Abstract
Aims
To assess healthcare costs and hospitalization rates associated with rifaximin therapy versus lactulose alone among patients at risk for hepatic encephalopathy (HE).
Methods and materials
IBM Marketscan Commercial and Optum’s de-identified Clinformatics Data Mart databases were used separately to identify commercially insured HE patients treated with rifaximin or lactulose alone, using an algorithm developed with clinical experts. HE-related hospitalizations were defined based on an algorithm using diagnosis codes and diagnosis-related group codes. HE-related/all-cause hospital admissions/days and healthcare costs were compared between rifaximin and lactulose episodes using incidence rate ratios and adjusted cost differences.
Results
In Marketscan, there were 13,515 [Optum: 5,217] rifaximin episodes and 9,946 [4,897] lactulose alone episodes included. Yearly rates of HE-related hospital admissions decreased by 33% [34%] when treated with rifaximin versus lactulose alone, and rates of HE-related hospital days similarly decreased by 43% [57%]. Yearly rates of all-cause hospital admissions decreased by 27% [27%]; rates of all-cause hospital days decreased by 33% [37%] during rifaximin episodes versus lactulose alone. This translated to $2,417 [$2,301] and $173 [$397] lower total mean medical costs and HE-related hospital costs per-patient-per-month, respectively (p < .05). Despite increased pharmacy costs associated with rifaximin, there was no change in total healthcare costs. Patients adherent to rifaximin incurred $2,891 [$2,340] lower total healthcare costs than non-adherent patients. In a simulated plan of 1 million lives, if 50% of HE patients treated with lactulose alone had rifaximin added on and were adherent to rifaximin therapy, the total cost savings would be $7.5 [$6.1] million per year ($0.62 [$0.50] per-member-per-month).
Conclusions
Patients incurred significantly lower rates of HE-related and all-cause hospitalizations during rifaximin versus lactulose episodes, resulting in lower facility and professional costs. Cost savings may be possible if rifaximin adherence is improved in HE patients.
Limitations
The study is subject to limitations common to claims-based analyses.
Introduction
Hepatic encephalopathy (HE) is a common complication of liver cirrhosis, affecting up to 70% of patients with cirrhosisCitation1. HE is characterized by reversible neurological impairments that result from the accumulation of toxins, such as inflammatory cytokines and elevated levels of gut-derived neurotoxins (e.g. ammonia), that are not cleared by the cirrhotic liver and eventually enter the brainCitation2–4. Symptoms are highly heterogeneous and can range from cognitive defects to altered conscious state and impaired neuromuscular functionCitation2,Citation3. As such, the severity spectrum is represented by the categories of minimal HE and the more severe overt HECitation3; however, while minimal HE may affect 30%–80% of patients with liver cirrhosis, it is seldom diagnosed and frequently untreated due to its covert natureCitation5,Citation6.
The disabling nature of HE negatively affects multiple aspects of patients’ quality of life (e.g. physical, social, cognitive)Citation1, increases the risk of road traffic accidents and fallsCitation7,Citation8, and represents a significant burden to caregiversCitation9. Furthermore, hospitalizations occur frequently to manage the debilitating symptomsCitation10. Indeed, HE accounted for approximately 100,000 hospitalizations per year from 2005 to 2009, with a mortality rate of 14%–16% over the 5-year periodCitation11. This translated to total HE-related hospitalization charges of $7.2 billion in 2009 in the United States (US), highlighting the large economic burden of this disease.
The clinical management of HE focuses on inhibiting precipitating events such as infection, gastrointestinal bleeding, and electrolyte disordersCitation10 Xifaxan (rifaximin) is the only approved treatment for reducing the risk of overt HE recurrence in adultsCitation12. Treatment guidelines from the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) recommend the use of lactulose for the treatment of episodic HE, with rifaximin as an effective add-on therapy to prevent HE recurrenceCitation10. Indeed, rifaximin with or without lactulose has been shown to be associated with reductions in HE-related hospital admissions and hospitalization charges compared to lactulose aloneCitation13–17. Despite these benefits, because the drug cost of treatment with rifaximin in combination with lactulose is higher than that of lactulose alone, there is uncertainty regarding the effect of treatment with rifaximin on overall healthcare costs.
Therefore, this study aimed to assess the healthcare costs associated with rifaximin with or without lactulose compared to lactulose alone, considering the increased drug costs and reduced medical services associated with rifaximin. Additionally, the rates of HE-related and all-cause hospitalizations were assessed and compared between rifaximin with or without lactulose and lactulose alone. Lastly, the impact of adding on rifaximin for the patients receiving lactulose and the impact of improving rifaximin adherence on healthcare costs from a commercial health plan perspective were assessed.
Methods
Data source
Claims data from 1 July 2014 to 30 June 2019 from the IBM MarketscanFootnotei Commercial database were used (herein referred to as Marketscan). The database consists of medical and pharmacy claims of insured employees and their dependents from over 100 different insurance companies, representing approximately 43 million covered lives across all census regions. It includes detailed information on history of health plan enrollment, demographics, medical care received across different settings, diagnosis, procedures, and medications. In addition, claims data from 1 July 2014 to 31 March 2020 from the Optum’s de-identified ClinformaticsFootnoteii Data Mart Database commercial claims database, representing 15 million beneficiaries of commercial health plans per year from a single payer, were used (herein referred to as Optum). Analyses were conducted separately in each data source. The rationale for performing analyses in both data sources was to confirm that results were consistent across one single large payer, or multiple smaller payers.
