Abstract
Aims
Estimating the monetary value of the convenience of using influenza antivirals approved in Japan from a patient perspective using a conjoint analysis.
Methods
An online survey (August 2020) was performed on individuals aged 20–64 years living in Japan who had taken oral or inhalant antivirals for influenza treatment in the 2018/19 or 2019/20 seasons. Efficacy and safety were assumed to be equivalent among the antivirals. The attributes for the conjoint analysis included route (oral or inhalant), duration, frequency of administration, and out-of-pocket expenses. A conditional logit model was applied as a baseline model. The monetary value of each attribute was calculated by comparing the same utility of the linearly interpolated level of the out-of-pocket attribute. Another survey to determine the experiences of the latest antiviral intake was also conducted on the same respondents.
Results
Of the respondents, 1,550 were men and 1,587 were women. The monetary value for oral antivirals was estimated to be higher, saving JPY 741 (USD 7.06, as of August 2020), compared with inhalant. Regarding the length and frequency of administration, five days corresponds to an increase of JPY 2,072, compared with one day, and twice a day corresponds to a JPY 574 increase compared to once a day.
Conclusions
The results suggest that – among the antivirals approved in Japan – the monetary value of the utility is the highest in the single dose oral antiviral, baloxavir marboxil (baloxavir). Although the drug cost was highest in baloxavir among the brand antivirals, the difference in the value of utility for influenza patient was estimated to be larger than the difference in the drug costs.
Limitations
Although individuals with diverse attributes from all over the country were included in the survey, they are not necessarily a representative population of the Japanese society.
Introduction
Each year, the influenza virus attacks 10–20% of people worldwideCitation1, resulting serious symptoms in 3 to 5 millionCitation2. Although the duration of illness is only 1 to 2 weeks, influenza’s burden of illness is the highest among all infectious diseases due to its high incidenceCitation3. In addition, the current COVID-19 pandemic has raised concerns about the possibility of co-infection with influenzaCitation4. The Infectious Diseases Society of America’s clinical guidelines for managing influenza recommend the use of antiviral treatmentCitation5. Antiviral treatment within 48 h of illness onset can shorten the duration of fever and other symptomsCitation6,Citation7. Moreover, antivirals can reduce viral shedding, suggesting that they may lead to suppression of transmissionCitation8.
There are two classes of antivirals available for influenza A and influenza B. One is neuraminidase inhibitors (NAIs), such as oseltamivir, zanamivir, peramivir, and laninamivir; these inhibit the release of new virus from the cell surface. The other is the cap-dependent endonuclease inhibitor baloxavir, which inhibits the initiation of viral mRNA synthesis. Among the series of antivirals, there are various dosage forms for administration. Oral oseltamivir and inhaled zanamivir are required twice daily for 5 consecutive days, and adherence – which is an important factor of successful therapy – could influence the success of treatmentCitation9. Peramivir can be used intravenously once daily or repeated administration. Laninamivir and baloxavir are both single dose antivirals; the former is inhaled, and the latter taken orally.
In Japan, baloxavir and a generic drug of oseltamivir were approved in February and June 2018 respectively, and five brand-name antivirals and the generic oseltamivir were used for the treatment of 12.9 million people in the 2018/2019 seasonCitation10. Physicians most often choose a suitable drug for each patient; however, we think that each patient has their own preference for drug characteristics based on dosage forms. Quantification of patient preferences for the drugs can be used to enhance patient-centered care for influenza medication. However, to the best of our knowledge, no study has explicitly investigated the value of each antiviral based on patient preference.
This study aims to estimate the monetary value of the convenience of antivirals approved in Japan from the perspective of patients using a conjoint analysis. We performed the conjoint analysis on the combined dosage forms and out-of-pocket cost of the drugs and a survey of recent experiences of antiviral intake among Japanese adults who had taken antivirals in the recent two seasons. Thus, we revealed the differences in the values of patient preferences by the dosage forms of antivirals.
Methods
Study design
We evaluated the monetary value of the convenience of antivirals for influenza treatment in patients through the conjoint analysis based on an online survey. Conjoint analysis is an established research method and is increasingly applied in the medical field to assess value from the patient’s perspectiveCitation11. In addition, good research practices for the conjoint analysis have been identified by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR)Citation12. We assumed that efficacy and safety is equivalent among the antivirals in this survey. We also used a survey to examine the most recent experiences of antiviral intake during the period of taking antivirals and later.
This study was performed in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects by the Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and WelfareCitation13, and the checklist of good research practices for the conjoint analysis by ISPORCitation12. Informed consent was obtained from all respondents online. The study was approved by the Ethics Committee of the Research Institute of Healthcare Data Science (RI2020006) and registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN000041452).
