Abstract
Aims
Rivaroxaban reduces stroke compared with warfarin in patients with non-valvular atrial fibrillation (NVAF). This study compared healthcare costs before and after stroke in NVAF patients treated with rivaroxaban or warfarin.
Materials and methods
Using de-identified IBM MarketScan Commercial and Medicare databases, this retrospective cohort study (from 2011 to 2019) included patients with NVAF who initiated rivaroxaban or warfarin within 30 days after initial NVAF diagnosis. Patients who developed stroke were identified, and stroke severity was determined by the National Institutes of Health Stroke Scale (NIHSS) score, imputed by a random forest method. Total all-cause per-patient per-year (PPPY) costs of care were determined for patients: (1) who developed stroke during the pre- and post-stroke periods and (2) who remained stroke-free during the follow-up period. Treatment groups were balanced using inverse probability of treatment weighting.
Results
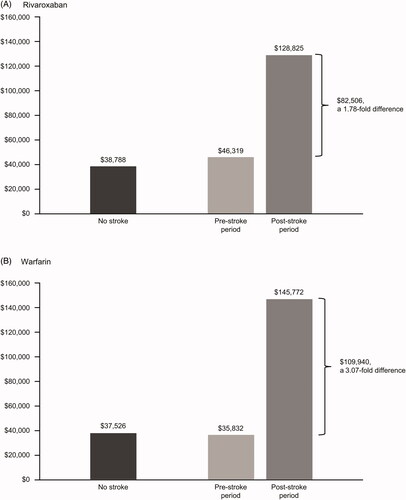
A total of 13,599 patients initiated rivaroxaban and 39,861 initiated warfarin, of which 272 (2.0%) and 1,303 (3.3%), respectively, developed stroke during a mean follow-up of 28 months. Among patients who developed stroke, PPPY costs increased from the pre-stroke to post-stroke period, with greater increases in the warfarin cohort relative to the rivaroxaban cohort. Overall, the costs increased by 1.78-fold for rivaroxaban vs 3.07-fold for warfarin; for less severe strokes (NIHSS < 5), costs increased 0.88-fold and 1.05-fold, respectively. Cost increases for more severe strokes (NIHSS ≥ 5) among rivaroxaban patients were half those for warfarin patients (3.19-fold vs 6.37-fold, respectively). Among patients without stroke, costs were similar during the follow-up period between the two treatment groups.
Conclusions
Total all-cause costs of care increased in the post-stroke period, and particularly in the patients treated with warfarin relative to those treated with rivaroxaban. The lower rate of stroke in the rivaroxaban cohort suggests that greater pre- to post-stroke cost increases result from more strokes occurring in the warfarin cohort.
Introduction
Atrial fibrillation (AF) affects an estimated 3–6 million people in the United States (US) and is the most common cardiac arrhythmiaCitation1,Citation2. Patients with AF have a 5-fold increased risk of stroke compared to patients in normal sinus rhythmCitation3. Non-valvular atrial fibrillation (NVAF) is associated with the use of substantial healthcare resources and cost burden, adding up to $26 billion in US healthcare spending each yearCitation2,Citation4. In a 2014 study of Medicare claims, the patient costs associated with stroke in newly diagnosed NVAF were nearly double those of people without NVAF, and approximately 50% of the costs were attributed to inpatient careCitation3. Stroke severity also impacts the healthcare cost and resource utilization following a stroke.
The National Institutes of Health Stroke Scale (NIHSS) is a widely used, validated tool for clinical assessment of stroke severity and to predict patient outcomes, with excellent discrimination of 30-day mortalityCitation5,Citation6. The scale evaluates 15 elements of a neurological exam for a sum score ranging from 0–42, with higher numbers indicating more severe stroke.