For both data sources, the data were de-identified and complied with the confidentiality requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Study design
A retrospective cohort study design was used. The study index date was defined as the first prescription fill of rifaximin or lactulose. The baseline period was defined as the 6-month period prior to the study index date. The observation period was the time between the study index until the earliest of the end of data availability or end of continuous enrollment. Observation periods were also censored if patients underwent a liver transplant.
Sample selection
Since an HE-specific code only exists under ICD-9-CM and not ICD-10-CMCitation18, patients with HE treated with rifaximin 550 mg (regardless of number of tablets per day) or lactulose were identified using an algorithm developed according to input from medical experts based on their real-world clinical practice of coding for HE in their respective electronic medical records and included four sequential criteria. The criteria were based on either a diagnosis for HE or treatment for HE (i.e. rifaximin and/or lactulose), combined with a diagnosis or hospitalization for liver-related complications, as described in Supplemental Figure S1 and Figure S2.
Additionally, patients identified through the four criteria were required to have no diagnoses for secondary malignant neoplasm of the liver at any time. Commercially insured patients were included for these analyses, and were required to be aged 18–64 years as of the study index date, with ≥6 months of continuous enrollment prior to and ≥30 days of follow-up after the study index date.
Definition of treatment episodes
Treatment episodes were defined by an algorithm developed in conjunction with the medical experts and were classified into two mutually exclusive treatment categories: (1) rifaximin with or without lactulose (herein referred to as rifaximin episodes), or (2) lactulose alone (referred to as lactulose episodes). The first treatment episode started at the study index date. New treatment episodes were defined by either the initiation of another treatment or discontinuation of a treatment (defined as a gap in supply of >30 days). In the case of a patient moving from a treated episode to an untreated episode, a tail of 15 days was considered to be included in the treated episode. A sensitivity analysis was also conducted where a tail of 30 days was considered. This adjustment was made based on medical expert opinion that some events shortly after the discontinuation of rifaximin or lactulose may potentially be associated with that treatment. If the created treatment episode was initiated during an inpatient (IP) stay, the treatment episode was adjusted to start after the discharge (as events during that IP stay could not reasonably be associated with the newly initiated treatment). The treatment episode end date was defined as the day before the next episode index date or the end of the observation period, whichever occurred earlier.
Treatment episodes were used rather than an intention-to-treat approach as rifaximin is a minimally absorbed oral antibiotic which is concentrated in the gastrointestinal tract, and therefore medical experts determined that any events beyond a certain point after exposure could not be associated with the drug (which an intention-to-treat approach would do). Although this study assessed association and not causation, an effort was made to identify events that could be prevented by the treatment.
Treatment episodes were then stratified based on adherence, defined as proportion of days covered calculated at the episode level (PDC; i.e. sum of the days with the treatment on hand divided by the number of days during the treatment episode)Citation19. Additionally, because of the variable dosing of lactulose (due to its formulation as a syrup), input from medical experts was used to assume an average daily dose of 15 mL/day.
Characteristics and study outcomes
Patient characteristics were evaluated and included the duration of the follow-up period, HE patient selection criteria, age at the study index date, sex, medically relevant baseline procedures (paracentesis, endoscopy, transvenous intrahepatic portosystemic shunt [TIPS], and dialysis) during the baseline period, Charlson comorbidity index (CCI) and other comorbidities during the baseline period, and treatment characteristics.
Characteristics of treatment episodes were also summarized and included the length of the treatment episode (i.e. from initiation of the treatment episode to either end of follow-up or next treatment episode) and treatment adherence (i.e. PDC) during the treatment episode.
Study outcomes measured in the rifaximin and lactulose episodes included healthcare resource utilization (HRU) and healthcare costs. HRU was measured as yearly incidence rates during each treatment episode and included HE-related and all-cause IP admissions and days. HE-related IP admissions and days were defined in consultation with medical experts as hospitalizations with HE as the primary diagnosis, as well as Diagnosis Related Group (DRG) codes 441, 442, or 443 (i.e. disorders of the liver except malignancy, cirrhosis, or alcoholic hepatitis, with or without complication, comorbidity or major complication). Given the real-world variance in how a patient with HE presents during an IP stay, medical experts also recommended the use of a secondary, more inclusive definition of HE-related hospitalizations, as hospitalizations with HE as primary or secondary diagnosis (with no restriction on DRG). Healthcare costs were reported per-patient-per-month (PPPM) and included HE-related IP costs, as well as all-cause medical (IP, emergency room, and outpatient) and outpatient pharmacy components. Costs were measured from a payer’s perspective (i.e. health plan payment + coordination of benefits, excluding patients’ payment), adjusted for inflation using the US Medical Care consumer price index (CPI) from the Bureau of Labor Statistics from the US Department of Labor, and reported in 2019 US dollarsCitation20.
