Abstract
Aims
Potentially life-threatening diagnosis of non-convulsive status epilepticus (NCSE) can only be confirmed with electroencephalography (EEG). When access to EEG is limited, physicians may empirically treat, risking unnecessary sedation and intubation, or not treat, increasing risk of refractory seizures. Either may prolong hospital length of stay (LOS). The current study aimed to examine the effect of a new EEG system (Ceribell Rapid Response EEG, Rapid-EEG) on hospital costs by enabling easy access to EEG and expedited seizure diagnosis and treatment.
Materials and methods
We built a two-armed decision-analytic cost–benefit model comparing Rapid-EEG with clinical suspicion alone for NCSE. Diagnostic parameters were informed by a multicenter clinical trial (DECIDE, NCT03534258), while LOS and cost parameters were from public US inpatient data, published literature, and Center for Medicare and Medicaid Services fee schedules. We calculated reference case estimates from mean values, while uncertainty was assessed using 95% prediction intervals (PI) generated by probabilistic sensitivity analysis (PSA) and ANCOVA sum of squares. All costs were indexed to 2019 US$.
Results
Each use case of Rapid-EEG saved $3,971 to $17,290 as it led to reduction in the hospital LOS by 1.2 days (6.1 vs. 7.4 days) and ICU LOS by 0.4 days (1.5 vs. 1.9 days). Using PSA, Rapid-EEG saving was $5,633 per use case (95% PI: $($4,649 to $6,617), as it led to diminished hospital LOS by 1.1 days (95% PI: 0.9–1.4 days) and reduced ICU LOS by 0.5 days (95% PI: 0.4–0.6 days). Cost-savings were demonstrated in 75% of replications. Sixty-four percent of variance in total costs was attributable to LOS for persons incorrectly diagnosed with seizures.
Limitations
Results were obtained from the analysis of existing data and not a prospective outcome trial.
Conclusions
Rapid-EEG alters the treatment course for patients with suspected seizures and will result in cost savings per patient.
Introduction
The diagnosis of suspected seizures or status epilepticus often confronts physicians with a guessing game. Patients may present with outright convulsions, which may be epileptic or non-epileptic (i.e. psychogenic), or with alterations in consciousness without any obvious motor signs (i.e. subclinical or non-convulsive). Experienced clinicians may be misled by convulsive psychogenic seizures or by non-convulsive status epilepticus (NCSE) presenting as subtle confusion or delirium. More formally, NCSE is a continuous state of seizures without convulsions, or a series of non-convulsive seizures without full recovery between seizures, lasting greater than 30 minCitation1. Failure to accurately diagnose NCSE can result in over-treatment with unnecessary administration of sedating antiseizure medications (ASMs), including benzodiazepines such as lorazepam, diazepam and midazolam, sedatives such as propofol, and other anticonvulsants such as levetiracetam, valproate, and phenytoin), which can result in intubation and prolonged encephalopathy, or under-treatment, which could lead to refractory NCSE and lengthy intensive care unit (ICU) admissionsCitation2,Citation3. Clinicians are often left with the unenviable choice of either presuming NCSE and treating maximally, or electing to delay treatment when faced with diagnostic uncertainty.
Because NCSE can only be confirmed with electroencephalography (EEG), the availability of this diagnostic tool should reduce clinicians’ uncertainty and lead to more accurate diagnosis and more timely management of NCSE. Unfortunately, conventional EEG requires a trained technologist to set up and specialized neurologists to interpret, limiting its availability to only those hospitals that can afford such a resource-intensive infrastructure. Even when available, substantial delays exist in initiating and interpreting EEGCitation4–6. During normal working hours, technologists may be committed to other tasks, and hours may elapse between the first suspicion of seizure and the initiation of EEG monitoring, let alone its initial interpretation by neurologists who may not be readily available due to outpatient clinic or inpatient rounds. On nights and weekends, when technologists and equipment are less available, or in community hospitals without 24/7 EEG technologist coverage, clinicians managing patients with possible NCSE are often left to their own suspicions.