The ROCKET-AF trial demonstrated a reduced number of strokes (ischemic or hemorrhagic) in NVAF patients treated with rivaroxaban compared with warfarinCitation7. Stroke or systemic embolism occurred in 1.7% and 2.2% of patients per year in the rivaroxaban and warfarin cohorts, respectively (p < 0.001). Rates of major bleeding were similar between treatment groups, but rivaroxaban was associated with a significantly lower rate of intracranial hemorrhage compared with warfarin (0.5% vs 0.7%, p = 0.02). The effectiveness of rivaroxaban in patients with NVAF has been demonstrated, but the economic impact of treatment and reduction in stroke occurrence over time in these patients remains unclear.
Using US health insurance claims data, this study examined pre- vs post-stroke costs of care, by stroke severity, among rivaroxaban- and warfarin-treated patients with NVAF who developed stroke, and total costs of care by treatment for patients who did not develop stroke.
Methods
Study design
This retrospective cohort study used the IBM MarketScan Commercial and Medicare databases from 2011 to 2019. These databases include de-identified records of > 250 million patients, providing information on medical resource utilization and total costs (medical and pharmacy) for a commercially insured US population.
Study population
Adult patients with ≥ 2 NVAF diagnosis codes that were ≥ 1 week apart between 1 January 2012 and 31 January 2019 to ensure accurate identification of NVAF were included in the study if they had ≥ 6 months of continuous health plan enrollment and CHA2DS2-VASc ≥ 2 during the 6 months prior to the first NVAF diagnosis. Both CHA2DS2-VASc and HAS-BLED scores were defined based on previously published studiesCitation8,Citation9. Briefly, the scores were determined from existing data fields, including age, diagnosis codes, and prescription fills (Supplementary Table 1). Patients were excluded if they had a valve-related diagnosis, stroke diagnosis, transient AF, or anticoagulant use prior to the first NVAF diagnosis.
Table 1. Demographics and baseline characteristics of rivaroxaban and warfarin treatment cohorts.
Enrolled patients had ≥ 1 prescription claim for rivaroxaban or warfarin within 30 days following NVAF diagnosis and demonstrated adequate treatment coverage following the index treatment date. To ensure ≥ 60% treatment coverage, patients were required to stay on index treatment for ≥ 54 days within the first 90 days in order to be included in this analysis. Patients were followed from the index treatment date to the earliest occurrence of a primary inpatient diagnosis of stroke, death, end of eligibility, or end of study.
Stroke was defined as the first observed stroke diagnosis using International Classification of Diseases, Ninth Edition (ICD-9) and International Classification of Diseases, Tenth Edition (ICD-10) codes for ischemic and hemorrhagic stroke and transient ischemic attack (TIA) in an inpatient setting or emergency room (ischemic: ICD-9: 433.XX–434.XX, 436; ICD-10: I63.XXX; hemorrhagic: ICD-9: 431; ICD-10 I61.XX; or TIA: ICD-9: 435.X; ICD-10: G45.9). Stroke severity according to NIHSS was imputed using a random forest method. Two categories of stroke severity were defined for evaluation based on imputed NIHSS scores: less severe (NIHSS < 5) and more severe (NIHSS ≥ 5) strokesCitation10,Citation11.
Total costs of care (medical and pharmacy costs) per-patient per-year (PPPY) were calculated for the pre- and post-stroke periods for each treatment cohort and inflated to 2018 US dollars. The pre-stroke period was defined as the time from treatment initiation to stroke occurrence. The post-stroke period was defined as the time from stroke occurrence until the end of the follow-up period. Total costs of care by treatment cohort during the follow-up period were also determined for NVAF patients who did not develop stroke.