Statistical analysis
For all outcomes, continuous variables were summarized using means, standard deviations (SD), and medians, while categorical variables were summarized using frequency counts and percentages.
HRU and healthcare costs were compared between the rifaximin and lactulose episodes. Additionally, healthcare costs were compared between adherent (i.e. PDC ≥80%) and non-adherent patients during rifaximin treatment episodes. For HRU, multivariate generalized linear mixed models (GLMM) with a negative binomial distribution, a log link, a random effect to account for multiple episodes by patient and adjusted for a priori selected potential confounding factors associated with HRU and costs (i.e. age, gender, region, health plan, baseline procedures, and CCI) were used to estimate adjusted incidence rate ratios (IRR) with 95% confidence intervals (CIs) and p-values. For healthcare costs, adjusted cost differences with 95% CIs and p-values were estimated using a multivariate GLMM with a gamma distribution, a log link, and a random effect, weighted on treatment episode duration and adjusted for the same a priori selected confounding factors.
Results
Treatment episode and patient characteristics
After patient selection, 7,675 commercially insured patients from the Marketscan database were included in the study, contributing 13,515 rifaximin and 9,946 lactulose treatment episodes. From the Optum database, 3,530 commercially insured patients were included, contributing 5,217 rifaximin and 4,897 lactulose treatment episodes. At the patient level, the mean (SD) duration of follow-up was 14.5 (12.6) months (Marketscan) and 13.9 (13.1) months (Optum; ). Of the 4 patient selection criteria, the majority of patients were selected based on an HE diagnosis (83.7% in the Marketscan database; 92.8% in the Optum database). The median age was 56.0 years in the Marketscan database and 55.0 years in the Optum database, and 41.2% were female in both samples. During the observation period, approximately half of patients (53.2% in the Marketscan database; 45.8% in the Optum database) had both rifaximin and lactulose treatment episodes at different times. More information on the patient clinical profile may be found in Supplemental Table S1.
Table 1. Patient characteristics.
At the treatment episode level, the mean (SD) length of a rifaximin treatment episode was 83.2 (114.2) days in the Marketscan database and 82.0 (114.6) days in the Optum database, whereas lactulose episodes had mean (SD) 63.1 (73.7) days and 65.8 (83.0) days, respectively. The mean (SD) PDC while on treatment during rifaximin episodes was 86% (18%) in the Marketscan database and 85% (18%) in the Optum database. Out of rifaximin episodes, on average, PDC ≥80% was achieved 76.8% (Marketscan) and 75.8% (Optum) of the time. By contrast, during lactulose episodes, mean (SD) PDC was lower at 75% (23%) in the Marketscan database and 75% (22%) in the Optum database. Among lactulose episodes, PDC ≥80% was achieved only 51.8% (Marketscan) and 50.0% (Optum) of the time, indicating lower adherence for lactulose use.
Healthcare resource utilization
When comparing treatment episodes of rifaximin versus lactulose, there was a 33% decrease in yearly rates of HE-related IP admissions in the Marketscan database (unadjusted 0.21 vs. 0.25; adjusted IRR [95% CI] = 0.67 [0.58, 0.79]; ) and a 34% decrease in the Optum database (unadjusted 0.16 vs. 0.21; adjusted IRR [95% CI] = 0.66 [0.51, 0.85]; ). Similarly, there was a 43% and 57% reduction in the yearly rates of HE-related IP days in the Marketscan (unadjusted 1.39 vs. 1.68; adjusted IRR [95% CI] = 0.57 [0.45, 0.73]; ) and Optum (unadjusted 1.37 vs. 2.33; adjusted IRR [95% CI] = 0.43 [0.27, 0.69]; ) databases, respectively, during rifaximin episodes compared to lactulose episodes. Similar reductions were observed when using the secondary definition of HE-related hospitalizations in both the Marketscan () and Optum () databases. In sensitivity analyses where a tail of 30 days was included when patients moved from treated to untreated episodes, results were nearly identical; for example, a reduction of HE-related IP admissions of 32% during rifaximin episodes compared to lactulose episodes was observed in the Marketscan database (adjusted IRR [95% CI] = 0.68 [0.58, 0.80]). In both the Marketscan and Optum databases, the yearly rates of all-cause IP admissions decreased by 27% during rifaximin episodes compared to lactulose episodes (Marketscan: unadjusted 1.48 vs. 1.96; adjusted IRR [95% CI] = 0.73 [0.69, 0.78]; ; Optum: unadjusted 1.47 vs. 2.02; adjusted IRR [95% CI] = 0.73 [0.66, 0.80]; ). Similar reductions in the yearly rate of IP days were observed in both databases, with rifaximin episodes being associated with a 33% decrease compared to lactulose episodes in the Marketscan database (unadjusted 11.22 vs. 16.71; adjusted IRR [95% CI] = 0.67 [0.61, 0.73]; ) and a 37% decrease in the Optum database (unadjusted 12.43 vs. 19.35; adjusted IRR [95% CI] = 0.63 [0.56, 0.71]; ).
