incremental costs of ADPKD by CKD stage while the patients were at each stageCitation6,Citation7. Conversely, the current study aimed to provide a comprehensive assessment of the costs of an average patient with ADPKD at different CKD stages, while allowing for the natural disease progression (i.e. given each patient has a specific probability of progression over one year and that, among those that progress, they may do so at different speeds, patients included in the current study were allowed to progress to later CKD stages during the study period). This approach resulted in slightly higher incremental costs among patients with advanced renal dysfunction compared to prior studies, as it included the cost of progression to higher stages. Cost information stratified by stage and accounting for progression is instrumental for the determination of optimal timing for the use of various interventions from a cost-saving perspective.
Third, the current study additionally presented findings for patients with an unknown CKD stage. A sizeable portion in both the commercially insured (46.2%) and Medicare Supplemental populations (29.1%) did not have any indicator of CKD stage. It is likely that this subpopulation of patients with unknown CKD stage represented asymptomatic patients (potentially in earlier stages of the disease), those with infrequent medical visits, or those with limited access to specialists, thus leading to a lack of CKD diagnosis. Indeed, commercially insured patients with an unknown CKD stage tended to be younger, have a lower ADPKD-related comorbidity burden, and have fewer visits to a kidney specialist than those with a known CKD stage. Nevertheless, patients with an unknown CKD stage in the ADPKD cohort still incurred substantial incremental healthcare costs, suggesting that these patients may benefit from closer monitoring. Of note, in the older Medicare population, patients with unknown CKD stage were less distinguishable from those with a known CKD stage in both characteristics and cost estimates. Given the progressive nature of the disease, patients in the Medicare population are expected to be at a relatively advanced stage and may present with ADPKD-related comorbidities that would require the use of medical services, regardless of the presence of ADPKD symptoms. Therefore, despite the unknown CKD stage, these patients still incurred high incremental costs. Taken together, the current study sheds light on the characteristics and economic burden of this important subpopulation of patients with ADPKD and unknown CKD stage, who have thus far not been thoroughly described in the literature.
This study also highlighted the substantial economic burden of patients with ADPKD receiving dialysis—incremental costs incurred by dialysis recipients was more than 30-times greater than those incurred by patients with CKD stage I. Knight et al. and Lentine et al. also reported considerable cost burdens for commercially insured patients with ADPKD on dialysisCitation7,Citation8, though both estimates (∼$57,000 over 6 months [2010 USD] and $131,890 PPPY [2008 USD], respectively) were lower than the $213,690 PPPY observed in the current study. Knight et al., estimated the costs of the first 6 months of dialysis, while Lentine et al. estimated the costs of patients who initiated dialysis during the course of the study period, thus capturing a period of time when the patients were not yet on dialysis; these differences may explain the lower costs estimated in the prior studies.
The findings of this study highlight a substantial unmet need for patients with ADPKD. Until recently, treatment options for ADPKD were limited to providing supportive care for its symptoms and complications rather than addressing the progressive nature of the disease and the severe consequences associated with its progressionCitation10,Citation11. In April 2018, tolvaptan became the first and only therapy approved by the Food and Drug Administration (FDA) to treat ADPKD by slowing down kidney function decline in patients at risk of rapidly progressingCitation12. In clinical trials, tolvaptan was shown to significantly reduce kidney growth, functional decline, and pain compared to placeboCitation13,Citation14, potentially providing a way to delay progression to the debilitating ESRD condition. Additional agents are currently being investigated for the treatment of ADPKD in clinical trials, including venglustat and bardoxolone methylCitation15,Citation16.
Considering the substantial burden associated with ADPKD and its progression, particularly at advanced stages and among patients at high risk of rapid progression, treatment that prevents the loss of renal function earlier in ADPKD may contribute to reducing the overall burden. However, little is known about the impact of these new treatments, and further studies assessing the economic impact of the introduction of newer therapies (and their costs) are needed to assess how they will impact the overall economic burden of ADPKD. In addition, it is important to better understand the optimal timing and stage to initiate therapy, which may yield substantial benefit if the intervention is carefully timed and planned based on evidence-based research.
Limitations
The results of this study should be interpreted in light of some limitations. The key limitation is that since the study population was limited to commercially insured and Medicare Supplemental patients, the results may not be representative of the overall US population. Second, CKD stage was based on diagnosis and procedure codes from claims and not laboratory values, which were not available in the databases. Third, since there is no established standard definition of ADPKD at high risk of rapid progression in claims data, the criteria used in the current study did not rely on laboratory test values and thus have not been clinically validated; however, the same definition has been used in previously published studies, with identified patients demonstrating faster progression to ESRDCitation5,Citation7. Lastly, as with all claims-based studies, billing inaccuracies and data omissions may have occurred.