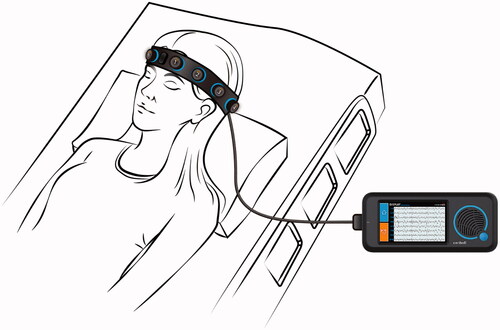
New innovations have the potential to ameliorate this problem, reducing uncertainty in diagnosis and treatment and improving the management of patients with suspected NCSE. Ceribell Rapid Response EEG System (Rapid-EEG) provides EEG with ten electrodes covering the head circumference () and requires minimal training to apply by non-EEG technologist healthcare providers. A series of recent clinical studies have demonstrated that Rapid-EEG with ten-electrode circumferential configuration yields high concordance with standard EEG in detecting and ruling out seizures; has concordant signal properties with standard EEG; has ease of application; and has potential impact on clinical decision makingCitation7–10. Recently, a multicenter prospective observational clinical trial of the Rapid-EEG system (DECIDE, NCT03534258) assessed the diagnostic and therapeutic decisions of 37 clinicians at 5 academic medical centers across the United States who participated in the care of 181 encephalopathic patients suspected of having non-convulsive seizuresCitation11. Clinicians were asked to describe their diagnostic suspicion for seizure and their decision to escalate treatment with ASMs both before and after reviewing Rapid-EEG data. A significant trend towards reducing ASM and ventilatory management escalation was observed due to earlier acquisition of EEG data to rule out NCSE, however clinicians were not obliged to treat the patient based on Rapid-EEG data and as such, treatment outcomes and related adverse effects and costs could not be assessed in this study. DECIDE was designed to test the diagnostic accuracy of Rapid-EEG primarily, with the possible effects on treatment as an only exploratory analysis. As many of the clinicians may have been initially less familiar with Rapid-EEG than traditional EEG, they were not expected to rely on this new technology for clinical management decisions.
Figure 1. Rapid Response EEG System. Rapid-EEG is a new EEG device developed by Ceribell, Inc. (Mountain View, CA) and cleared by US Food and Drug Administration (FDA) since 2018. It consists of a headband with 10 electrodes (5 left and 5 right), which enables eight-channel EEG acquisition with a pocket-size battery-operated EEG recording device. The EEG can be set up by anyone without needing specialized EEG techs. The acquired data is accessible at the bedside as well as on a cloud portal that can be accessed remotely in real time.
Given the pitfalls in clinical diagnosis of suspected NCSE and limitations in availability and timeliness of conventional EEG, the value of Rapid-EEG towards appropriate management of patients is potentially great. In addition to curbing clinical misadventures from poorly informed decision making, we hypothesized that the Rapid-EEG system would lead to cost savings to the hospital, third party payer, and patient from preventing ICU admissions or untreated NCSE. In the present study, we aimed to systematically test our hypothesis by utilizing data from the DECIDE clinical trial to build a decision-analytic model that details costs and benefits of Rapid-EEG compared to standard care to generate an assessment of the overall economic value of Rapid-EEG to the healthcare system.
Methods
Cost–benefit analysis considerations
Our decision analysis addresses cumulative costs incurred during inpatient hospitalization for suspected seizures in persons with altered consciousness who receive Rapid-EEG compared to costs incurred from treating based on clinical suspicion alone. Costs are assessed from the perspective of the health care system with a time horizon consisting of the window of inpatient care, from hospital admission to dischargeCitation12. We do not include further downstream outpatient expenditures or costs from post-discharge readmission. As the goal of the analysis is to examine the value that Rapid-EEG brings to a healthcare system, and the pricing for Rapid-EEG is variable depending on the selected hardware and software features each of which is subject to volume discount, we elected not to include an estimation of the retail price of the Rapid-EEG device. The model combines diagnostic characteristics from the DECIDE trial () with along with costs and length of stay information from nationally representative inpatient data. The analysis was crafted and reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) from the International Society of Pharmacoeconomics Outcomes Research (ISPOR)Citation13.
Table 1. DECIDE trial summary.
Decision analytic model structure
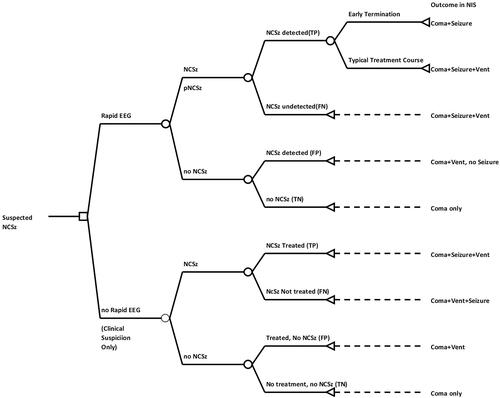
We built a two-armed decision tree model branching from a single decision node engaged at the time of suspected non-convulsive seizure activity ()Citation14,Citation15. The intervention arm represented the decision to use Rapid-EEG and act on its diagnostic results. The control arm represented acting on clinical suspicion alone, which encompasses treatment or non-treatment of suspected seizures in the absence of immediate electrographic diagnostic information. Both arms assumed that conventional EEG would be obtained but anticipated that the latency from time of ordering to recording actionable diagnostic information would necessitate clinically meaningful decision-making (including decision not to treat until conventional EEG acquisition) prior to recording and completion of conventional EEG. Subsequent event nodes logged the possible clinical events that occur because of the initial decision to perform Rapid-EEG or treat suspected seizures.