Figure 1. IRR of HE-related and all-cause IP admissions and days during rifaximin versus lactulose episodes in the Marketscan database. Abbreviations. CCI, Charlson comorbidity index; CI, confidence interval; DRG, diagnosis-related group; HE, hepatic encephalopathy; IP, inpatient; IRR, incidence rate ratio; PDC, proportion of days covered; SD, standard deviation.
Notes: aHE-related hospitalizations were defined as hospitalizations with HE as primary diagnosis, as well as DRG 441, 442, or 443 (liver-related diseases).
bFor the secondary scenario, HE-related hospitalizations were defined as hospitalizations with HE as primary or secondary diagnosis.
cModels were adjusted for age, gender, region, health plan, baseline procedures (paracentesis, endoscopy, transvenous intrahepatic portosystemic shunt, and dialysis) and Charlson comorbidity index.
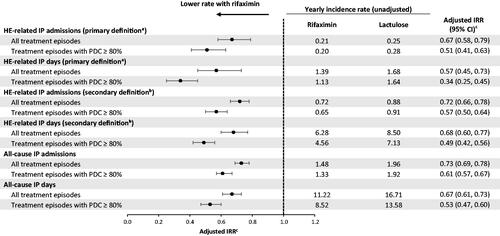
Figure 2. IRR of HE-related and all-cause IP admissions and days during rifaximin versus lactulose episodes in the Optum database. Abbreviations. CCI, Charlson comorbidity index; CI, confidence interval; DRG, diagnosis-related group; HE, hepatic encephalopathy; IP, inpatient; IRR, incidence rate ratio; PDC, proportion of days covered; SD, standard deviation.
Notes: aHE-related hospitalizations were defined as hospitalizations with HE as primary diagnosis, as well as DRG 441, 442, or 443 (liver-related diseases).
bFor the secondary scenario, HE-related hospitalizations were defined as hospitalizations with HE as primary or secondary diagnosis.
cModels were adjusted for age, gender, region, health plan, baseline procedures (paracentesis, endoscopy, transvenous intrahepatic portosystemic shunt, and dialysis) and Charlson comorbidity index.
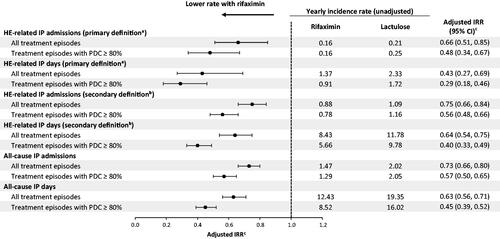
When considering the subset of treatment episodes with high adherence (PDC ≥ 80%), the yearly rates of HE-related IP admissions decreased by 49% during rifaximin episodes compared to lactulose episodes in the Marketscan database (unadjusted 0.20 vs. 0.28; adjusted IRR [95% CI] = 0.51 [0.41, 0.63]; ) and by 52% in the Optum database (unadjusted 0.16 vs. 0.25; adjusted IRR [95% CI] = 0.48 [0.34, 0.67]; ). Similarly, the yearly rates of HE-related IP days decreased by 66% during rifaximin episodes with high adherence compared to lactulose episodes with high adherence in the Marketscan database (unadjusted 1.13 vs. 1.64; adjusted IRR [95% CI] = 0.34 [0.25, 0.45]; ) and by 71% in the Optum database (unadjusted 0.91 vs. 1.72; adjusted IRR [95% CI] = 0.29 [0.18, 0.46]; ). Similar results were seen when using the secondary definition of HE-related hospitalizations during treatment episodes with high adherence in both the Marketscan () and Optum () databases. With regards to all-cause hospitalizations during treatment episodes with high adherence, there was a 39% decrease in the yearly rates of all-cause IP admissions during rifaximin episodes compared to lactulose episodes in the Marketscan database (unadjusted 1.33 vs. 1.92; adjusted IRR [95% CI] = 0.61 [0.57, 0.67]; ) and a 43% decrease in the Optum database (unadjusted 1.29 vs. 2.05; adjusted IRR [95% CI] = 0.57 [0.50, 0.65]; ). Similarly, the yearly rates of all-cause IP days decreased by 47% during rifaximin episodes with high adherence compared to lactulose episodes with high adherence in the Marketscan database (unadjusted 8.52 vs. 13.58; adjusted IRR [95% CI] = 0.53 [0.47, 0.60]; () and by 55% in the Optum database (unadjusted 8.52 vs. 16.02; adjusted IRR [95% CI] = 0.45 [0.39, 0.52]; ).
Healthcare costs
On average, a HE-related hospitalization incurred a mean (SD) cost of $28,063 ($37,776), with a mean duration of 6.8 days in the Marketscan database. In the Optum database, a HE-related hospitalization incurred a mean (SD) cost of $34,810 ($60,353), with a mean duration of 10 days.
In the Marketscan database, mean medical costs were lower during rifaximin episodes compared to lactulose episodes (unadjusted $6,938 vs. $8,616 PPPM; adjusted cost difference [95% CI] = –$2,417 [–$3,057; –$1,776]; p < .001), mainly driven by lower IP costs (unadjusted $3,990 vs. $5,679 PPPM; adjusted cost difference [95% CI] = –$2,054 [–$2,613; –$1,495]; p < .001; ). In the Optum database, similar reductions in mean medical costs were seen during rifaximin episodes compared to lactulose episodes (unadjusted $6,455 vs. $7,983 PPPM; adjusted cost difference [95% CI] = –$2,301 [–$3,051; –$1,551]; p < .001), mainly driven by lower IP costs (unadjusted $3,497 vs. $4,901 PPPM; adjusted cost difference [95% CI] = –$2,113 [–$2,765; –$1,462]; p < .001; ).