Statistical analysis
Balance between rivaroxaban and warfarin groups was performed by inverse probability of treatment weighting (IPTW). Specifically, the treatment probability was estimated by regressing treatment assignment (i.e. rivaroxaban) against baseline risk factors, including age, sex, baseline Quan-Charlson comorbidity index, residence region, health insurance type, health plan type, baseline CHA2DS2-VASc score, baseline HAS-BLED score, and presence of other baseline risk factors, including hypertension, diabetes mellitus, hyperlipidemia, obesity, coronary artery disease, and heart failure. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated for stroke incidence in each treatment group. Among patients with stroke, total healthcare cost was calculated separately for pre-stroke and post-stroke periods, and on a PPPY basis. Pre-stroke and post-stroke costs were assessed for rivaroxaban and warfarin cohorts separately.
Data availability
Deidentified data were available to the authors via license between IBM MarketScan, a commercial data provider in the US, and Janssen Pharmaceuticals. As such, the authors cannot provide the raw data; however, interested researchers may access the data through IBM, and the methods applied previously would allow study replication.
Results
Patient attrition is shown in Supplementary Table 2. The treatment cohorts included 13,599 patients who initiated rivaroxaban and 39,861 patients who initiated warfarin. Compared to patients in the rivaroxaban cohort, patients in the warfarin cohort, on average, were older, had slightly higher Quan-Charlson comorbidity index scores, and more often had comorbid coronary artery disease (). After IPTW, the baseline demographics and clinical characteristics were well balanced.
Table 2. Total costs of care (PPPY) by stroke severitya.
Stroke occurred in 272 (2.0%) rivaroxaban patients and in 1,303 (3.3%) warfarin patients during a mean follow-up of 28 months. The event rates for less severe stroke (NIHSS < 5) and more severe stroke (NIHSS ≥ 5) were lower in the rivaroxaban cohort compared with the warfarin cohort (). The risk of less severe and more severe strokes was significantly reduced by 21% (HR = 0.79; 95% CI = 0.72–0.87; p < 0.0001) and 14% (HR = 0.86; 95% CI = 0.77–0.97; p = 0.012), respectively, in the rivaroxaban cohort compared to the warfarin cohort.
Among patients who had a stroke, direct healthcare costs increased from the pre-stroke period to the post-stroke period in both treatment groups, with a 1.78-fold increase for rivaroxaban and a 3.07-fold increase for warfarin (). Detailed costs for rivaroxaban and warfarin in the pre-stroke and post-stroke periods are shown in Supplementary Table 3. For less severe stroke, there was an increase of 0.88-fold with rivaroxaban and 1.05-fold with warfarin from the pre-stroke period to the post-stroke period. For patients with more severe strokes, the rivaroxaban cohort cost increase was half of that in the warfarin cohort (3.19-fold increase vs 6.37-fold increase, respectively). The total costs of care for patients who did not develop stroke were similar in the rivaroxaban and warfarin cohorts during the follow-up period.
Discussion
In this analysis, we examined pre- vs post-stroke costs of care, by stroke severity, among rivaroxaban- and warfarin-treated patients with NVAF who developed stroke and total costs of care by treatment for patients who did not develop stroke. As anticipated, this analysis of the development of stroke led to higher overall total costs in both treatment groups; however, post-stroke cost increases were substantially greater in the warfarin cohort relative to the rivaroxaban cohort. Warfarin patients incurred even higher healthcare costs for strokes of greater severity. Total costs of care were similar between treatment cohorts of NVAF patients who were stroke-free.
Favorable post-stroke cost benefits were identified for rivaroxaban, particularly for patients with more severe strokes in which post-stroke cost increases were approximately half of those observed with warfarin. In a post hoc analysis of the ROCKET-AF trial, rivaroxaban was associated with a 42% (HR = 0.58; 95% CI = 0.39–0.88) reduction in the risk of disabling or fatal stroke vs warfarin in patients with no prior history of stroke or TIACitation12. These findings underscore the importance of anticoagulant selection (e.g. rivaroxaban as opposed to warfarin) for NVAF management to reduce stroke burden. In Markov decision models using clinical trial data, rivaroxaban has been shown to be a cost-effective alternative to warfarin for stroke in patients with AFCitation13,Citation14. Favorable incremental cost-effectiveness ratios were attributed to the decreased risk of ischemic stroke as well as intracranial hemorrhage associated with rivaroxaban compared with warfarin in the ROCKET-AF trialCitation7.