Data source and participants
We used an online panel managed by INTAGE Inc. (Tokyo, Japan) for the online survey. The panel included individuals who had registered as members to participate in the online surveys in advance. The respondents for the survey were individuals aged 20–64 years living in Japan who had taken oral or inhalant antivirals for influenza treatment in the 2018/19 or 2019/20 season. It is noted that the respondents were limited to those who were treated with oral or inhalant antivirals; those who had taken intravenous infusion antivirals, which has also been available for influenza treatment, were excluded. This is because we considered that intravenous infusion antivirals may be used for hospitalized patients or those with difficulty in oral ingestion. In the guidelines of the Japanese Association for Infectious Diseases, it is suggested that the peramivir, which is an intravenous infusion antiviral, should be prescribed for outpatients when the physician determine that intravenous infusion treatment is applicable after careful consideration of the indications for other antivirals, such as oral and inhaled antiviralsCitation14. Consequently, patients treated with it may have different characteristics from those who had taken oral or inhalant antivirals. The age restriction was applied because people outside the specified age range might have difficulty in appropriately answering all the questions in both surveys of the conjoint analysis and their experiences of antiviral intake. We planned to include at least 3,000 individuals to obtain robust results. Because these surveys included variety of questions, the sample size was not set based on a calculation of sample size power, but set at as many as possible within the budget. The online survey was conducted in August 2020.
Survey for the conjoint analysis
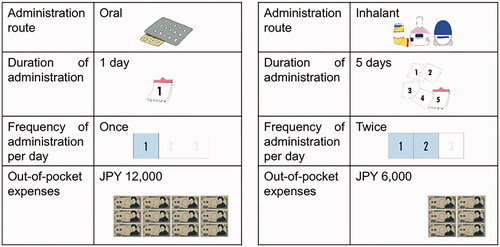
A conjoint analysis was performed to estimate the monetary value of the convenience of antivirals. describes the attributes (dosage forms [administration route, duration of administration, and frequency of administration per day] and out-of-pocket expenses) and the levels for each attribute for the analysis. The attributes and levels were selected through a discussion with clinicians and an expert in medical economics. Sixteen choice tasks were created from 72 possible combinations based on an orthogonal design. A representative example of the choice task questions is shown in . Respondents were provided with two options with question sentences and were required to choose the preferable one between them. The question sentences are as follows:
Table 1. Attributes and attribute levels in the survey for the conjoint analysis.
You are a patient with influenza and going to be treated with an antiviral drug. Which of the following drugs do you choose for the treatment? It takes 3 days to recover from the influenza symptoms, such as fever and a sore throat, from the initiation of the treatment with either drug. Note that there are two types of administration routes in the treatment options.
The administration routes were explained with pictures along with the sentences. Each respondent completed 20 choice tasks.
Survey about the experiences of antiviral intake
We conducted a survey to determine the experiences of the latest antiviral intake, including the period of taking the drug, dosage form of the drug, whether all the drugs were taken or not (percentage of drugs taken of all the prescribed drugs), situation and feeling when taking the drugs, number of days until breaking the fever, and number of days until returning to usual life. The options for dosage form were based on the available antivirals in Japan. These include baloxavir: oral and single dose, oseltamivir: oral and twice daily for 5 days (hereafter called “5-day dose”), laninamivir: inhalant and single dose, and zanamivir: inhalant and 5-day dose. The actual question sentences are listed in Table S1 of the Supplementary material.
Statistical analysis
In conjoint analysis, the utility of each level of each attribute was estimated. We applied a conditional logit model as a baseline model for the conjoint analysis. The model elucidates the hidden utility behind each level of each attribute by inverse estimation, based on each choice between the two options. In this model, the utility was assumed to be the same among the respondents. The utility other than the monetary attribute (out-of-pocket expenses) was converted to monetary equivalent by comparing the same utility of the linearly interpolated level of the monetary attribute. Statistical significance was defined as p<.05. We also applied the models with interaction terms, and these models were stratified by groups based on the attributes of the respondents for the analyses.
The answers to questions about experiences of antiviral intake were tabulated by dosage forms of the latest antivirals that the respondents took and the attributes of the respondents. We also calculated the average and standard error (SE) of the number of days until fever resolution and patients returning to their usual life from the first medication based on the answers. In these calculations, we considered “5 days or longer” as 5 days, and “7 days or longer” as 7 days. We excluded respondents who answered, “not sure”.
To support an interpretation of the results, we calculated the productivity loss for each available antiviral in Japan by applying the average number of days until returning to usual life from initiation of the antiviral treatment (calculated above) according to the Japanese Guideline for Cost-Effectiveness EvaluationCitation15. We assumed the monthly average wage for each person to be Japanese Yen (JPY) 307,700, which is the average wage across all industries, all ages, and both genders obtained from the Japanese Basic Survey on Wage Structure in 2019Citation16.
We used MS Excel 2016 (Microsoft, Redmond, WA) and R software ver. 3.6.3 (The R Foundation for Statistical Computing, Vienna, Austria) for the analyses.