Figure 2. Decision tree model for cost–benefit analysis. Two-armed decision model with intervention (Rapid-EEG vs clinical suspicion only) at the point of suspected seizure and subsequent event nodes corresponding with diagnostic categories of Rapid-EEG interpretation (true positive, false positive, true negative, false negative). Length of stay data at terminal nodes correspond to Nationwide Inpatient Sample 2017 cross-sectional cohorts for coma (ICD10 codes R40.2*), seizure (Clinical Classification Software diagnostic grouper CCS-83), and mechanical ventilation (ICD10 procedure = 5A1935Z, 5A1945Z, 5A1955Z). Abbreviations. EEG, electroencephalography; FN, false negative; FP, false positive; NCSz, non-convulsive seizure; NIS, National Inpatient Sample; TN, true negative; TP, true positive.
For the intervention arm, the true positive and false positive detection of electrographic seizure was determined from the sensitivity and specificity, respectively, of Rapid-EEG from DECIDE trial. Identification of seizure (whether true or false positive) was assumed to lead to escalation of treatment with ASMs, which would result in the possibility of early seizure termination with benzodiazepines in the case of accurate seizure detection, or in the case of false positive seizure detection, erroneous administration of second-line ASMs and intubation with mechanical ventilation. Failure of early seizure termination with benzodiazepines was assumed to result in intubation, mechanical ventilation, and loading with further non-benzodiazepine ASMs.
For the control arm, we assumed that clinicians would act on their clinical suspicions, treating seizures when the level of suspicion was high, without the benefit of Rapid-EEG as described by the sensitivity and specificity of clinicians prior to Rapid-EEG in the DECIDE trial. In this case, we assumed that patients with non-convulsive seizures who were not treated prior to conventional EEG had no opportunity for early seizure termination with benzodiazepines, and these patients would require intubation, mechanical ventilation, and non-benzodiazepine ASM loading.
Model parameters
Model parameters, namely probabilities at each event node, length of stay metrics, and healthcare costs, are detailed in . We modeled the risk of NCSE from the weighted average of the prevalence of electrographic seizure from four cohort studies of NCSE in encephalopathic patientsCitation16–18,Citation22. The sensitivity and specificity of clinicians’ diagnostic suspicions before and after reviewing Rapid-EEG, compared to gold standard conventional EEG, from the DECIDE trial were used to define the diagnostic parameters for clinical suspicion alone (control arm) and for Rapid-EEG (intervention arm), respectivelyCitation11. The probability of early termination with benzodiazepines was taken from the results of a trial of a benzodiazepine seizure cessation protocol given at the time of suspected non-convulsive seizureCitation26. For treatment expendituresCitation24, we calculated intubation costs as the sum of reimbursed labor costs using Current Procedure Terminology (CPT) codesCitation25, disposable equipment costs (endotracheal tube, oxygen), and associated drug costs (sedatives, paralytics). Drug costs were estimated from most commonly used non-benzodiazepine intravenously administered ASMs for treatment of SECitation20. Length of stay (LOS) data was calculated as the mean LOS for persons in the National Inpatient Sample (NIS) dataset for 2017Citation23 with coding for coma (ICD10 code R422) with and without seizure and mechanical ventilation (the latter as a proxy for ICU treatment). Daily hospitalization costs were based on the 2019 Medicare rate for non-traumatic coma without major complications or comorbid conditions from the Medicare Part A Inpatient Prospective Payment SystemCitation21 for Medical Severity Diagnosis Related Group (MS-DRG code 81) as the national payment amount divided by the geometric mean LOS to give cost per inpatient day. Length of ICU stay was treated as a proportion of overall stayCitation27 and costs per day of ICU stay were the additional aggregated daily costs of a mechanically ventilated patient added to the daily hospitalization amountCitation28. All costs were inflated to 2019 dollars using the Consumer Price Index published by the US Bureau of Labor StatisticsCitation29.
Table 2. Model input parameters.
Model assumptions
Our model assumed that Rapid-EEG results would be acted upon, such that when non-convulsive seizure is determined, that patients will receive initial benzodiazepine treatment in hopes of early seizure termination, and failing this, will go on to parenteral loading of second-line ASMs, intubation, and mechanical ventilation with associated ICU stay as a function of treatment for seizure. We assumed that successful early seizure termination includes costs of hospitalization, but not the incremental costs of parenteral (non-benzodiazepine) ASMs, intubation or mechanical ventilation and affected LOS and ICU LOS. We further assumed that all patients in both arms would also eventually receive conventional (routine) EEG, and if found to have status epilepticus, would undergo continuous EEG monitoring. The costs of these services would be identical in each arm and therefore are not incorporated into the model.
