Abstract
Objective
The mortality benefit of implantable cardioverter defibrillators (ICDs) for primary prevention (PP) of sudden cardiac arrest (SCA) has been well-established, but ICD therapy remains globally underutilized. The results of the Improve SCA study showed a 49% relative risk reduction in all-cause mortality among ICD patients with 1.5 primary prevention (1.5PP) characteristics (patients with one or more risk factors, p < 0.0001). We evaluated the cost-effectiveness of ICD compared to no ICD therapy among patients with 1.5PP characteristics in three Latin American countries and analyzed the factors involved in cost-effectiveness.
Methods
We used a published Markov model that compares costs and outcomes of ICD to no ICD therapy from local payers’ perspective and included country-specific and disease-specific inputs from the Improve SCA study and current literature. We used WHO-recommended willingness-to-pay (WTP) thresholds to assess cost-effectiveness and compared model outcomes between countries.
Results
Incremental costs per QALY (quality-adjusted life year) saved by ICD compared to no ICD therapy are Colombian Pesos COP$46,729,026 in Colombia, Mexican Pesos MXN$246,016 in Mexico, and Uruguayan Pesos UYU$1,213,614 in Uruguay in the base case scenario; all three figures are between 1–3-times GDP per capita for each country. One-way and probabilistic sensitivity analyses confirm the base case scenario results. Non-cardiac accumulated deaths are lower in Mexico, resulting in a comparatively increased cost-effective ICD therapy.
Limitations
The Improve SCA study was not randomized, so clinical results could be biased; however, measures were taken to reduce this bias. Costs and benefits were modelled beyond the timeline of direct observation in the Improve SCA study.
Conclusions
ICD therapy is cost-effective in Mexico and Uruguay and potentially cost-effective in Colombia for a 1.5PP population. Variability in ICER estimates by country can be explained by differences in non-cardiac deaths and cost inputs.
JEL Classification Codes:
Introduction
Implantable cardioverter defibrillators (ICD) therapy has been well-established as the gold standard for prevention of sudden cardiac death (SCD) in a primary and secondary prevention population with a high risk of ventricular tachyarrhythmias (VT/VF)Citation1–3. Primary prevention ICD therapy efficacy in patients with moderately symptomatic heart failure and reduced systolic function is well-established through multiple randomized clinical trialsCitation4,Citation5 and confirmed in real-world observational evidenceCitation1. This evidence has been used to establish strong recommendations for ICD use in society guidelines internationallyCitation2,Citation3 and has been leveraged to establish the cost-effectiveness of ICD therapy in multiple healthcare systemsCitation6,Citation7. However, ICD therapy for SCD prevention remains underutilized globally, due in part to a lack of reimbursement and cost considerations.
The Improve SCA studyCitation8 was a prospective, non-randomized, non-blinded, multi-center, global study which enrolled (n = 3,889) patients from Asia, Latin America, Eastern Europe, the Middle East, and Africa, and categorized patients by their prevention level (primary and secondary) and by ICD implantation condition (with and without an ICD). This study identified a high-risk subset of patients in primary prevention called 1.5 primary prevention (1.5PP) based on the presence of at least one of the following documented risk factors: non-sustained ventricular tachycardia (NSVT), frequent premature ventricular contractions (PVCs) >10/h, left ventricular ejection fraction (LVEF) <25%, pre-syncope, or syncopeCitation9. The objectives of this study were to compare the time to first therapy between patients with 1.5PP characteristics and patients with primary (risk of sustained ventricular arrhythmias) and secondary prevention characteristics (history of sustained ventricular arrhythmias), and to compare the mortality rates between patients with 1.5PP characteristics with an ICD and those without an ICD. Improve SCA patients with 1.5PP characteristics were found to have a higher rate of appropriate therapy than patients with primary prevention characteristics. Moreover, patients with 1.5PP characteristics with an ICD experienced a 49% relative risk reduction in all-cause mortality compared to patients without an ICD (HR = 0.51; 95% CI = 0.40–0.66, p < 0.001)Citation8.