Conclusions
This study demonstrated the substantial healthcare costs associated with ADPKD, which increased as patients progressed through more severe CKD stages. With the especially large economic burden associated with ESRD-RRT, dialysis, and certain high-risk subgroups, further research is warranted to evaluate whether early interventions to delay disease progression may result in significant cost savings among patients with ADPKD.
Transparency
Declaration of funding
This study was funded by Otsuka Pharmaceutical Development & Commercialization, Inc. The study sponsor contributed to and approved the study design, participated in the interpretation of data, and reviewed and approved the manuscript; all authors contributed to the development of the manuscript and maintained control over the final content.
Declaration of financial/other interests
AG, MC, PGS, and YL are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Otsuka Pharmaceutical Development & Commercialization, Inc., which funded the development and conduct of this study and manuscript. MS and DO are employees of Otsuka Pharmaceutical Development & Commercialization, Inc. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contributions
PGS, YL, AG, MS, DO, and MC contributed to the design of the study and interpretation of the data. PGS, YL, AG, and MC contributed to the data collection and data analysis. All authors critically revised the draft manuscript and approved the final content.
Previous presentations
Part of this analysis was presented at the National Kidney Foundation Spring Clinical Meeting held from 25–29 March 2020 in New Orleans, LA.
Supplemental Material
Download MS Word (25.1 KB)Acknowledgements
Medical writing assistance was provided by Christine Tam, an employee of Analysis Group, Inc.
Notes
i. MarketScan is a registered trademark of IBM, Armonk, NY, USA.
References
- Willey CJ, Kamat S, Stellhorn R, et al. Analysis of nationwide data to determine the incidence and diagnosed prevalence of autosomal dominant polycystic Kidney Disease in the USA: 2013–2015. Kidney Dis. 2019;5(2):107–117.
- Lanktree MB, Chapman AB. Autosomal dominant polycystic kidney disease. Can Med Assoc J. 2017;189(45):E1396. Nephrology Jared J. Grantham Research Fellowship in Polycystic Kidney Disease and is a member of the Kidney Foundation of Canada KRESCENT program. Arlene Chapman has served as a consultant to Otsuka and Kadmon Pharmaceutical.
- Sommerer C, Zeier M. Clinical manifestation and management of ADPKD in Western Countries. Kidney Dis. 2016;2(3):120–127.
- Ong AC, Devuyst O, Knebelmann B, et al. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet. 2015;385(9981):1993–2002.
- Blanchette CM, Liang C, Lubeck DP, et al. Progression of autosomal dominant kidney disease: measurement of the stage transitions of chronic kidney disease. Drugs Context. 2015;4:212275.
- Blanchette CM, Iorga SR, Altan A, et al. Healthcare resource utilization and costs associated with autosomal dominant polycystic Kidney Disease. J Health Eco Ootcom Res. 2014;2(1):63–74.
- Knight T, Schaefer C, Krasa H, et al. Medical resource utilization and costs associated with autosomal dominant polycystic kidney disease in the USA: a retrospective matched cohort analysis of private insurer data. Clin Eco Outcomes Res. 2015;7:123–132.
- Lentine KL, Xiao H, Machnicki G, et al. Renal function and healthcare costs in patients with polycystic kidney disease. Clin J Am Soc Nephrol. 2010;5(8):1471–1479.
- US Department of Labor Bureau of Labor Statistics. Consumer Price Index 2018; [cited 2018 June 13]. Available from: https://www.bls.gov/cpi/home.htm.
- Chebib FT, Torres VE. Recent advances in the management of autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol. 2018;13(11):1765–1776.
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369(9569):1287–1301.
- Otsuka. Otsuka’s JYNARQUE™ (tolvaptan) Approved by U.S. FDA as the First Treatment to Slow Kidney Function Decline in Adults at Risk of Rapidly Progressing Autosomal Dominant Polycystic Kidney Disease (ADPKD) 2018; [cited 2019 November 11]. Available from: https://www.otsuka-us.com/discover/articles-1188.
- Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–2418.
- Torres VE, Chapman AB, Devuyst O, et al. Tolvaptan in Later-Stage Autosomal Dominant Polycystic Kidney Disease. N Engl J Med. 2017;377(20):1930–1942.
- A Trial of Bardoxolone Methyl in Patients With ADPKD – FALCON (FALCON): ClinicalTrials.gov; [updated November 16, 2020; [cited 2020 November 26]. Available from: https://clinicaltrials.gov/ct2/show/NCT03918447.
- A Medical Research Study Designed to Determine if Venglustat Can be a Future Treatment for ADPKD Patients (STAGED-PKD): ClinicalTrials.gov; [updated November 24, 2020]; cited 2020 November 26]. Available from: https://clinicaltrials.gov/ct2/show/NCT03523728.