Figure 3. Healthcare costs among patients during rifaximin versus lactulose episodes in the Marketscan databasea,b. Abbreviations. CI, confidence interval; DRG, diagnosis-related group; HE, hepatic encephalopathy; IP, inpatient; PPPM, per-patient-per-month; USD, United States dollar.
Notes: aMean costs presented are unadjusted, while cost differences were weighted using episode duration and adjusted for age, gender, region, health plan, Charlson comorbidity index during baseline, and procedures during baseline (i.e. paracentesis, dialysis, endoscopy, transvenous intrahepatic portosystemic shunt).
bThe mean medical and pharmacy costs were estimated from separate unadjusted models and therefore do not sum to the total healthcare costs.
cHE-related IP costs were defined as those from hospitalizations with HE as primary diagnosis, as well as DRG 441, 442, or 443 (liver-related diseases). For the secondary scenario (hospitalizations with HE as primary or secondary diagnosis), unadjusted HE-related IP costs were $2,219 in the rifaximin cohort and $3,108 in the lactulose cohort, with an adjusted cost difference (95% CI) of –$1,235 (–$1,655; –$814); p < .001. Unadjusted all-cause IP costs were $3,990 in the rifaximin cohort and $5,679 in the lactulose cohort, with an adjusted cost difference (95% CI) of –$2,054 (–$2,613; –$1,495); p < .001.
dUnadjusted pharmacy costs were $2,760 in the rifaximin cohort and $921 in the lactulose cohort, with an adjusted cost difference (95% CI) of $1,878 ($1,742; $2,014); p < .001. Unadjusted outpatient costs were $2,283 in the rifaximin cohort and $2,255 in the lactulose cohort, with an adjusted cost difference (95% CI) of –$180 (–$343; –$17); p = .030.
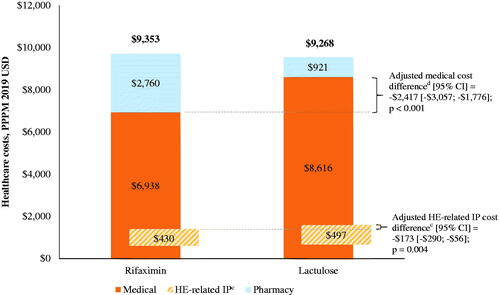
Figure 4. Healthcare costs among patients during rifaximin versus lactulose episodes in the Optum databasea,b. Abbreviations. CI, confidence interval; HE, hepatic encephalopathy; IP, inpatient; PPPM, per-patient-per-month; USD, United States dollar.
Notes: aMean costs presented are unadjusted, while cost differences were weighted using episode duration and adjusted for age, gender, region, health plan, Charlson comorbidity index during baseline, and procedures during baseline (i.e. paracentesis, dialysis, endoscopy, transvenous intrahepatic portosystemic shunt).
bThe mean medical and pharmacy costs were estimated from separate unadjusted models and therefore do not sum to the total healthcare costs.
cHE-related IP costs were defined as those from hospitalizations with HE as primary diagnosis, as well as DRG 441, 442, or 443 (liver-related diseases). For the secondary scenario (hospitalizations with HE as primary or secondary diagnosis), unadjusted HE-related IP costs were $2,372 in the rifaximin cohort and $3,175 in the lactulose cohort, with an adjusted cost difference (95% CI) of –$1,693 (–$2,312; –$1,075); p < .001. Unadjusted all-cause IP costs were $3,497 in the rifaximin cohort and $4,901 in the lactulose cohort, with an adjusted cost difference (95% CI) of –$2,113 (–$2,765; –$1,462); p < .001.
dUnadjusted pharmacy costs were $2,738 in the rifaximin cohort and $763 in the lactulose cohort, with an adjusted cost difference (95% CI) of $1,978 ($1,802; $2,155); p < .001. Unadjusted outpatient costs were $2,295 in the rifaximin cohort and $2,307 in the lactulose cohort, with an adjusted cost difference (95% CI) of –$35 (–$280; $211); p = .782.
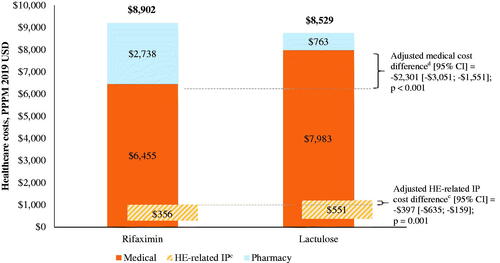
However, in the Marketscan database, the lower medical costs were offset by higher pharmacy costs during rifaximin treatment episodes compared to lactulose episodes (unadjusted $2,760 vs. $921 PPPM; adjusted cost difference [95% CI] = $1,878 [$1,742; $2,014]; p < .001), resulting in no change in the total healthcare costs (unadjusted $9,353 vs. $9,268 PPPM; adjusted cost difference [95% CI] = –$92 [–$653; $468]; p = .747; ). Similarly, in the Optum database, higher pharmacy costs during rifaximin treatment episodes compared to lactulose episodes (unadjusted $2,738 vs. $763 PPPM; adjusted cost difference [95% CI] = $1,978 [$1,802; $2,155]; p < .001) led to no change in the total healthcare costs (unadjusted $8,902 vs. $8,529 PPPM; adjusted cost difference [95% CI] = $30 [–$682; $742]; p = .934; ).