Reference case
The model examines the net value of Rapid-EEG for the reference case of a single hospitalization, comparing the use and non-use of Rapid-EEG, accounting for the likely outcomes in each arm. As such, the cost–benefit model calculates net cost as the arithmetic difference in costs of the control arm (clinical suspicion alone, no Rapid-EEG) and the intervention arm (Rapid-EEG), where each possible outcome within treatment arms is weighted to likelihood of occurrence then summed to give an average cost per patient for each arm. We further evaluate length of stay in the hospital and ICU, the number of unnecessary intubations and parenteral ASMs given.
Uncertainty analysis
We created a probabilistic sensitivity analysis (PSA), randomly drawing from probability distributions for each parameter to model the possible outcomes. We utilized the method of moments to characterize each probability distribution. Summed intubation costs and daily hospitalization costs from fee schedules were varied on a uniform distribution (±20%), while other aggregated costs were modeled on lognormal and gamma (γ) distributions. Binomial proportions were modeled with beta (β) distributionsCitation30. We ran a Monte Carlo simulation (MCS) with 1,000 replications of Rapid-EEG versus clinical suspicion alone examining the outcomes detailed in the deterministic model. Each replication of the Monte Carlo simulation is a random draw of each parameter along its probability distribution, such that an individual replication could have, for example, a control sensitivity in the 97th percentile (Sensitivity = 1.0), or less than the third percentile Sensitivity = 0.42. Estimates are the means with 95% prediction intervals (PI) calculated from the standard errors from the replications. The simulation sample was analyzed using ANCOVA sum of squares to evaluate the effects of individual parameters on the overall outcome of net costs. In addition to the PSA, we performed a one-way sensitivity analysis evaluating the effects on the model conclusions from varying individual parameters across a range of values (0.5–1.0 for diagnostic parameters, ±50% of costs and length of stay, and plausible range for actual NCSE rates.) Simulation and statistical analyses were performed using Stata 16.0 (StataCorp, College Park, TX) and Microsoft Excel 365 (Microsoft Corp, Redmond, WA).
Results
Assuming a non-convulsive seizure prevalence of 21% in critically ill neurological patients that Rapid EEG is designed to be used for and given the findings from the DECIDE trial describing improved clinician diagnostic accuracy for non-convulsive seizures using Rapid-EEG compared to clinical suspicion alone, we calculated that use of Rapid-EEG would save an average of $3,971 ($21,261 in control arm vs. $17,290 in intervention arm) per patient during the course of a hospitalization for coma or encephalopathy. Rapid-EEG use was associated with a reduction in overall hospital LOS by 1.2 days (7.4 days in control arm vs. 6.2 days in intervention arm) and reduction in ICU LOS by 0.4 days (1.9 days in control arm vs. 1.5 days in intervention arm). Rapid-EEG also led to a 51% reduction in intubations and parenteral ASM treatments (45% in control arm vs. 22% in intervention arm). See for details.
Table 3. Results of reference case and probabilistic sensitivity analysis (PSA).
In the PSA, cost savings attributable to using Rapid-EEG instead of relying on clinical suspicion alone was $5,633 (95% PI: $4,649, $6,617) with reduced total hospital costs for the Rapid-EEG arm seen in 75% of replications (). This analysis also revealed that Rapid-EEG use was associated with reduction in hospital LOS by 1.1 days (95% PI: 0.9–1.4 days) and in ICU LOS by 0.5 days (95% PI: 0.4–0.6 days).
Figure 3. Monte Carlo simulation. This figure depicts results of Monte Carlo simulation with 1,000 replications of difference in costs between Rapid-EEG and clinical suspicion.