Results
Respondents
We collected the answers from 3,137 individuals including 1,550 men (25 percentile, median, 75 percentile, and average ± standard deviation (SD) of age: 32, 42, 52, and 42.1 ± 12.1 years) and 1,587 women (32, 42, 51, and 41.9 ± 11.6 years; ). The number of respondents by attributes – including living prefectures, marital status, occupation, and number of children living together – are shown in Table S2.
Table 2. Number of respondents by age and gender.
describes the number of respondents by dosage forms as a combination of route and dose. The number of respondents with the single dose inhalant antiviral was the largest (916), followed by that with 5-day dose oral antiviral (908), single dose oral antiviral (579), and 5-day dose inhalant antiviral (266).
Table 3. Number of respondents by dosage forms after cross-tabulation based on answers to questions (Q5) “Which type of antiviral agent did you take the last time?” and (Q6) “What kind of dosage did you take of (or how to use) the antiviral agent?”
Monetary value for utility of antivirals based on the conjoint analysis
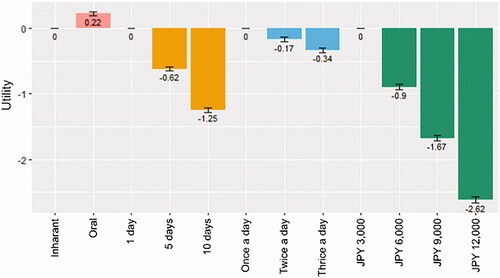
The utility of each attribute and level based on the logit model is shown in . We found that higher out-of-pocket expenses, longer duration of administration, and more frequent administration per day were associated with larger negative utility; moreover, the impact of the length of duration on the utility was shown to be greater than that of the frequency. The utility was larger in the oral than in the inhalant antiviral. The differences in the utility between levels were statistically significant (p<.001) for all attributes.
We estimated the monetary value for each attribute by level, as shown in . The oral antiviral saves JPY 741 (US Dollar 7.06, as of August 2020) compared with the inhalant. Regarding the length and frequency of administration, compared to 1 day, 5 days and 10 days correspond to a JPY 2,072 increase and JPY 4,172 increase, respectively; moreover, compared to once a day, twice a day and thrice a day correspond to a JPY 574 increase and JPY 1,129 increase, respectively.
Table 4. Monetary value of each level of each attribute estimated by the baseline model.
We obtained the utility of attributes including the combination of the administration route and duration of administration (Figure S1A) and the combination of the duration and frequency of administration (Figure S1B) from the model with the minimum Akaike information criterion (AIC) and second minimum AIC of all 64 models with possible combinations, respectively. The superiority of oral to inhalant tended to be reduced with an increase in duration (from 0.23 for 1 day to 0.11 for 10 days as utility; Figure S1A), and a shorter duration tended to be preferred, regardless of the frequency of administration (Figure S1B).
Almost the same tendency in the value of convenience related to the dosage form was found regardless of the attributes of respondents; however, some differences in the size of the values were observed in some sub-groups stratified by the attributes (Figure S2). The size of the values of convenience related to the dosage forms was relatively similar among age and gender groups, but the utility value for out-of-pocket expenses tended to be more negative in groups of women aged 20–49 years compared with women in other age bands and groups of men (Figure S2A). In analyses for more stratified groups, the tendency of greater negative value of the utility for out-of-pocket expenses was shown to be marked when the groups of women aged 20–49 years were married (Figure S2B) or had children living together (Figure S2C). By stratifying respondents by the number of children living together, the value for oral to inhalant tended to decrease, corresponding to the number of children (Figure S2D). By stratifying occupations, a greater negative value for an increase in out-of-pocket expenses tended to be shown for self-employed individuals (including freelance and professional workers), part-time employees, and house-wives/husbands (Figure S2E). The value for oral to inhalant tended to be greater in directors/managers and the self-employed; relatively small in regular employees, civil servants, and contractors; and negative in students (Figure S2E). We stratified the respondents into three groups according to the level of average salary in the prefectures where they livedCitation17, and we compared the values for the attributes among the groups. The negative value for out-of-pocket expenses tended to be higher in the lower average salary prefecture group; no such tendency was seen for attributes related to dosage forms (Figure S2F).
Experience of antiviral intake based on the survey
We examined the experience of antiviral intake – including medication adherence, difficulty in taking the drug, feeling of worry for vomiting or taking not enough the drug, and number of days to break the fever and return to the usual life – by attributes and dosage forms of the antivirals most recently taken by the respondents. In respondents who took the 5-day dose antivirals, the percentage of those who answered that they took all drugs as instructed was 94.6% for oral and 93.6% for inhalant. In those who did not take all the drugs, the percentage of drugs they took was 70% or larger in more than half of those with oral antivirals and 60% or larger in more than half of those with inhalants (Figure S3).
Figure 3. Difficulty in taking the drug (answer to Q 8-1: “Was it easy to take the antiviral that you took the last time?”) (A), and reasons for the difficulty (answer to Q 8-2: “Why did you answer ‘Neither’ or ‘No’ to Q8-1 regarding the ease of taking the antiviral that you took the last time?”) by type of drug (B). Multiple choices were allowed. Denominator: All respondents according to the type of dosage form of the drugs.