With regards to rifaximin adherence, adherent patients (i.e. PDC ≥ 80%) incurred lower total healthcare costs PPPM than non-adherent patients during rifaximin episodes in the Marketscan database (adjusted cost difference [95% CI] = –$2,891 [–$4,034; –$1,747; p < .001) and Optum database (adjusted cost difference [95% CI] = –$2,340 [–$3,558; –$1,123; p < .001).
Impact on health plan
In a simulated plan of 1 million lives, taking the results from MarketScan into account, if payers and physicians ensured proper adherence to rifaximin, the total cost savings would be $5.9 million per year or $0.49 per-member-per-month (PMPM). Additionally, if 50% of patients with HE who were treated with lactulose alone had rifaximin added on and were adherent to their rifaximin therapy, the total cost savings would be $7.5 million per year or about $0.62 PMPM. The same simulated savings from the Optum results when ensuring adherence of rifaximin would result in cost savings of $4.4 million per year or $0.37 PMPM. If 50% of patients treated with lactulose were also given rifaximin and were adherent, the total cost savings would be $6.1 million per year or $0.50 PMPM.
Discussion
In this retrospective cohort study of data spanning from 2014 to 2020 in two large claims databases, patients had 43%–57% lower rates of HE-related IP days during rifaximin (with or without lactulose) episodes compared to lactulose episodes. Similarly, the rates of all-cause IP days were 33%–37% lower during rifaximin versus lactulose episodes. Importantly, this decrease in rates was even more pronounced during treatment episodes with high treatment adherence. This reduction in HRU translated to $2,301– $2,417 lower medical (facility and professional) costs PPPM, including $173–$397 lower HE-related IP costs, associated with rifaximin treatment compared to lactulose. Despite the higher pharmacy costs associated with rifaximin versus lactulose treatment, total healthcare costs were similar between the two treatments, and total costs were significantly lower among patients with high rifaximin adherence.
The reduced rates of hospitalization associated with rifaximin treatment in the current real-world study have also been observed in prior literature, including clinical trialsCitation13–17. The 49%–52% reduction in the rate of HE-related IP admissions during treatment episodes with high adherence aligns with the rifaximin clinical trial, where treatment with rifaximin reduced the risk of HE-related hospitalization by 50% compared to placebo (>90% of patients receiving placebo were also treated with lactulose)Citation12,Citation17. Rifaximin has also been shown to be associated with reduced hospital readmissionsCitation13,Citation16. In a retrospective chart review study, the rate of readmissions after an initial IP admission for HE was lower among patients treated with rifaximin and lactulose combination therapy compared to those receiving lactulose (2.5% vs. 16.2%; p = .02)Citation13. Additionally, in a separate claims-based study of Medicare enrollees with cirrhosis by Tapper et al., treatment with rifaximin was associated with a lower rate of all-cause readmissions (IRR [95% CI] = 0.18 [0.08, 0.40])Citation16. Of note, this outcome differs from the current study, in that Tapper et al. evaluated re-hospitalizations following discharge from an HE-related hospitalization rather than the rate of hospitalizations on a treatment episode basisCitation16. Furthermore, Tapper et al. found that rifaximin and lactulose combination therapy relative to no therapy demonstrated the largest reduction in rate of hospital days (IRR [95% CI] = 0.28 [0.27, 0.30]) compared to lactulose alone (IRR [95% CI] = 0.31 [0.30, 0.32]) or rifaximin alone (IRR [95% CI] = 0.49 [0.45, 0.53])Citation16. While untreated patients were not evaluated in the current study, a separate analysis by Vadhariya et al. did not identify any significant difference in the rate of all-cause hospital readmissions between treated and untreated Medicare patients with HE, though the sample sizes were limited (117 treated and 67 untreated patients) and Medicare patients may differ from their commercially insured counterpartsCitation21.
As shown in the current study, the reduction in hospitalization rates translated to a decrease in IP costs of $2,054–$2,113 PPPM with rifaximin versus lactulose treatment. This finding is corroborated by a retrospective chart review study of patients with HE who were originally treated with lactulose prior to being switched to rifaximin in 2004 (i.e. first availability in the US) by Leevy et al.Citation14. From the 6-month lactulose treatment period to the 6-month rifaximin treatment period, hospitalization charges decreased by $42,413 per patient (2005 USD). This cost difference is higher than the cost difference observed in the current study, which may be partly due to the fact that Leevy reported hospital charges while the present study reported costs from a payer’s perspective (i.e. health plan payment + coordination of benefits, excluding patients’ payment). The payer’s perspective more accurately reflects the paid amount, as charges for a unit of healthcare often does not reflect the actual paid amountCitation14.