Countries in Latin America allocate sizeable public resources towards the financing of defined health benefit plans (of public health expenditures, more than 70% in Colombia, 72% in Uruguay, and 28.1% in Mexico are allocated towards this goal), which have become instruments of equity in heath spendingCitation10. The healthcare systems of Colombia, Mexico, and Uruguay, despite having different levels of segmentation, centrally define benefits and reimbursement levels within their health benefit plansCitation10. Health technology assessments and cost-effective analysis are policy mechanisms that improve the efficiency of these plans. Previous analyses have found ICD therapy to be cost-effective for the primary prevention population in developed countries, but it is not clear how measures of cost-effectiveness of ICD therapy differ by country, or which factors explain these differences. This study calculates and compares the cost-effectiveness of ICD therapy in the 1.5PP population in Colombia, Mexico, and Uruguay using a combination of global clinical inputs and local costs and competing mortality inputs, following local guidelines for health technology assessments.
Methods
In this analysis, we used an existing Markov decision modelCitation6 to estimate the lifetime cost, quality-of-life, survival, and incremental cost-effectiveness of ICD therapy versus no ICD therapy for a population at risk for SCA (1.5PP) for each country. A previous study (SCD-HeFT) found no significant difference in the risk of death between treatment with amiodarone and treatment with a placeboCitation4; hence, no ICD therapy was selected as the control instead of pharmacologic based therapy, as the latter only increases costs compared to the former. Model inputs are shown in and described in detail below. The model analysis was performed in Microsoft Excel.
Table 1. Model input parameters.
Model structure and model inputs
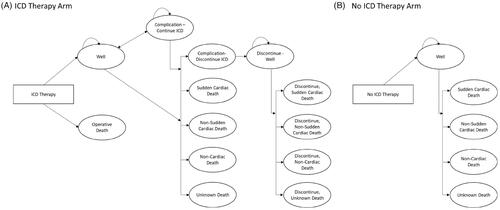
A standard indication for primary prevention ICD therapy and at least one 1.5PP risk factor in a simulated cohort of 1,000 patients is used in this model. The starting age for this cohort is 60.2 years old, and it is 77.6% male; these characteristics follow the cohort of patients with 1.5PP characteristics in the Improve SCA study. The model is structured as a Markov model with two treatment arms, ICD therapy or no ICD therapy (); patients in the ICD therapy arm can face ICD complications and decide to continue or discontinue ICD therapy. A more detailed account of the model paths is described in the literatureCitation6. We assumed that ICD patients who discontinue ICD therapy have the same overall mortality risk as patients in the no ICD arm. Patients incur costs and outcomes by progressing through the model in monthly increments over a lifetime (420 months), which allows the model to account for all costs incurred by patients that survive without a sudden cardiac arrest event.
Inputs to the model that are shared in all country analyses were based on the Improve SCA clinical studyCitation8 results, current literature, and administrative claims-based analyses. The probability of implant-related operative death (0.0002) was based on the US National ICD Registry and applied only to the ICD treatment armCitation11, and the probabilities of other forms of death during the study follow-up duration (sudden cardiac death, non-sudden cardiac death, non-cardiac death, and unknown death) were based on results from the Improve SCA studyCitation8; it is assumed that these probabilities are the same in all three countries in the analysis. Inappropriate shock probability was derived from a weighted average based on results from existing clinical trials (MADIT RIT, ADVANCE III, PROVIDE, and PainFree SST) that showed reductions in inappropriate shock rates due to device programmingCitation12–14. Probabilities of lead failure or dislodgement after initial implant were based on studies of the annual incidence of lead failure and ICD lead dislodgement at 1 year after implantCitation15,Citation16. The probability of lead dislodgement or replacement after ICD replacement was based on data from the REPLACE registryCitation17. Given the lack of information about the specific probabilities of lead failure and lead dislodgement, in order to not prioritize one complication over the other it was assumed that half of the combined rate reported in the REPLACE registry could be attributed to lead failure and half could be attributed to lead dislodgement. The probabilities of lead infection after initial implant and after replacement were estimated with administrative healthcare claims from a large US insurerCitation18. After the first year of an initial or replacement implant, the lifetime risk of lead infection was double the value of the 1-year claims-based probabilityCitation19. Lifetime mortality rates were obtained from local actuarial life tables and are used to adjust the non-cardiac mortality rates. All model inputs are listed in .