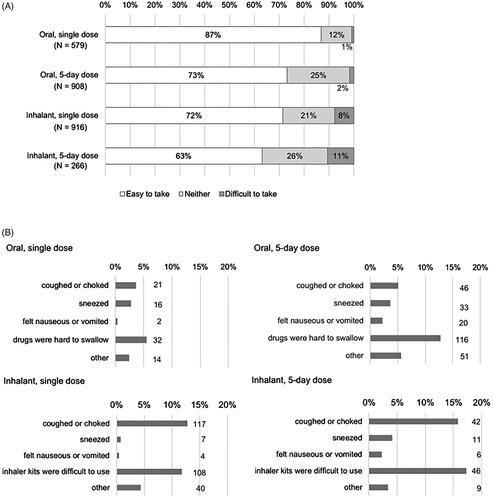
illustrates the difficulty of taking the drug in different dosage forms. The percentage of respondents who answered “easy to take” was larger in those who took oral drugs (87% for the single dose and 73% for the 5-day dose) than those who used inhalants (72 and 63%, respectively), and larger for those with a single dose than for those with a 5-day dose. The reasons for the answer “difficult to take” or “neither” were similar between those with the same route of administration, regardless of the dose; more common reasons were “hard to swallow” for oral drugs and “cough or choke” and “difficulty to use the inhaler kits” for inhalants ().
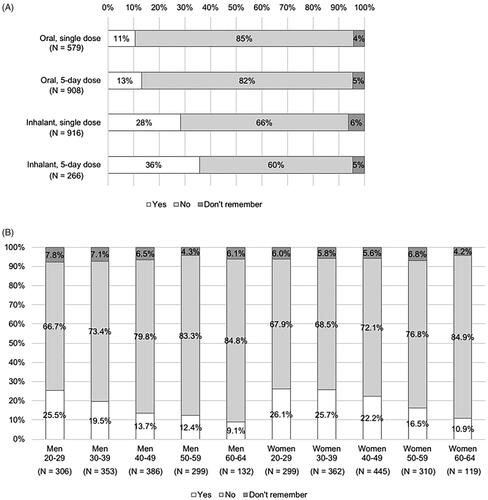
The percentage of respondents who felt worried when taking the drugs was lower in those taking oral antivirals (11% for the single dose and 13% for the 5-day dose) than those taking inhalants (28% for the single dose and 36% for the 5-day dose), as well as in those with a single dose than those with a 5-day dose (). Considering age and gender, younger respondents tended to be associated with a higher percentage of those who felt worried in both genders (). This tendency was observed in both oral and inhalant antivirals.
Figure 4. Feeling of worry about taking the drug (answer to Q 9: “When you were taking the antiviral for influenza the last time, were you worried that you would vomit out the drugs or you did not take (inhale) enough of the drugs?”) by type of dosage form of the drugs (A) or by age and gender (B).
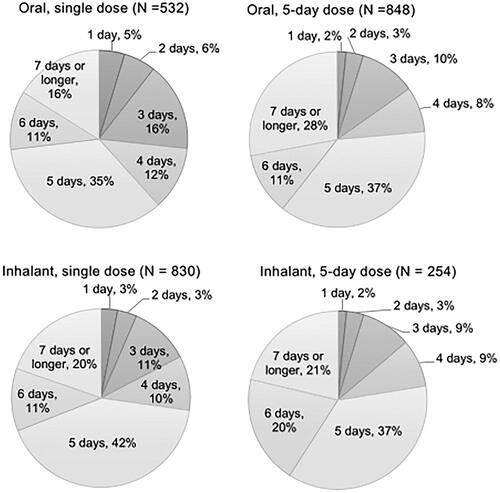
Considering the number of days to fever resolution from initiation of the antiviral, the most frequent answer was 1 day in respondents with oral single dose antiviral, and 2 days in those with other antivirals (). The number of respondents who answered “not sure” was 55 for single dose oral antiviral, 82 for 5-day dose oral antiviral, 98 for single dose inhalant antiviral, and 19 for 5-day dose inhalant antiviral. When comparing the demographics of the respondents between those who answered, “not sure” and others, the age was similar; average ± SD of age was 41.9 ± 11.9 and 41.9 ± 11.6 (p=.50), but the percentage of woman was higher in those who answered “not sure”, 59%, than others, 49% (p<.01). After excluding the respondents who answered “not sure”, the 25 percentile, median, 75 percentile, and average ± SE of the number of days was 1, 2, 2, and 1.87 ± 0.04 days for single dose oral antiviral, 2, 2, 3, and 2.47 ± 0.04 days for 5-day dose oral antiviral, 1, 2, 3, and 1.99 ± 0.03 days for single dose inhalant antiviral, and 2, 2, 3, and 2.40 ± 0.06 days for 5-day dose inhalant antiviral.
