Abstract
Aims
To determine the effect of antiviral agents on influenza-related complications, health care resource utilization (HRU), and costs over three influenza seasons (2014–2016).
Methods
This retrospective cohort study used claims data from the U.S. MarketScan Research Databases. Patients with a diagnosis code for influenza during the 2014–2016 seasons in an outpatient setting, with continuous enrollment from 1 year before to 91 d after diagnosis, were included. Patients who received an antiviral within 48 h of diagnosis were identified and propensity score–matched to a comparator cohort of untreated patients on baseline demographics, comorbid conditions, and HRU. Outcomes were assessed at days 30 and 90 after diagnosis and included respiratory-related complications (all respiratory-related and selected respiratory-related conditions [influenza, asthma, chronic obstructive pulmonary disease, or infection]), HRU, and costs.
Results
Treated and matched untreated cohorts each consisted of 362,818 patients. HRU was significantly lower in the treated cohort compared with the untreated cohort at 30 and 90 d after diagnosis, respectively (hospitalizations: 0.6% vs. 0.8% and 1.2% vs. 1.6%; emergency department [ED] visits: 4.1% vs. 4.9% and 7.9% vs. 9.2%; intensive care unit/critical care unit (ICU/CCU) admissions: 0.2% vs. 0.4% and 0.4% vs. 0.6%). Respiratory-related HRU was lower in the treated cohort at both 30 and 90 d after diagnosis (p < .0001 for both periods). Mean all-cause total costs (including prescription costs) were significantly reduced in the treated group (day 30: $633 vs. $778; day 90: $1778 vs. $2119), despite higher prescription costs in the treated group.
Limitations
The study was retrospective and subject to residual selection bias, despite propensity score matching. Additionally, despite its potential relevance to influenza severity, vaccination status was not available in our data.
Conclusions
Antiviral influenza treatment is associated with a significant reduction in complications, HRU, and costs at 30 and 90 d after diagnosis.
Introduction
Seasonal influenza is an important public health burden worldwide, with substantial variation in disease burden over seasons and across populations. The virulence, type, and spread of circulating influenza strains varies seasonallyCitation1. The 2017–2018 influenza season had a high severity in the United States, with high rates of outpatient and emergency department (ED) visits and hospitalizationsCitation2. Based on surveillance data, the cumulative incidence of hospitalization for 2017–2018 was 102.9 per 100,000 population overall, and among Americans aged 50–64 years was 112.8 per 100,000 population, compared with 62.7, 45.1, and 53.4 in the preceding three influenza seasonsCitation3. Pneumonia- and influenza-associated mortality rates exceeded 10.0% over four consecutive weeks during the 2017–2018 influenza season in the United StatesCitation2.
Every year, a substantial burden of lower respiratory infections and other respiratory conditions (such as chronic obstructive pulmonary disease [COPD]) are attributable to seasonal influenzaCitation4,Citation5. Among adults hospitalized for influenza, >90% have an underlying medical condition that increases the risk for complicationsCitation2. Worldwide, in 2017, an estimated 55 million episodes of lower respiratory tract infections were attributable to influenza, of which approximately 8 million were severe, leading to almost 1.5 million deaths and 9.5 million hospitalizations; the greatest number of episodes, hospitalizations, and deaths were in younger children and elderly adultsCitation4. Persons at increased risk for influenza-related complications such as pneumonia include children <2 years and adults ≥65 years, residents of long-term care facilities, pregnant women, and persons with certain chronic medical conditionsCitation6,Citation7. Associated health care costs and productivity losses due to influenza are greatest among high-risk groups and are also not trivial in non-high-risk groups. In 2015, the estimated average annual economic burden of influenza to the health care system was $11.2 billion, with $3.2 billion, and $8.0 billion attributed to direct and indirect costs, respectivelyCitation8. Across eight influenza seasons from the 2001–2002 to 2008–2009 seasons, annual U.S. health care costs attributable to influenza ranged from $2.0 billion to $5.8 billion, averaging $3.5 billion annuallyCitation9.
One of the primary strategies for reducing the effect of influenza in the community is vaccination, which is known to both prevent and limit the severity of influenza infection, although coverage and seasonal effectiveness remain suboptimalCitation10. Neuraminidase inhibitors may also be prescribed to reduce disease duration and improve clinical outcomes. The Infectious Diseases Society of America recommends that clinicians consider antiviral treatment with neuraminidase inhibitors within 48 h of illness onset not only for individuals at higher risk for influenza complications but also for uncomplicated influenza in non-high-risk persons in contact with high-risk individualsCitation11. Early treatment with neuraminidase inhibitors relieves influenza symptoms, reducing both their severity and durationCitation12–14. Several studies have also shown that antiviral treatment lowers the risk of complications, reduces health care resource utilization (HRU), and decreases mortality in hospitalized patientsCitation15–21. However, one review reported no significant effect of neuraminidase inhibitors on hospitalizations for adults or children and called into question the effectiveness of early antiviral treatment in reducing complications of influenzaCitation13.