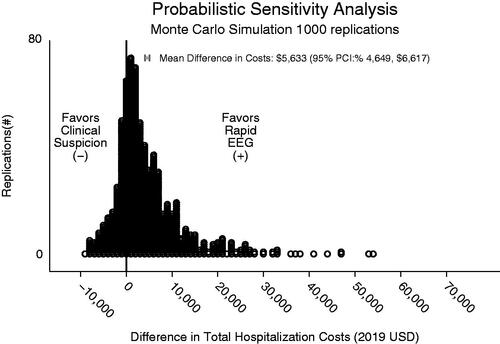
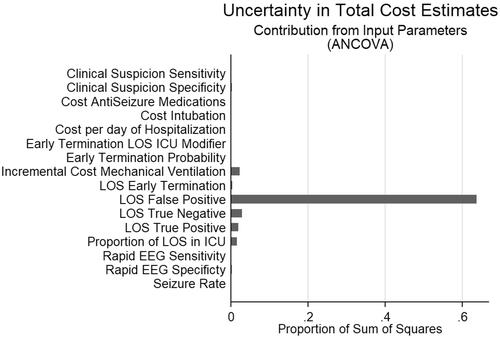
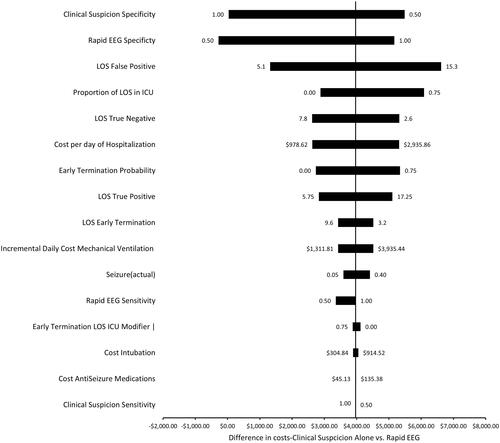
In ANCOVA sum of squares analysis of the effects of the model parameters on the primary outcome of difference in total costs (), 64% of the variance in total costs was explained by the LOS of persons who were incorrectly diagnosed with seizures (false positives) and therefore treated with parenteral ASMs, intubated, and mechanically ventilated. This was followed by the LOS of persons who were correctly diagnosed as not having seizures (true negatives, 3% of variance) and daily added cost of ICU stays/mechanical ventilation (2% of variance). The one-way sensitivity analysis likewise showed that factors influencing costs of false positives (LOS), including diagnostic specific and length of stay had the largest effect on the difference in total costs between intervention and control ().
Discussion
This decision analytic model comparing management of suspected non-convulsive seizure using Rapid Response EEG to conventional care relying on clinical suspicion alone pending availability of conventional EEG systems indicates average cost savings in downstream costs nearly $4,000 per patient due to early seizure termination from rapid diagnosis and avoidance of over-treatment that leads to prolonged hospital and ICU LOS. Through deploying a highly accurate and portable reduced EEG device with automatic seizure detection and remote review, clinicians have access to much needed information to confirm or deny their suspicions of electrographic seizure and act accordingly. Compare this to conventional care, in which a trained technologist has to navigate crowded inpatient wards with a bulky EEG machine consisting of a monitor and hardware on a wheeled cart, affix leads to the scalp with collodion, and troubleshoot impedance and signal issues before obtaining a single epoch of usable data that requires review by a trained neurologist. The process of acquiring EEG may take hours, followed by additional hours of delay until EEG interpretationCitation4,Citation6. Given the significant delays associated with confirming clinical suspicions with EEG, it is unsurprising that clinicians may opt for the better part of valor in treating suspected NCSE empirically.
From a business perspective, the information provided by Rapid-EEG allows for avoidance of opportunity costs that arise from either over-treatment or under-treatment. NCSE in the absence of available EEG is a diagnostic dilemma with the extremes of indecision (waiting for EEG) or maximal treatment (parenteral ASMs, intubation, mechanical ventilation) as the only viable options. While overtreatment, (even including excessive doses of benzodiazepines) increases the risk of intubation and mechanical ventilation, prolonging hospitalization and ICU staysCitation19, failure to treat SE early can lead to greater refractoriness when the seizures are treated.
NCSE may progress to refractory NCSE that becomes less responsive to first-line treatment (benzodiazepines) and second-line treatment (other non-benzodiazepine anticonvulsants) and requires sedative drips. Status epilepticus that has become refractory to treatment or even super-refractory (lasting > 24 h) can have profound consequences for length of stay and cost, as well as mortality (the latter not modeled here)Citation31. Given the timeliness with which Rapid-EEG can be acquired, we assumed that early seizure termination with carefully monitored doses of benzodiazepine observed in previously published data could be a plausible outcome, and we found that this could reduce ICU and overall hospital stay both in the literature and in our model. For cases in the intervention arm in which NCSE occurred but early seizure termination did not, we assumed the same treatment as in the non-Rapid-EEG arm (parenteral ASM loading, intubation, and mechanical ventilation).