Figure 5. Number of days to fever resolution from initiation of the antivirals (answer to Q 10: “When you were taking the antiviral for influenza the last time, how long did it take to break your fever after the first dose?”) by dosage form of the drugs identifying the percentages of respondents excluding those who answered “not sure” for each answer. N: number of respondents excluding “not sure” (oral, single dose: 55; oral, 5-day dose: 82; inhalant, single dose: 98, and inhalant, 5-day dose: 19).
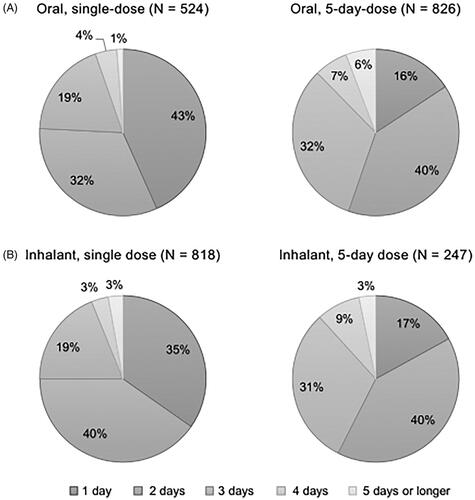
The most frequent answer for the number of days to return to usual life (working or attending school) from initiation of the antiviral was 5 days for all types of antivirals (). Respondents with single dose oral antiviral had the highest percentage of those who answered 1–3 days (27%) to the question among the antivirals (). After excluding patients who answered “not sure” (47and 60 individuals for single and 5-day dose oral antivirals, and 86 and 12 individuals for single and 5-day dose inhalant antivirals, respectively), the average ± SE of the number of days was 3, 5, 6, and 4.63 ± 0.07 days for single dose oral antiviral, 5, 5, 7, and 5.22 ± 0.05 days for 5-day dose oral antiviral, 4, 5, 6, and 4.97 ± 0.05 days for single dose inhalant antiviral, and 5, 5, 6, and 5.20 ± 0.09 days for 5-day dose inhalant antiviral. Notably the age was similar between the respondents who answered, “not sure” and others (41.4 ± 11.4 and 42.0 ± 11.7, p=.20), while the percentage of woman was higher in those who answered “not sure” than others (60 and 49%, p<.01).
Figure 6. Number of days to return to the usual life from initiation of the antivirals (answer to Q 11: “When you were taking the antiviral for influenza the last time, how long did it take to return to your usual life (working or attending school) after the first dose?”) by type of dosage form of the drugs identifying the percentages of respondents excluding those who answered “not sure” for each answer. N: number of respondents excluding “not sure” (oral, single dose: 47; oral, 5-day dose: 60; inhalant, single dose: 86, and inhalant, 5-day dose: 12).
Discussion
We estimated the monetary value of the convenience of antivirals based on the conjoint analysis. Higher out-of-pocket expenses, longer duration of administration, and more frequent administration per day were shown to be associated with lower utility value. The impact on the value was greater based on the duration compared with the frequency per day. These results are reasonable when considering the convenience of the patients. In addition, results of the survey about the experience of antiviral intake in this study suggested that longer duration with more frequent administration is associated with difficulty and feeling of worry to take the drugs even between the same routes of administration. The likely reason for these survey results is the number of times patients experienced difficulty and feelings of worry. The differences in the devices to inhale the drugs for different dosage forms may also be associated with the results.
When comparing oral and inhalant antivirals, a higher value of convenience was estimated in oral administration by conjoint analysis (). This result is consistent with the results regarding the difficulty and feelings of worry about taking the drugs in the surveyCitation18. Moreover, the answer about the feelings of worry for inhalants is consistent with the complaints often made by patients stating that they are not sure if they could inhale the drugs properly in clinical practiceCitation19,Citation20.
The trend in the value for convenience was not much different between all respondents and those in each sub-group stratified by the attributes of the respondents; however, the size of the value was diverse among the sub-groups (Figure S2). A reduction in the value for oral associated with the number of children living together is probably because the inhalants are often used for children in JapanCitation21, which means that parents are used to the inhalants. The relatively lower and negative value for oral to inhalant in the respondents in their 20 s and students, respectively, may also be for the same reason; they easily remember the experiences when they were children. We found differences in the size of utility of the dosage forms and out-of-pocket expenses among occupations of the respondents. In regular employees, directors/managers, and civil servants, which could be considered to have higher income and/or employment stability, lower negative values of out-of-pocket expenses were shown in all three categories of occupations; however, the size of the value of dosage forms differed among the occupations. These results suggest that the value of convenience related to dosage forms may be associated with factors other than the amount of income and employment stability. Regarding the amount of income, no association was observed between the value of dosage forms and level of salary in the prefectures.