In a comparison of healthcare costs using the Humana database, all-cause hospitalization and outpatient costs were shown to be significantly lower for AF patients treated with rivaroxaban vs warfarinCitation15. Despite higher drug costs with rivaroxaban, overall costs associated with rivaroxaban have been reported as similar or lower than warfarin. This difference may stem from several of the following factors: (1) the anticoagulant effect of rivaroxaban does not require routine monitoring, in contrast to the frequent monitoring required to maintain warfarin within the therapeutic INR range and manage food–drug interactions; (2) the time to reach the therapeutic range may be longer with warfarin; or (3) rivaroxaban has been shown to provide better protection against strokes, leading to poor outcomes and deathCitation16.
Compared to other reports, this study offers valuable information on costs related to stroke as well as costs by stroke severity, the latter of which is generally not reported in real-world studies because NIHSS scores are unavailable in structured claims databases. For our study, use of a validated machine learning algorithm allowed determination of NIHSS scoresCitation10,Citation11. Due to variations that may arise in clinical practice, we assigned these values to categories representing a range of scores to help mitigate any lack of specificity associated with imputation of a single score. Our model performance was consistent with that seen in previous studiesCitation16–18, which suggests that imputed NIHSS based on our model appears to be a valid proxy for stroke severity in patients with a primary stroke diagnosis.
In the current study, we focused on the costs before and after a first stroke over more than 2 years of follow-up time. Very high costs following stroke may be related, in part, to an increased risk of recurrent stroke events. In an incremental analysis up to 4 years after the first stroke event in NVAF patients, each additional year of follow-up was associated with an incremental cost for patients with stroke or systemic embolismCitation19. The authors concluded that recurrent stroke may partly explain the higher long-term costs. NVAF patients are known to be at higher risk of more severe and debilitating strokes. These factors should be considered when selecting anticoagulation therapy for long-term stroke prevention and to avoid poor outcomes associated with stroke in NVAF patients. We did not include an analysis of bleeding risks, which are associated with all anticoagulants, because the focus of this report was on stroke outcomes by stroke severity. In addition, bleeding risks associated with anticoagulant therapy have been thoroughly reviewed, including a post-marketing surveillance safety study that determined that the risks of bleeding associated with direct oral anticoagulants are in line with the results observed in randomized controlled trialsCitation20.
Studies using claims data may be limited by potential misclassification of information that results from incomplete or inaccurate claims. Data are based on financial claims filed for reimbursement purposes, and disease coding may reflect financial incentives for reimbursement rather than clinically verified diagnoses. Prescription data represents filled prescriptions, but there is no confirmation that medication was taken as directed. In addition, INR values were not available to determine whether warfarin patients were within therapeutic range. Although we used IPTW that incorporated 14 baseline covariates including age and numerous comorbidities, there may be residual confounding by unmeasured covariates. There is also a time lag in data availability because the IBM MarketScan data reflect only records that are completely paid. The generalizability of these findings is limited to similar commercially insured populations.
Conclusion
Costs of stroke prevention in NVAF patients did not vary markedly between rivaroxaban- and warfarin-treated patients without stroke and in patients before a stroke event. However, a substantial increase in the costs of care from pre-stroke to post-stroke costs was observed in warfarin patients who suffered a more severe stroke. The event rates for both less severe and more severe strokes were significantly higher in the warfarin cohort compared to the rivaroxaban cohort. Thus, the increase in costs of care is likely due to significantly higher cases of severe stroke observed in the warfarin cohort. These findings suggest the importance of anticoagulation selection in managing NVAF to optimize both clinical and healthcare cost outcomes.