Economic data
Device-related and the long-term healthcare utilization costs associated with heart disease were modelled over a patient’s lifetime. Cost inputs are country-specific and reflect the healthcare prices and productivity of each country (). In Colombia, procedural costs of initial ICD implant, subsequent revision or replacement, and ICD-related complications (infection and dislodgement) which include: cost of devices, admission fee, drug fee, examination etc., were derived from the 2001 fee schedule from the Social Security Institute (Instituto de Seguro Sociales – ISS) and increased by 30% according to the local HTA guidelines. Ongoing inpatient and outpatient costs were estimated from a local publication and adjusted by inflationCitation20. For Mexico, costs were derived from fee schedules available from the Mexican Social Security Institute (Instituto Mexicano del Seguro Social – IMSS). Uruguayan costs come from the National Resource Fund (Fondo Nacional de Recursos – FNR) fee schedules. Costs of inappropriate shock were derived from an analysis of procedures commonly performed at encounters for shocksCitation21, and priced following local fee schedules. Long-term inpatient and outpatient costs were estimated from a publication on the costs of heart failure in ColombiaCitation20; costs for Mexico were adjusted using local fee schedules, while these costs for Uruguay were obtained from the National Health Observatory (Observatorio del Sistema de Salud del Uruguay). To obtain long-term care costs, the long-term inpatient costs were multiplied by the average number of hospitalizations per year for patients indicated for ICD therapy based on the SCD-HeFT trialCitation7. These costs reflect the perspective of a public payer of high complexity services in Uruguay, and the payer of an employee-based plan in Colombia and Mexico. Costs and outcomes discount rates follow local guidelines for health technology assessmentsCitation22–24. All costs are expressed in local currency units (Colombian Pesos COP$, Mexican Pesos MXN$, and Uruguayan Pesos UYU$), and were adjusted to constant 2017 prices (in Colombia and Mexico) or 2019 prices (in Uruguay) using local general price indices; for the international comparison, costs in local currency units were converted to purchasing power parity (PPP) adjusted 2019 dollars using the World Bank’s PPP conversion factor.
Table 2. Base case scenario results.
Health-related quality-of-life
An analysis of EQ-5D data collected in the PainFree SST clinical trialCitation25 was used for the quality-of-life basis. Preference weights for EQ-5D health states were obtained from the quality-of-life literature for Latin AmericaCitation6,Citation26. We assumed the baseline utility for ICD patients and no ICD patients to be the same. The patients who experienced an ICD-related complication, which usually affect patients in the short-term and don’t have permanent effects on the patient, received a utility decrement of 0.096 that is equivalent to a decrement of 3.5 days of quality-adjusted life yearsCitation27.
Construction of the ICER (w/WTP) and sensitivity analysis
The total lifetime costs and quality-adjusted life years (QALYs) between ICD therapy and no ICD therapy were simulated to calculate the incremental cost-effectiveness ratio (ICER). The undiscounted and discounted results were calculated to best represent the time value of costs and outcomes. One-way sensitivity analysis and probabilistic sensitivity analysis were conducted to assess the impact of model inputs and parameter uncertainty. Willingness-to-pay (WTP) threshold values of one- and three-times the Gross Domestic Product (GDP) per capita in Colombia and Mexico for 2017 and in Uruguay for 2019 were used, as recommended by the World Health Organization (WHO) for countries without an established WTP for healthcare technology adoptionCitation28.