The diagnostic parameters of Rapid-EEG and clinical suspicion from DECIDE allowed for assessment of uncertainty in the model. The model conclusions were most sensitive to parameters affecting the false positive rates and costs, highlighting that false positives are a primary major driver of costs in NCSE. When specificity in the control group neared 100% or dropped below 50% in the intervention group, Rapid-EEG was no longer cost-3saving. Greater expertise in SE in the control group could account for a gain in specificity in the controls, as in DECIDE non-EEG expert neurologists were asked to make treatment decisions on the basis of their own interpretation of the EEG at the bedside. In our model, Rapid-EEG sensitivity was important insofar as it allowed for the early treatment of NCSE, avoiding later intubation, hospitalization, and ICU costs. The sensitivity of clinical suspicion had little effect because the control arm had no means of determining the success of any early treatment, we assumed maximal treatment (non-benzodiazepine ASM, intubation, ventilation) would be necessary in this arm, with little functional difference in LOS and costs between false negatives and true positives. From the DECIDE trial, we found no instances where Rapid-EEG could not be applied or yielded no viable information. While the trial found that Rapid-EEG was 100% sensitive compared to the gold standard of conventional EEG, the device did not always yield perfect results as shown by its 89% specificity. In real-life situations, when EEG expert readers are available to evaluate the EEG remotely through Ceribell cloud portal, the accuracy of the EEG diagnosis (and the accuracy of physicians’ treatment choices) would be anticipated to improve the numbers in the trial and in the model.
In our current analysis, we assumed that Rapid-EEG will be used primarily by non-experts. With this assumption, we can reduce opportunity losses with Rapid-EEG, but some over-treatment will necessarily occur in the Rapid-EEG arm since EEG-non-expert physicians will make overcalls (especially when the EEG contains seizure-like activity but not actual seizure activity). Nevertheless, in the deterministic model, the number of patients receiving parenteral ASM loading, intubation, and mechanical ventilation in the Rapid-EEG arm was only one-third that of standard care. Likewise, unnecessary treatment overall was decreased by over two-thirds in the Rapid-EEG arm.
Our cost–benefit model is predicated on the idea that the gains in accurate diagnosis and correct treatment lead to cost savings for the hospital treating status epilepticus. Reduced healthcare expenditures are mediated by 20% shorter average ICU and total LOS and less expenditure in pharmaceutical and intubation costs. On net, costs to the hospital would be over 25% less when Rapid-EEG was utilized. We assume in all cases that conventional EEG will eventually be utilized, so the estimates given are only for the addition, not the substitution, of Rapid-EEG to standard care including conventional EEG. In hospitals where Rapid-EEG is being used to triage patients rapidly and decide that the patient may not need continuous monitoring, we predict that cost savings will be significantly higher.
Cost-effectiveness and cost–benefit models have been utilized to illustrate the clinical and financial benefits of neurodiagnostic interventions including intraoperative monitoring and continuous video-EEG monitoring. Relevant outcomes include cost per positive health event gained or adverse event avoided, effect on overall costs, or cost per quality adjusted life year (QALY), taken from a patient, hospital, payer, or societal perspective. A 3-year prospective multi-center randomized control trial assessing tele-continuous EEG compared to tele-routine EEG is ongoing, with a cost per QALY analysis as a secondary outcomeCitation32. Most economic evaluations in seizure management have focused on therapeutics, demonstrated by a systematic review by Wijnen et al.Citation33 where 73% of included studies evaluated pharmacotherapies, with the remainder examining epilepsy surgery, self management, and vagal nerve stimulation. In our case, we assess a new diagnostic intervention in the inpatient setting, modeling the impact on overall costs of hospitalization. We take the perspective of the hospital system providing the care, where expenditures here are derived from reimbursement costs from CMS (rather than hospital charges) and include the additional expenses of mechanical ventilation and ICU stays, which are markedly greater than a non-ICU bed stay. Consistent with the analysis from the hospital perspective, post-discharge expenses are not included.
Our analysis has several limitations. First, diagnostic parameters in the model are based on real-world data, but from a limited number of academic hospitals, and these may not be representative of Rapid-EEG’s performance in other venues. However, we assume that standard care at these facilities, where tertiary or quaternary epilepsy care is available with state-of-the-art equipment and technologists, meets or exceeds care in community care hospitals in terms of availability and optimal management. Second, our study is based on the hypothetical that Rapid-EEG would guide NCSE management, leading to better outcomes. We assume that clinicians would take the information obtained from Rapid-EEG at face value, such that seizure and lack of electrographic seizure activity diagnosed with Rapid-EEG would be sufficient for clinical decision-making. This model would not account for decisions to ignore Rapid-EEG results. Third, actual management of NCSE is dependent on the individual clinician, and adherence to guidelines may or may not occur, which can affect health care utilization and costs. Fourth, the estimated retail cost of the Rapid-EEG device was outside of the scope of this model which attempts to ascertain the cost-savings that can be accrued to a healthcare system when the device is available for use. Pricing for Rapid-EEG is in flux at the time of this writing and we did not want to provide a value that was inaccurate at publication. Fifth, our assumption that continuous EEG monitoring costs would be identical in both arms may be incorrect, as early seizure termination and aborting NCSE would have the benefit of not requiring continuous EEG, resulting in thousands of additional dollars of savings by Rapid-EEG. Therefore, our results presented here may be more conservative than those actually experienced in the real world. Our model is only as good as the data powering the parameters therein, and new data could contradict our results. In the words of mathematician George Box: “All models are wrong, but some are useful.”