Transparency
Declaration of funding
This work was supported by Janssen Scientific Affairs, LLC, USA.
Declaration of financial/other interests
DM and YWC are employees of Janssen Scientific Affairs, LLC. JHL was an employee of Janssen Scientific Affairs, LLC at the time of study conduct. EK and ES are employees of Janssen Research & Development, LLC. SS has received consultancy fees from Janssen Pharmaceuticals, LLC. MA has not received compensation for this project but received consultancy fees in the past from Janssen Pharmaceuticals, LLC.
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
Preliminary data presented at the International Stroke Conference (ISC); 19–21 February 2020; Los Angeles, CA, USA.
Supplemental Material
Download MS Word (81.7 KB)Acknowledgements
Medical writing support was provided by Michelle McDermott, PharmD, of MedErgy (Yardley, PA, USA), and was funded by Janssen Scientific Affairs, LLC (Titusville, NJ, USA). The authors thank Nancy Connolly for her support during manuscript development.
References
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics–2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492.
- Centers for Disease Control and Prevention. Atrial fibrillation fact sheet 2017; [May 4, 2018]. Available from: https://www.cdc.gov/dhdsp/data_statistics/.
- Fitch K, Broulette J, Kwong WJ. The economic burden of ischemic stroke and major hemorrhage in Medicare beneficiaries with nonvalvular atrial fibrillation: a retrospective claims analysis. Am Health Drug Benefits. 2014;7(4):200–209.
- Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–320.
- Rost NS, Bottle A, Lee JM, et al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc. 2016;5(1):e002433.
- Fonarow GC, Saver JL, Smith EE, et al. Relationship of National Institutes of Health Stroke Scale to 30-day mortality in Medicare beneficiaries with acute ischemic stroke. J Am Heart Assoc. 2012;1(1):42–50.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891.
- Lip GY, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41(12):2731–2738.
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–1100.
- Kogan E, Twyman K, Heap J, et al. Assessing stroke severity using electronic health record data: a machine learning approach. BMC Med Inform Decis Mak. 2020;20(1):8.
- Kogan E, Sjoeland E, Milentijevic D, et al. Validation of a machine learning approach to determine stroke severity of patients diagnosed with stroke in claims data. Stroke. 2020;51(suppl 1):ATP307.
- Hankey GJ, Patel MR, Stevens SR, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11(4):315–322.
- Harrington AR, Armstrong EP, Nolan PE Jr, et al. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44(6):1676–1681.
- Lee S, Anglade MW, Pham D, et al. Cost-effectiveness of rivaroxaban compared to warfarin for stroke prevention in atrial fibrillation. Am J Cardiol. 2012;110(6):845–851.
- Laliberte F, Cloutier M, Crivera C, et al. Effect of rivaroxaban versus warfarin on health care costs among nonvalvular atrial fibrillation patients: observations from rivaroxaban users and matched warfarin users. Adv Ther. 2015;32(3):216–227.
- Alberts M, Chen YW, Lin JH, et al. Risks of stroke and mortality in atrial fibrillation patients treated with rivaroxaban and warfarin. Stroke. 2020;51(2):549–555.
- Sung SF, Hsieh CY, Kao Yang YH, et al. Developing a stroke severity index based on administrative data was feasible using data mining techniques. J Clin Epidemiol. 2015;68(11):1292–1300.
- Hung LC, Sung SF, Hsieh CY, et al. Validation of a novel claims-based stroke severity index in patients with intracerebral hemorrhage. J Epidemiol. 2017;27(1):24–29.
- Rozjabek HM, Coleman CI, Ashton V, et al. Healthcare costs of stroke and major bleeding in patients with atrial fibrillation treated with non-vitamin K antagonist oral anticoagulants. J Med Econ. 2019;22(8):751–759.
- Villines TC, Peacock WF. Safety of direct oral anticoagulants: insights from postmarketing studies. Am J Med. 2016;129(11S):S41–S46.