Adherence of more than 90% was indicated for drugs with a 5-day dose in the survey. This is rather high, compared with that of oseltamivir, which is reported as ranging from 30 to 88% for all and 70–80% for most studies in an existing systematic review including both treatment and prophylaxis for influenzaCitation22; and as 78.9% in a study based on a surveyCitation23. Considering the reported number of days until fever resolution, about 2 days in this survey, most respondents were supposed to continue taking the medication after fever resolution. In Japan, people developing influenza are suspended from the workplace or school until 2 or 3 days after fever resolutionCitation24,Citation25. Therefore, it is reasonable that patients continue the medication during recuperation at home, even after fever resolution. Another possible reason for the high compliance is the respondents’ characteristics; people who answer the survey may tend to be earnest and take the drugs properly. If so, adherence in this study could be higher than that in Japanese patients. As described above, the results regarding the difficulty and feeling of worry when taking the drug could be considered consistent with the results of the conjoint analysis. In addition, we found that younger respondents tended to have feelings of worry, probably due to the amount of experience in the past; generally, older people have more opportunities to take drugsCitation26.
The average number of days until fever resolution was shorter in single dose antivirals than in 5-day dose antivirals in both oral and inhalant, and also that was shorter in single dose oral administration than in single dose inhalant antiviral (). This result is similar to that of a 2020 Japanese multicenter observational study that included 295 patients aged 0–91 years oldCitation27. In that study, the average ± SD time to the alleviation of the fever was reported to be 1.94 ± 0.09 days for 111 patients prescribed baloxavir (a single dose oral antiviral) and 2.35 ± 0.08 days for those prescribed NAIs (oseltamivir: 74 patients, zanamivir: 24 patients, peramivir: 4 patients, and laninamivir: 77 patients), and the difference was statistically significant (p=.002). Shorter time to fever resolution in baloxavir (median [interquartile range]: 1.0 [1.0–2.0] days) than in laninamivir (2.0 [1.5–3.5] days, p=.032) was also reported in another observational study including 43 adult patients treated with baloxavir (14 patients), laninamivir (16 patients), or oseltamivir (13 patients) Citation28. The median time for baloxavir was also shorter than that for oseltamivir (3.0 [1.0–3.0] days), although the difference was not statistically significant (p=.067).
The time to return to usual life was also shorter in respondents with the single dose oral antiviral than in those with others in our study (). Because we did not examine factors indicating the severity of influenza in this study, the comparison while adjusting for confounding factors could not be conducted for the differences in the number of days among different dosage forms. Considering the situation in Japanese clinical practice, it is unlikely that baloxavir is more often prescribed for patients who have mild symptoms and/or are easy to recover than other antivirals. We should also consider the possibility of the effect of recall bias; patients who took antivirals with shorter duration of administration might recall the length of duration to be shorter. However, the duration until returning to usual life was not so different between inhalants with different durations of administration.
To interpret the results, we calculated the cost for influenza per patient for available antivirals as follows (). Here, we assumed that there were no confounding factors for treatment choice among respondents:
Table 5. Estimated total cost of the drug, utility, and productivity loss for influenza per patient for available antivirals in Japan.
total drug costCitation29,Citation30 – monetary value of the utility of convenience estimated by the conjoint analysis + productivity loss estimated based on the survey.
The drug cost of baloxavir was calculated with the prescription dosage at 40 mg based on the average body weight of Japanese individualsCitation31. The dosage increases to 80 mg for patients weighing more than 80 kg. The cost, calculated as
drug cost – monetary value of utility
was JPY 1,491 for baloxavir, which is lower than that of other brand drugs (JPY 1,931 for oseltamivir, JPY 1,713 for laninamivir, and JPY 2,882 for zanamivir) but higher than that of generic oseltamivir, JPY 540. The cost, after including the productivity loss, was the lowest for baloxavir (JPY 48,306) among all the antivirals, including generic oseltamivir, JPY 53,352.
This study has several limitations. First, it was based on an online survey of internet panels, who were registered as members to participate in online surveys in advance, provided by a survey company. Although we included respondents with a wide range of attributes – men and women aged 20–64 years from all over the country – they are not necessarily a representative population of Japanese society. In fact, when comparing the age distribution (20–60s) between the respondents of this study and the whole population of patients with influenza in Japan based on the national surveillanceCitation32, the percentage of the respondents in the 20 s was higher (20 vs. 16%, p<.01), and that in the 40 s and the 60 s was lower (27 vs. 30%, p=.03, and 8 vs. 12%, p=.02, respectively) in the respondents of this study than that in the whole population. The difference might be caused by the characteristics of the survey using internet. Second, reliability of the study results depends on whether the respondents accurately understood and to what extent they veraciously answered the questions. Particularly in the survey about the latest antiviral intake, we should consider the possibility that the respondents remembered concomitant drugs taken as needed, other than the antivirals, or that they were influenced by their recall bias. Third, we targeted people who had taken antivirals for influenza for not only the survey about the experience but also the conjoint analysis. Although, for the conjoint analysis, it could be another option that not to limit to those who had experience in taking the antivirals, we considered that the people who had experiences in taking antivirals were supposed to give more reliable answers when selecting their preference from two options of choice tasks because they could imagine the actual treatment. It should be noted that in Japan, most patients with influenza received antivirals as treatmentCitation33; therefore, we considered that limiting the sample to them would not have a significant negative effect on this study’s generality. Finally, we showed the results from the survey about the experiences of antiviral intake based on the assumption of no confounding effects between the choice of the antivirals and answers to the questions; thus, we did not adjust for confounding factors in this analysis.