Despite these limitations, this decision analytic model demonstrates that Rapid-EEG, a novel, quickly-applied EEG device, has the potential to reduce uncertainty in the time from suspected seizure to confirmed electrographic seizure, where clinicians may be making decisions without the informational benefit that conventional EEG provides. As this period may represent multiple hours of under-treatment for ongoing NCSE or over-treatment including parenteral ASM loading, intubation, and mechanical ventilation for non-seizing patients, the urgency of accurate and reliable EEG information is paramount and has substantial implications in inpatient cost savings.
Our current study joins a handful of other analyses in assessing the economic value of diagnostic interventions in clinical neurophysiologyCitation34–36. In a study by Hill et al.Citation36, it was found that use of continuous conventional EEG led to an increase in the cost and length of hospitalization and was not associated with reduced in-hospital mortality for patients with seizure/status epilepticus subgroup. Given the association between delayed diagnosis of seizures and poor prognosisCitation37,Citation38, and given that conventional EEG set up is labor intensive and delayed by several hours even in some of the best American hospitals,Citation11 Neligan and colleaguesCitation39 showed that the mortality of status epilepticus has not changed in the last 30 years, and as Guterman and BetjemanCitation40 commented in an accompanying editorial, faster diagnosis of seizures with new technologies may lead to different prognostic trajectory for seizures in the years ahead. In this context, while we have estimated the effect of Rapid-EEG on overall hospitalization costs, much work remains to be done to determine longitudinal effects of early and precise diagnosis of seizures including mortality and morbidity. We are mindful that any calculated benefit for Rapid-EEG to hospital costs may only be telling a portion of the story, as the ultimate beneficiary of optimally treated seizures and status epilepticus is the patient, and in aggregate, the US healthcare system and society as a whole.
Transparency
Declaration of funding
Funding was provided by Ceribell Inc.
Declaration of financial/other relationships
JP is inventor of Rapid EEG and founder of Ceribell Inc., a startup company based in Silicon Valley, CA that is commercializing the Rapid Response EEG system for clinical use. JPN and KG serve as scientific advisors to Ceribell and both have received consulting fees from Ceribell. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
JPN and JP designed the study. JPN performed statistical analysis and interpretation, and drafted the initial manuscript. All authors critically revised the manuscript and approved the final version. JPN takes full responsibility for the integrity of the data presented.
Previous presentation
Results from this study were presented at the 2020 American Clinical Neurophysiology Society (ACNS) Annual Meeting (Feb 5–9, New Orleans, LA).
Acknowledgements
Ceribell Inc. provided data from the DECIDE trial to inform statistical model parameters.
References
- Sutter R, Semmlack S, Kaplan PW. Nonconvulsive status epilepticus in adults – insights into the invisible. Nat Rev Neurol. 2016;12(5):281–293.
- Uppal P, Cardamone M, Lawson JA. Outcomes of deviation from treatment guidelines in status epilepticus: a systematic review. Seizure. 2018;58:147–153.
- Viarasilpa T, Panyavachiraporn N, Osman G, et al. Intubation for psychogenic non-epileptic attacks: frequency, risk factors, and impact on outcome. Seizure. 2020;76:17–21.
- Quigg M, Shneker B, Domer P. Current practice in administration and clinical criteria of emergent EEG. J Clin Neurophysiol. 2001;18(2):162–165.
- Kämppi L, Mustonen H, Soinila S. Analysis of the delay components in the treatment of status epilepticus. Neurocrit Care. 2013;19(1):10–18.
- Gururangan K, Razavi B, Parvizi J. Utility of electroencephalography: experience from a U.S. tertiary care medical center. Clin Neurophysiol. 2016;127(10):3335–3340.
- Gururangan K, Razavi B, Parvizi J. Diagnostic utility of eight-channel EEG for detecting generalized or hemispheric seizures and rhythmic periodic patterns. Clin Neurophysiol Pract. 2018;3:65–73.
- Westover MB, Gururangan K, Markert MS, et al. Diagnostic value of electroencephalography with ten electrodes in critically ill patients. Neurocrit Care. 2020;33(2):479–490.
- Kamousi B, Grant AM, Bachelder B, et al. Comparing the quality of signals recorded with a rapid response EEG and conventional clinical EEG systems. Clin Neurophysiol Pract. 2019;4:69–75.
- Hobbs K, Krishnamohan P, Legault C, et al. Rapid bedside evaluation of seizures in the ICU by listening to the sound of brainwaves: a prospective observational clinical trial of Ceribell’s brain stethoscope function. Neurocrit Care. 2018;29(2):302–312.