Results
Base case scenario
shows the results of the base-case scenario for each country. In Colombia, ICD therapy for 1.5PP resulted in a benefit of 7.36 (discounted) and 9.72 (undiscounted) life-years, while no ICD therapy resulted in a benefit of 5.97 and 7.56 life-years, respectively. Measured in QALYs, the discounted benefit from ICD therapy is 6.20 and 5.04 from no ICD therapy, resulting in an incremental effectiveness of 1.15 QALYs. Discounted costs from ICD therapy and no ICD therapy account for COP $132,937,755 and COP $79,017,109, respectively. The ICER for ICD therapy is COP $46,729,026 per QALY. Following Colombian guidelines for health technology assessmentsCitation23, we find that ICD therapy for 1.5PP is potentially cost-effective at COP $58,903,902, 3-times the Colombian GDP per capita WTP threshold in the base case scenario. Undiscounted results for Mexico show a differential cost between ICD therapy and no ICD therapy of MXN $689,423, and a difference of 3.52 QALYs saved with ICD therapy. Discounted results show differential costs of MXN $455,387, and a difference of 1.85 QALYs saved, which leads to an ICER for ICD therapy of MXN $246,016. At a WTP threshold of 3-times the Mexican GDP per capita of MXN $594,383, we find ICD therapy cost-effective in Mexico in the base case scenario. Undiscounted results for Uruguay show a differential cost between ICD therapy and no ICD therapy of UYU $1,730,802, and a difference of 1.74 QALYs saved with ICD therapy. Discounted results show differential costs of UYU $1,358,114 and a difference of 1.12 QALYs saved, which leads to an ICER for ICD therapy of UYU $1,214,937. At a WTP threshold of 3-times the Uruguayan GDP per capita of UYU $1,802,860, we find ICD therapy cost-effective in Uruguay in the base case scenario.
Sensitivity analyses
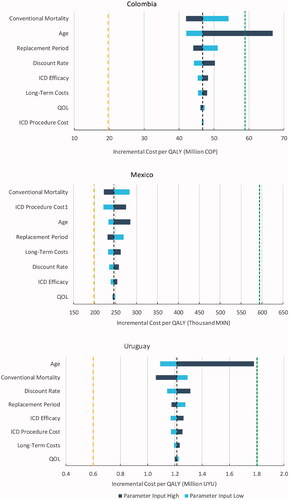
shows Tornado charts resulting from one-way sensitivity analysis per country. Each bar represents the resulting ICER of changing the parameter of the Markov model on the y-axis, while the remaining parameters are constant and the short-dashed and the long-dashed lines represent the 3-times and 1-time GDP per capita WTP thresholds, respectively. Most results confirm the base case scenario in all three countries; only the high value for age in Colombia results in an ICER above the 3-times WTP threshold. In Colombia and Mexico, the results are most sensitive to changes in conventional mortality, while in Uruguay it is most sensitive to age. In addition, in Mexico the results are particularly sensitive to ICD procedure costs.
Figure 2. One-way sensitivity tornado charts. 1Includes device cost. Abbreviations. ICD, Implantable Cardioverter-Defibrillator; QoL, Quality-of-Life; COP, Colombian Pesos; MXN, Mexican Pesos.
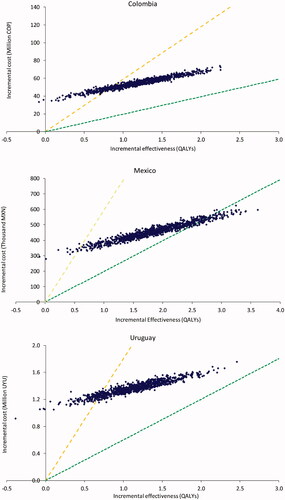
shows the simulated costs per QALY of the probabilistic sensitivity analysis for each country, where each dot corresponds to the resulting cost per QALY of a model iteration, and the dashed lines show the WTP thresholds of 1- and 3-times the GDP per capita of the corresponding country. Results for Colombia show a mean cost per QALY of COP $46,389,154 (median cost per QALY COP $46,483,046, 95% Credible Interval [COP $33,780,726–$95,957,807] per QALY) after 1,000 iterations. A total of 81.4% of simulations result in costs per QALY below the 3-times GDP per capita WTP threshold; no iteration resulted in costs per QALY below the 1-time GDP per capita WTP threshold. Results from the Mexico PSA indicate that after 1,000 simulations, the mean cost per QALY was MXN $245,482, with a median cost per QALY of MXN $244,843, and a 95% Credible Interval of [MXN $183,382−$490,506]. Also, 98.7% of simulations result in costs per QALY below the 3-times GDP per capita WTP threshold, and 9% of iterations resulted in costs per QALY below the 1-time GDP per capita WTP threshold. Results from the Uruguay PSA indicate that after 1,000 simulations, the mean cost per QALY was UYU $1,215,895, with a median cost per QALY of UYU $1,214,379 and a 95% Credible Interval of [UYU $809,047−$2,795,561]. Also, 86.4% of simulations result in costs per QALY below the 3-times GDP per capita WTP threshold, but no iterations resulted in costs per QALY below the 1-time GDP per capita WTP threshold.