Conclusions
Higher monetary value of convenience was estimated in oral antivirals than inhalants based on the conjoint analysis. Higher value was also estimated for those with single dose intake compared with those with a 5-day dose, in both oral and inhalant antivirals. Among the brand antivirals approved in Japan, the monetary value of the utility of baloxavir was suggested to be the highest and the difference in the value of utility was larger than the difference in drug costs with all the brand antivirals. Moreover, the monetary value of utility is lower than the difference in drug cost between baloxavir and generic drug of oseltamivir. When assuming that there were no confounding factors for treatment choice among respondents, the total cost for influenza per patient – including productivity loss calculated based on the survey – was estimated to be the lowest in baloxavir among all antivirals, including generic oseltamivir. From the perspective of patient-centered care, a treatment choice considering the convenience for each patient is important. Thus, our results will help physicians choose antivirals for influenza patients.
Transparency
Declaration of funding
This study was supported by Shionogi & Co., Ltd.
Declaration of financial/other relationships
AY, SH, and HI are or were employed by Shionogi & Co., Ltd. KI and TT are employees of Milliman Inc., which has received consultancy fees from Shionogi & Co., Ltd. AY, SH, and HI report company stock in Shionogi & Co., Ltd. TT reports grants and personal fees from MSD Co Ltd and Sumitomo Dainippon Pharma Co, Ltd and personal fees from Pfizer Inc outside the submitted work. HM has received honoraria for lecturing and research grants from Shionogi and Chugai Pharmaceutical Co, Ltd and grants and honoraria for lecturing from Daiichi Sankyo Co, Ltd. HM also reports personal fees from AbbVie GK, Asahi Kasei Pharma Corporation, Astellas Pharma Inc, AstraZeneca K.K., Bristol-Myers Squibb, Eli Lilly Japan K.K., FUJIFILM Toyama Chemical Co, Ltd, Gilead Sciences Inc, Insmed Incorporated, Janssen Pharmaceutical K.K., Kyorin Pharmaceutical Co, Ltd, Meiji Seika Pharma Co, Ltd, Mitsubishi Tanabe Pharma Corporation, MSD Co, Ltd, Nihon Pharmaceutical Co, Ltd, Nippon Boehringer Ingelheim Co, Ltd, Novartis Pharma K.K, Pfizer Inc, Sumitomo Dainippon Pharma Co, Ltd, Taiho Pharmaceutical Co, Ltd, Taisho Pharma Co, Ltd, Teijin Home Healthcare Ltd, and Toa Shinyaku Co, Ltd and grants from Asahi Kasei Pharma Corporation, Astellas Pharma Inc, FUJIFILM Toyama Chemical Co, Ltd, Kyorin Pharmaceutical Co, Ltd, Meiji Seika Pharma Co, Ltd, Pfizer Inc, Taiho Pharmaceutical Co, Ltd, Taisho Pharma Co, Ltd, Teijin Pharma Ltd, Toa Shinyaku Co, Ltd, and Torii Pharmaceutical Co, Ltd outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
NH, TT, AY, SH, HI, and HM contributed to the study conception. All authors contributed to the study design and interpretation of data. Data analysis was performed by KI. The first draft of the manuscript was written by TT and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (742.5 KB)Acknowledgements
The authors are grateful to Dr. Ataru Igarashi for valuable advice and discussion on the conjoint analysis. We thank INTAGE healthcare Inc. for execution of online survey and Editage for English language editing.
References
- Somes MP, Turner RM, Dwyer LJ, et al. Estimating the annual attack rate of seasonal influenza among unvaccinated individuals: a systematic review and meta-analysis. Vaccine. 2018;36(23):3199–3207.
- World Health Organization. Influenza (Seasonal). Geneva (Switzerland): World Health Organization; 2018.
- Cassini A, Colzani E, Pini A, et al. Impact of infectious diseases on population health using incidence-based disability-adjusted life years (DALYs): results from the Burden of Communicable Diseases in Europe study, European Union and European Economic Area countries, 2009 to 2013. Euro Surveill. 2018;23(16):17–00454.
- Cuadrado-Payán E, Montagud-Marrahi E, Torres-Elorza M, et al. SARS-CoV-2 and influenza virus co-infection. Lancet. 2020;395(10236):e84.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal Influenzaa. Clin Infect Dis. 2019;68(6):895–902.