Although the drug cost of rifaximin is significant, inclusion of rifaximin in the HE treatment paradigm did not result in a total healthcare cost increase compared to treatment with lactulose alone, even after accounting for the increased pharmacy costs. The increased pharmacy costs were offset by the cost impact of the reduction in HE-related and all-cause hospitalizations. From a patient perspective, a reduction in hospitalizations would likely correlate with improved health-related quality of lifeCitation22,Citation23. Indeed, in a survey-based study of patients with cirrhosis, recent hospital admission was associated with poor quality of life health status scoresCitation23. When considering the health benefits gained with rifaximin treatment in the context of costs, a recent cost-effectiveness study found that rifaximin with or without lactulose was cost-effective compared to lactulose alone, with an incremental cost effectiveness ratio of $29,161 per quality-adjusted life-year gainedCitation24. Our findings suggest that adding rifaximin may improve quality of life at no extra net healthcare cost, thus being a dominant strategy from a cost-effectiveness standpoint. In addition, a study by Tapper et al. suggested that there may also be a survival benefit of treating with rifaximin, however, this analysis was performed in a Medicare population; further studies are warranted to confirm these findings in a commercially insured populationCitation16.
In addition, hospitalizations not only incur direct healthcare costs, but also indirect costs due to work lossCitation25. While the indirect costs associated with HE have not been quantified in the literature, the indirect cost of lost wages due to hospitalization among patients with chronic liver disease was estimated to be over $225 million in 2004Citation25. Future research is required to evaluate the indirect costs associated with HE and the impact of rifaximin treatment on these costs.
Further influencing the economic burden of HE is treatment adherence. In the current study, patients were not adherent (i.e. PDC < 80%) in more than half of lactulose episodes, which is aligned with the literature demonstrating low adherence with lactuloseCitation14,Citation26,Citation27. While rifaximin has been shown to be associated with superior adherenceCitation14,Citation21, patients were not adherent in approximately 30% of rifaximin episodes in this study. Based on the cost analyses, there may be potential cost savings associated with improving adherence to rifaximin. Indeed, each additional patient that uses rifaximin properly would account for savings of about $2,340–$2,891 in total healthcare costs per month, even after taking into account the increased drug costs. These cost savings would be driven by the reduction in HE-related and all-cause hospitalizations associated with rifaximin treatment. Therefore, in a typical health insurance plan of 1 million members, if payers and physicians ensured proper adherence to rifaximin, the total cost savings would be $4.4–5.9 million per year or $0.37–0.49 PMPM. Additionally, if 50% of patients with HE treated with lactulose alone had rifaximin added on (in line with treatment guidelinesCitation10) and were adherent, the total cost savings would be $6.1–7.5 million per year or about $0.50–0.62 PMPM. It is thus instrumental to improve medication adherence through the implementation of patient education programs that may emphasize the long-term value of compliance, from both a quality of life and economic perspective.
Limitations
The findings of this study should be interpreted in light of some limitations. HE-related hospitalizations were identified using a conservative algorithm that may have led to the underestimation of HE-related medical services used and consequently, HE-related costs. As such, the focus of the cost analyses was on all-cause healthcare costs. However, this is the first study to our knowledge that systematically attempts to identify HE-related hospitalizations based on all available data in US claims, as well as medical expert opinion. Additionally, a less conservative definition of HE-related hospitalization was considered as a secondary definition. Similarly, due to the lack of a specific ICD-10-CM diagnosis code for HE, patients with HE were identified using an algorithm (Supplemental Figure S1) that has not been clinically validated; however, the algorithm was developed together with medical experts using all data available in US claims and aligns with prior publicationsCitation18 as well as what they observe in real-world clinical practice, ensuring its clinical applicability. As with all claims-based studies, the data used in the study may have included billing inaccuracies or omissions in coded procedures, diagnoses, and pharmacy claims. Additionally, despite adjustments for patient covariates in the HRU and cost comparisons, residual confounding due to unmeasured confounders unavailable in administrative claims data, such as disease severity, may have remained.
Conclusions
This real-world study demonstrated that patients incurred significantly lower rates of all-cause and HE-related hospitalizations during treatment with rifaximin with or without lactulose compared to lactulose alone, resulting in lower medical costs. Despite the higher pharmacy costs associated with rifaximin, the cost impact of the reduction in hospitalizations resulted in no change to the overall healthcare costs. There is an important potential for considerable cost savings if adherence to rifaximin can be improved among patients with HE in clinical practice.
Transparency
Declaration of funding
This study was funded by Bausch Health US, LLC; Xifaxan (rifaximin) is distributed in the US by Salix Pharmaceuticals, which is a division of Bausch Health US, LLC. The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Declaration of financial/other relationships
GJ and ZH are employees of Bausch Health US, LLC; Xifaxan (rifaximin) is distributed in the US by Salix Pharmaceuticals, which is a division of Bausch Health US, LLC. AG, RB, and SS are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Bausch Health US, LLC, which funded the development and conduct of this study and manuscript. MLV and MA received consulting fees from Bausch Health US, LLC.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors participated in the conception and design of the study. RB and SS participated in the data analysis. All authors participated in the interpretation of the data. All authors revised the manuscript critically for intellectual content and approved the final version to be published. All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (69.5 KB)Acknowledgements
Medical writing assistance was provided by Christine Tam, an employee of Analysis Group, Inc.