Discussion
Results show that costs per QALY of ICD therapy in a 1.5PP population are between 1- and 3-times GDP per capita WTP threshold in Colombia, Mexico, and Uruguay; sensitivity analyses confirm these results. However, Colombian guidelines for health technology assessment consider costs per QALY between 1- and 3-times GDP per capita as potentially cost-effective. These results are in line with the literature on the cost-effectiveness of ICD therapy. There is established evidence of the cost-effectiveness of ICD therapy in the primary prevention population. An analysis of the randomized SCD-HeFT trial by Mark et al.Citation7 found ICD therapy to be economically attractive at $41,530/QALY (at a WTP of $100,000) in the US healthcare system. Similar results were shown within the healthcare system of a European country, using the same model in this study and a meta-analysis of six randomized primary prevention trialsCitation6. In addition, ICD therapy for the 1.5 prevention population has been found to be cost-effective in BrazilCitation29. This cost-effectiveness evidence has been validated in a real world setting outside of clinical trialsCitation30.
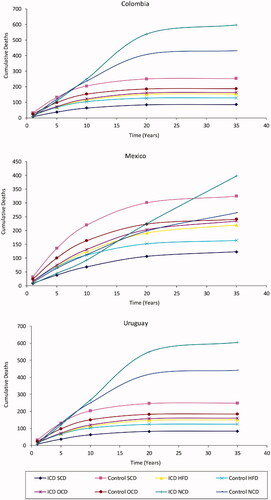
Even though the results coincide in the cost-effectiveness of ICD therapy in all three countries, there are differences in costs and effectiveness between these countries. shows that, despite using the same mortality risks in the Markov model, results for Mexico have a higher effectiveness in both ICD Therapy and No ICD Therapy arms of the model. Discounted effectiveness results in Mexico are 0.90 and 1.59 QALYs more than in Colombia, and 0.97 and 1.70 more than in Uruguay in the No ICD Therapy and ICD Therapy arms, respectively. These differences can be explained by differences in population health among countries (the model used country-specific data for this input), particularly differences in non-cardiac death rates; results from the model show non-cardiac cumulative deaths in both arms overtake any cardiac deaths at the end of the model time in the Mexican case (). In Colombia and Uruguay, non-cardiac deaths overtake any cardiac deaths early in the model timeline and are the most prevalent cause of death. These results are in line with the Tornado charts, where the high value for age at implant leads to high ICERs in Colombia and Uruguay, but not in Mexico: all else equal, as older patients are more susceptible to non-cardiac death in Colombia and Uruguay than in Mexico, an older cohort in Colombia or Uruguay receives less utility from ICD therapy than in Mexico, hence the resulting ICER for an older cohort is higher in Colombia or Uruguay than in Mexico. Discounted costs of ICD therapy are similar between countries (within $10,000 purchase-parity adjusted dollars), but No ICD therapy costs in Colombia are 24.1% and 54.8% higher than in Mexico and Uruguay, respectively (). These differences in costs and effectiveness highlight the importance of using local inputs in cost-effectiveness analyses.
Figure 4. Cumulative deaths by country. Abbreviations. ICD, Implantable Cardioverter-Defibrillator; SCD, Sudden Cardiac Death; HFD, Heart Failure Cardiac Death; OCD, Other Cardiac Death; NCD, Non-Cardiac Death.