- Taieb V, Ikeoka H, Ma FF, et al. A network meta-analysis of the efficacy and safety of baloxavir marboxil versus neuraminidase inhibitors for the treatment of influenza in otherwise healthy patients. Curr Med Res Opin. 2019;35(8):1355–1364.
- Taieb V, Ikeoka H, Wojciechowski P, et al. Efficacy and safety of baloxavir marboxil versus neuraminidase inhibitors in the treatment of influenza virus infection in high-risk and uncomplicated patients – a Bayesian network meta-analysis. Curr Med Res Opin. 2020. DOI:https://doi.org/10.1080/03007995.2020.1839400
- Du Z, Nugent C, Galvani AP, et al. Modeling mitigation of influenza epidemics by baloxavir. Nat Commun. 2020;11(1):2750.
- Flicoteaux R, Protopopescu C, Tibi A, et al. Factors associated with non-persistence to oral and inhaled antiviral therapies for seasonal influenza: a secondary analysis of a double-blind, multicentre, randomised clinical trial. BMJ Open. 2017;7(7):e014546.
- Ministry of Health, Labour and Welfare. Status of the use of antivirals for influenza in the 2018/2019 season. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2019.
- Lee PY, Matchar DB, Clements DA, et al. Economic analysis of influenza vaccination and antiviral treatment for healthy working adults. Ann Intern Med. 2002;137(4):225–231.
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health-a checklist: a report of the ISPOR good research practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413.
- Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare. Ethical guidelines for medical and health research involving human subjects. Tokyo (Japan): Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labor and Welfare; 2014.
- The Japanese Association for Infectious Diseases. Guidelines for the use of anti-influenza drugs. Tokyo (Japan): The Japanese Association for Infectious Diseases; 2019.
- Center for Outcomes Research and Economic Evaluation for Health. Guideline for preparing cost-effectiveness evaluation to the Central Social Insurance Medical Council. Wako (Japan): Center for Outcomes Research and Economic Evaluation for Health; 2019.
- Ministry of Health, Labour and Welfare. Japanese basic survey on wage structure on 2019. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2020.
- Ministry of Health, Labour and Welfare. Japanese basic survey on wage structure on 2018 by prefectures. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2019.
- Mäkelä MJ, Backer V, Hedegaard M, et al. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938.
- Laube BL, Janssens HM, de Jongh FH, International Society for Aerosols in Medicine, et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331.
- Arakawa H, Hamasaki Y, Kohno Y, et al. Japanese guidelines for childhood asthma 2017. Allergol Int. 2017;66(2):190–204.
- Smith LE, D'Antoni D, Jain V, et al. A systematic review of factors affecting intended and actual adherence with antiviral medication as treatment or prophylaxis in seasonal and pandemic flu. Influenza Other Respir Viruses. 2016;10(6):462–478.
- Ushijima K, Higuchi S, Fujimura A. Survey of compliance with oseltamivir phosphate therapy in Japan. Am J Ther. 2009;16(1):8–10.
- Ministry of Education, Culture, Sports, Science, and Technology. Enforcement of the School Health and Safety Act, Chapter 3. Tokyo (Japan): Ministry of Education, Culture, Sports, Science, and Technology; 2019.
- Ministry of Health, Labour and Welfare. Q&A on influenza, FY 2019. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2019.
- Mabuchi T, Hosomi K, Yokoyama S, et al. Polypharmacy in elderly patients in Japan: analysis of Japanese real-world databases. J Clin Pharm Ther. 2020;45(5):991–996.
- Yoshii N, Tochino Y, Fujioka M, et al. The comparison of the efficacy of baloxavir and neuraminidase inhibitors for patients with influenza A in clinical practice. Intern Med. 2020;59(12):1509–1513.
- Yoshino Y, Kitazawa T, Ota Y. Clinical efficacy of baloxavir marboxil in the treatment of seasonal influenza in adult patients: a prospective observational study. Int J Gen Med. 2020;13:735–741.
- Ministry of Health, Labour and Welfare. National health insurance drug price list 2020 August (oral). Tokyo (Japan): Ministry of Health, Labour and Welfare; 2020.
- Ministry of Health, Labour and Welfare. National health insurance drug price list 2020 August (topical agent). Tokyo (Japan): Ministry of Health, Labour and Welfare; 2020.
- Ministry of Health, Labour and Welfare. The national health and nutrition survey in Japan, 2018. Tokyo (Japan): Ministry of Health, Labour and Welfare; 2020.
- National Institute of Infectious Diseases; Division of Tuberculosis and Infectious Disease Control, Ministry of Health, Labour and Welfare. Influenza in this season (2019/2020 season). Tokyo (Japan): National Institute of Infectious Diseases; Division of Tuberculosis and Infectious Disease Control, Ministry of Health, Labour and Welfare; 2020.
- Zaraket H, Saito R. Japanese surveillance systems and treatment for influenza. Curr Treat Options Infect Dis. 2016;8(4):311–328.