Certain data used in this study were supplied by International Business Machines Corporation. Any analysis, interpretation, or conclusion based on these data is solely that of the authors and not International Business Machines Corporation.
Notes
i Marketscan is a registered trademark of IBM, Armonk, NY, USA.
ii Clinformatics is a registered trademark of Optum, Eden Prairie, MN, USA.
References
- Neff G, Zachry W. III. Systematic review of the economic burden of overt hepatic encephalopathy and pharmacoeconomic impact of rifaximin. Pharmacoeconomics. 2018;36(7):809–822.
- Elwir S, Rahimi RS. Hepatic encephalopathy: an update on the pathophysiology and therapeutic options. J Clin Transl Hepatol. 2017;5(2):142–151.
- Hadjihambi A, Arias N, Sheikh M, et al. Hepatic encephalopathy: a critical current review. Hepatol Int. 2018;12(Suppl 1):135–147.
- Haj M, Rockey DC. Ammonia levels do not guide clinical management of patients with hepatic encephalopathy caused by cirrhosis. Am J Gastroenterol. 2020;115(5):723–728.
- Dhiman RK, Chawla YK. Minimal hepatic encephalopathy: time to recognise and treat. Trop Gastroenterol. 2008;29(1):6–12.
- Stinton L, Jayakumar S. Minimal hepatic encephalopathy. Can J Gastroenterol. 2013;27(10):572–574.
- Ezaz G, Murphy SL, Mellinger J, et al. Increased morbidity and mortality associated with falls among patients with cirrhosis. Am J Med. 2018;131(6):645–650 e2.
- Tapper EB, Romero-Gómez M, Bajaj JS. Hepatic encephalopathy and traffic accidents: vigilance is needed!. J Hepatol. 2019;70(4):590–592.
- Bajaj JS, Wade JB, Gibson DP, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106(9):1646–1653.
- Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735.
- Stepanova M, Mishra A, Venkatesan C, et al. In-hospital mortality and economic burden associated with hepatic encephalopathy in the United States from 2005 to 2009. Clin Gastroenterol Hepatol. 2012;10(9):1034–1041 e1.
- Food and Drug Administration. XIFAXAN® (rifaximin) label. 2017.
- Courson A, Jones GM, Twilla JD. Treatment of acute hepatic encephalopathy: comparing the effects of adding rifaximin to lactulose on patient outcomes. J Pharm Pract. 2016;29(3):212–217.
- Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52(3):737–741.
- Neff GW, Kemmer N, Zacharias VC, et al. Analysis of hospitalizations comparing rifaximin versus lactulose in the management of hepatic encephalopathy. Transplant Proc. 2006;38(10):3552–3555.
- Tapper EB, Aberasturi D, Zhao Z, et al. Outcomes after hepatic encephalopathy in population-based cohorts of patients with cirrhosis. Aliment Pharmacol Ther. 2020;51(12):1397–1405.
- Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081.
- Tapper EB, Korovaichuk S, Baki J, et al. Identifying patients with hepatic encephalopathy using administrative data in the ICD-10 Era. Clin Gastroenterol Hepatol. 2019. Available from: https://www.cghjournal.org/article/S1542-3565(19)31496-X/fulltext
- Karve S, Cleves MA, Helm M, et al. Prospective validation of eight different adherence measures for use with administrative claims data among patients with schizophrenia. Value Health. 2009;12(6):989–995.
- Bureau of Labor Statistics. Consumer Price Index 2019; [cited 2020 Jan 18]. Available from: https://www.bls.gov/cpi/tables/supplemental-files/home.htm.
- Vadhariya A, Chen H, Serna O, et al. A retrospective study of drug utilization and hospital readmissions among medicare patients with hepatic encephalopathy. Medicine. 2020;99(16):e19603.
- Chronic Liver Disease Foundation. The multidimensional burden of hepatic encephalopathy. 2012. Available from: http://www.chronicliverdisease.org/disease_focus/enewsletters/HepCoEE_eNewsLetter_Multidimensional_burden.pdf
- Marchesini G, Bianchi G, Amodio P, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120(1):170–178.
- Jesudian AB, Ahmad M, Bozkaya D, et al. Cost-effectiveness of rifaximin treatment in patients with hepatic encephalopathy. J Manag Care Spec Pharm. 2020;26(6):750–757.
- Neff G. Pharmacoeconomics of hepatic encephalopathy. Pharmacotherapy. 2010;30(5 Pt 2):28S–32S.
- Bajaj JS, Sanyal AJ, Bell D, et al. Predictors of the recurrence of hepatic encephalopathy in lactulose-treated patients. Aliment Pharmacol Ther. 2010;31(9):1012–1017.
- Hudson M, Schuchmann M. Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence. Eur J Gastroenterol Hepatol. 2019;31(4):434–450.