Despite convincing evidence from multiple randomized clinical trialsCitation1,Citation4,Citation14 and strong recommendations in international society guidelinesCitation2, ICD utilization is relatively low in Latin America, ranging from one implant per million in Perú to 56 implants per million in ArgentinaCitation31. These numbers are starkly low relative to other areas. The average rate of ICD implantation in Europe is approximately 100 implants per millionCitation32. Regarding economic factors, it remains cost-effective to treat the primary prevention population with ICD therapy; a budget impact model could provide additional information on the effect of treating a primary prevention population. This study provides information from an economic standpoint for decision-makers to first direct scarce resources toward those who can benefit the most. Priority should be placed on treating patients with a 1.5PP indication.
It is important to acknowledge the limitations of this analysis. The Improve SCA study was a non-randomized trial and may produce biased results; however, the mortality analysis from the trial was adjusted for baseline characteristics that are associated with mortality, reducing the potential bias. Moreover, the effectiveness of ICD therapy is replicated in non-randomized observational trials. Additionally, mortality rates from the study could be misclassified, as deaths in the Improve SCA study were not adjudicated by a central committee. Costs and benefits were modelled beyond the timeline of direct observation in the Improve SCA study, however this is a standard approach in economic modelling and necessary for the proper perspective for decision-makers.
Conclusions
ICD therapy is cost-effective in Mexico and Uruguay and potentially cost-effective in Colombia for the 1.5 primary prevention population identified in the Improve SCA trial. There is variability in the ICER estimates with respect to the willingness-to-pay thresholds that can be explained by differences in local health technology assessment guidelines, population health, and healthcare costs.
Transparency
Declaration of funding
Medtronic, Inc funded this study.
Declaration of financial/other interests
Medtronic; DAR: proctor/lecture fees: Boston Scientific, proctorship: Biosense Webster, St. Jude Medical/Abbott, steering committee fees: Medtronic. AC: registration and transfer to medical congresses: St. Jude Medical/Abbott, Medtronic.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Results for Mexico were presented at the 2018 ISPOR Annual Conference, and results for Colombia were presented at the 2019 ISPOR Latin America Conference.
Acknowledgements
We are grateful to Brian Van Dorn of Medtronic for providing the utility estimates based on the Improve SCACitation8 study of life data, and Janet E O’Brien, of Medtronic for medical writing assistance.
References
- Al-Khatib SM, Hellkamp A, Bardy GH, et al. Survival of patients receiving a primary prevention implantable cardioverter-defibrillator in clinical practice vs clinical trials. JAMA. 2013;309(1):55–62.
- Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117(21):e350-408–2840.
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–2867.
- Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237.
- Moss AJ, Schuger C, Beck CA, et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med. 2012;367(24):2275–2283.
- Cowie MR, Marshall D, Drummond M, et al. Lifetime cost-effectiveness of prophylactic implantation of a cardioverter defibrillator in patients with reduced left ventricular systolic function: results of Markov modelling in a European population. Europace. 2009;11(6):716–726.
- Mark DB, Nelson CL, Anstrom KJ, et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Circulation. 2006;114(2):135–142.
- Zhang S, Ching C-K, Huang D, et al. Utilization of implantable cardioverter-defibrillators for the prevention of sudden cardiac death in emerging countries: improve SCA clinical trial. Heart Rhythm. 2020;17(3):468–475.
- Zhang S, Singh B, Rodriguez DA, et al. Improve the prevention of sudden cardiac arrest in emerging countries: the Improve SCA clinical study design. Europace. 2015;17(11):1720–1726.
- Giedion U, Tristao I, Escobar L, et al. Health benefit plans in Latin America: a regional comparison. Washington DC. Inter-american Development Bank; 2014.
- Hammill SC, Kremers MS, Stevenson LW, et al. Review of the registry's fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7(9):1340–1345.
- Saeed M, Hanna I, Robotis D, et al. Programming implantable cardioverter-defibrillators in patients with primary prevention indication to prolong time to first shock: results from the PROVIDE study. J Cardiovasc Electrophysiol. 2014;25(1):52–59.
- Gasparini M, Proclemer A, Klersy C, et al. Effect of long-detection interval vs standard-detection interval for implantable cardioverter-defibrillators on antitachycardia pacing and shock delivery: the ADVANCE III randomized clinical trial. JAMA. 2013;309(18):1903–1911.
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883.