- Vespa PM, Olson DM, John S, et al. Evaluating the clinical impact of rapid response electroencephalography: the DECIDE multicenter prospective observational clinical study. Crit Care Med. 2020;48(9):1249–1257.
- Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in health and medicine. New York (NY): Oxford University Press; 1996.
- Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)-explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250.
- Philips Z, Bojke L, Sculpher M, et al. Good practice guidelines for decision-analytic modelling in health technology assessment: a review and consolidation of quality assessment. Pharmacoeconomics. 2006;24(4):355–371.
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. New York (NY): Oxford University Press; 2006.
- Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62(10):1743–1748.
- Kurtz P, Gaspard N, Wahl AS, et al. Continuous electroencephalography in a surgical intensive care unit. Intensive Care Med. 2014;40(2):228–234.
- Oddo M, Carrera E, Claassen J, et al. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37(6):2051–2056.
- Spatola M, Alvarez V, Rossetti AO. Benzodiazepine overtreatment in status epilepticus is related to higher need of intubation and longer hospitalization. Epilepsia. 2013;54(8):e99–e102.
- Sánchez Fernández I, Gaínza-Lein M, Lamb N, et al. Meta-analysis and cost-effectiveness of second-line antiepileptic drugs for status epilepticus. Neurology. 2019;92(20):e2339–e2348.
- DRG data files, files for FY 2019 final rule and correction notice [Internet]. Rockville, MD: Centers for Medicare and Medicaid Services; 2019 [cited 2020 May 1]. Available from: http://www.cms.gov
- Schmitt SE. Utility of clinical features for the diagnosis of seizures in the intensive care unit. J Clin Neurophysiol. 2017;34(2):158–161.
- National inpatient sample: Healthcare Cost and Utilization Project (HCUP) [Internet]. Rockville (MD): Agency for Healthcare Research and Quality; 2017 [cited 2020 May 1]. Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp
- Mihaylova B, Briggs A, O’Hagan A, et al. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20(8):897–916.
- Physician fee schedule, by HCPCS code [Internet]. Baltimore (MD): Center for Medicare and Medicaid Services; 2020 [cited 2020 May 1]. Available from: www.cms.gov
- Aranda A, Foucart G, Ducassé JL, et al. Generalized convulsive status epilepticus management in adults: a cohort study with evaluation of professional practice. Epilepsia. 2010;51(10):2159–2167.
- Hay A, Bellomo R, Pilcher D, et al. Characteristics and outcome of patients with the ICU Admission diagnosis of status epilepticus in Australia and New Zealand. J Crit Care. 2016;34:146–153.
- Dasta JF, McLaughlin TP, Mody SH, et al. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271.
- Consumer price index [Internet]. Washington (DC): Bureau of Labor Statistics, U.S. Department of Labor; 2020 [cited 2020 August 17]. Available from: http://www.bls.gov/cpi/
- Briggs AH, Goeree R, Blackhouse G, et al. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making. 2002;22(4):290–308.
- Strzelczyk A, Ansorge S, Hapfelmeier J, et al. Costs, length of stay, and mortality of super-refractory status epilepticus: a population-based study from Germany. Epilepsia. 2017;58(9):1533–1541.
- Limotai C, Ingsathit A, Thadanipon K, et al. Efficacy and economic evaluation of delivery of care with tele-continuous EEG in critically ill patients: a multicentre, randomised controlled trial (Tele-cRCT) study protocol. BMJ Open. 2020;10(3):e033195.
- Wijnen BFM, van Mastrigt G, Evers S, et al. A systematic review of economic evaluations of treatments for patients with epilepsy. Epilepsia. 2017;58(5):706–726.
- Abend NS, Topjian AA, Williams S. Could EEG monitoring in critically ill children be a cost-effective neuroprotective strategy? J Clin Neurophysiol. 2015;32(6):486–494.
- Ney JP, van der Goes DN, Nuwer MR, et al. Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005-2009. Neurology. 2013;81(23):2002–2008.
- Hill CE, Blank LJ, Thibault D, et al. Continuous EEG is associated with favorable hospitalization outcomes for critically ill patients. Neurology. 2019;92(1):e9–e18.
- Lowenstein DH, Alldredge BK. Status epilepticus at an urban public hospital in the 1980s. Neurology. 1993;43(3):483–488.
- Gainza-Lein M, Sanchez Fernandez I, Jackson M, et al. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol. 2018;75(4):410–418.
- Neligan A, Noyce AJ, Gosavi TD, et al. Change in mortality of generalized convulsive status epilepticus in high-income countries over time: a systematic review and meta-analysis. JAMA Neurol. 2019;76(8):897.
- Guterman EL, Betjemann JP. Status epilepticus mortality-improving or status quo? JAMA Neurol. 2019;76(8):885.