- Ghani A, Delnoy PPHM, Ramdat Misier AR, et al. Incidence of lead dislodgement, malfunction and perforation during the first year following device implantation. Neth Heart J. 2014;22(6):286–291.
- Providencia R, Kramer DB, Pimenta D, et al. Transvenous implantable cardioverter-defibrillator (ICD) lead performance: a meta-analysis of observational studies. J Am Heart Assoc. 2015;4(11):2047–9980.
- Li H, Natale A, Zhu W, et al. Causes and consequences of discontinuation of the implantable cardioverter-defibrillator therapy in non-terminally ill patients. Am J Cardiol. 1998;81(10):1203–1205.
- EL E, Lgs B, Mp J. Economic impact of cardiac implantable electronic device infections: cost analysis at one year in a large US health insurer. J Med Econ. 2020;23(7):698–705.
- Hussein AA, Baghdy Y, Wazni OM, et al. Microbiology of cardiac implantable electronic device infections. JACC Clin Electrophysiol. 2016;2(4):498–505.
- Tamayo D, Rodríguez V, Rojas M. Outpatient and inpatient costs of heart failure in two hospitals in Bogotá. Acta Med Colomb. 2013;38(4):208–212.
- Poole JE, Gleva MJ, Mela T, et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122(16):1553–1561.
- Evaluación de Tecnologías para la Salud: Documento Metodologico. Centro Nacional de Excelencia Tecnologica en Salud; 2017. Available from: http://www.cenetec.salud.gob.mx/descargas/detes/metodologico_ETES.pdf
- Mabel MV, Aurelio MM, Hectro ECJ. Manual para la elaboracion de evaluaciones economicas en salud. Bogotá DC: Instituo de Evaluacion Tecnologica en Salud; 2014.
- Guía metodológica para estudios de evaluación económica de tecnologías sanitarias. MERCOSUR; 2013. (Resolución N° 03/13). Available from: http://www.sice.oas.org/trade/mrcsrs/resolutions/Res0313_s.pdf
- Auricchio A, Schloss EJ, Kurita T, et al. Low inappropriate shock rates in patients with single- and dual/triple-chamber implantable cardioverter-defibrillators using a novel suite of detection algorithms: PainFree SST trial primary results. Heart Rhythm. 2015;12(5):926–936.
- Zarate V, Kind P, Chuang L-H. Hispanic valuation of the EQ-5D health states: a social value set for Latin Americans. Value Health. 2008;11(7):1170–1177.
- Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353(14):1471–1480.
- Hutubessy R, Chisholm D, Edejer TT-T. Generalized cost-effectiveness analysis for national-level priority-setting in the health sector. Cost Eff Resour Alloc. 2003;1(1):8.
- Wherry K, Holbrook R, Higuera L, et al. Cost-Effectiveness analysis of implantable cardioverter defibrillator therapy for primary prevention patients with additional risk factors in Brazil. Int J Cardiovasc Sci. Forthcoming.
- Thijssen J, van den Akker van Marle ME, Borleffs CJW, et al. Cost-effectiveness of primary prevention implantable cardioverter defibrillator treatment: data from a large clinical registry. Pacing Clin Electrophysiol. 2014;37(1):25–34.
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009-a World Society of Arrhythmia's project. Pacing Clin Electrophysiol. 2011;34(8):1013–1027.
- Raatikainen MJP, Arnar DO, Zeppenfeld K, et al. Statistics on the use of cardiac electronic devices and electrophysiological procedures in the European Society of Cardiology countries: 2014 report from the European Heart Rhythm Association. Europace. 2015;17 Suppl 1 (Suppl 1(16):i1–75.
- Sweeney MO, Wathen MS, Volosin K, et al. Appropriate and inappropriate ventricular therapies, quality of life, and mortality among primary and secondary prevention implantable cardioverter defibrillator patients: results from the Pacing Fast VT REduces Shock ThErapies (PainFREE Rx II) trial. Circulation. 2005;111(22):2898–2905.
- Turakhia MP, Zweibel S, Swain AL, et al. Healthcare utilization and expenditures associated with appropriate and inappropriate implantable defibrillator shocks. Circ Cardiovasc Qual Outcomes. 2017;10(2):1941–7713.