Effective control and management of seasonal influenza is a high public health priorityCitation22. Although symptom relief has been seen as the most common measure of influenza treatment efficacy, understanding how antiviral treatment affects rates and severity of influenza complications is also important, as this information can help guide treatment decisions both in clinical practice and at a health system level. This study used real-world U.S. claims data from three influenza seasons (2014, 2015, and 2016) to determine the frequency of influenza complications and to understand how intervention with antiviral agents may affect their occurrence. The objectives were to examine the effect of antiviral use on HRU and costs, including those related to respiratory conditions, in treated and untreated influenza patients over three influenza seasons.
Methods
Data source
Data were extracted from the MarketScan Commercial Claims and Encounters Database and the MarketScan Medicare Supplemental and Coordination of Benefits Database (IBM Watson Health, Cambridge, MA). The databases include information on health insurance claims of employees, dependents, and retirees insured by employer-sponsored commercial and Medicare insurance. The claims files capture inpatient and outpatient care, use of facilities and services, prescription fills, and payment information. Each medical claim has up to 15 diagnosis codes (ICD-9-CM [International Classification of Diseases, 9th Revision, Clinical Modification], ICD-10) and 15 procedure codes (ICD Procedure Coding System [PCS], Current Procedural Terminology [CPT], and Healthcare Common Procedure Coding System [HCPCS]) documented for billing purposes. Pharmacy claims record the National Drug Code, dispense date, quantity and days supplied, and payments. The study used de-identified data and was exempt from Institutional Review Board review. The research was compliant with the Health Insurance Portability and Accountability Act.
Study cohorts
The study sample included patients with a claim associated with a diagnosis of influenza (ICD-9 codes 487.xx or 488.xx; ICD-10 codes J09.xx, J10.xx, or J11.xx) during three influenza seasons: 2014 season: 1 May 2014–30 April 2015; 2015 season: 1 May 2015–30 April 2016; 2016 season: 1 May 2016–30 April 2017. The index date was defined as the date of the first influenza diagnosis in the season. Patients had to be continuously enrolled from 1 year before to 91 d after the index date. Patients who had their first influenza diagnosis in the inpatient setting were excluded from the study.
Patients were grouped into the treated cohort if they had a claim for one of the antiviral treatments (oseltamivir, zanamivir, rimantadine, or peramivir) within 2 d of the index date.
For each season, influenza patients who did not receive treatment were propensity score–matched to treated patients to create the untreated cohort. Propensity scores were derived from logistic regression models that included the following variables in each influenza season: age categories, sex, geographic region, type of health plan, Charlson comorbidity index (CCI) score, month of index event (influenza diagnosis), HRU in preceding 12 months (any inpatient or ED claim), and any HRU for asthma, COPD, cystic fibrosis, overweight/obesity, and pregnancy in the preceding 12 months.
Outcomes
The main outcomes included all-cause and respiratory-related HRU and costs.
Outcomes were cumulated over days 30 and 90 after the index date for the three influenza seasons and included events starting 1 d after the index date. Two types of respiratory-related HRU were assessed: HRU for all-respiratory-related conditions, which included a broad range of conditions (ICD-9 codes 460–519; or ICD-10 codes J00–J99) and HRU that focused on selected respiratory-related conditions: influenza (ICD-9 codes 487 or 488; or ICD-10 codes J10 or J11), asthma (ICD-9 code 493; or ICD-10 code J45), COPD (ICD-9 codes 490–492, 494–496; or ICD-10 codes J40–J44, J47, J67), or infection (ICD-9 codes 464, 466, 480–487; or ICD-10 codes J10–J16, J18, J20–J21). For all respiratory-related and selected respiratory-related conditions, hospitalizations were identified using the primary diagnosis, while ED or outpatient visits were identified using all diagnosis fields in the claims. HRU and costs were assessed by healthcare setting (inpatient, outpatient, ED, and pharmacy). With regard to HRU for disease-specific selected respiratory-related conditions, inpatient claims for a primary diagnosis were counted if they had a matching diagnosis code; outpatient claims were counted if there was a matching diagnosis in one of five diagnosis fields.
We further assessed intensive care unit/critical care unit (ICU/CCU) and mechanical ventilator use during the follow-up periods. Pharmacy encounters were recorded as the number of fills within the specified time period.
Costs were calculated as medical costs by care settings (inpatient, ED, and outpatient), prescription costs (outpatient pharmacy services), and total healthcare costs (medical plus prescription). Costs were adjusted to 2017 U.S. dollars using the medical care component of the Consumer Price IndexCitation23.
Statistical analysis
Complications and HRU 30 and 90 d after diagnosis were compared between patients who received antiviral treatment and patients who did not. Cohorts were compared with the nonparametric chi-squared test for categorical measures, nonparametric Wilcoxon rank-sum test for counts and costs, and Student’s t-test for age. Analyses were conducted using SAS version 9.4 software (SAS Institute Inc., Cary, NC).
Results
Study population
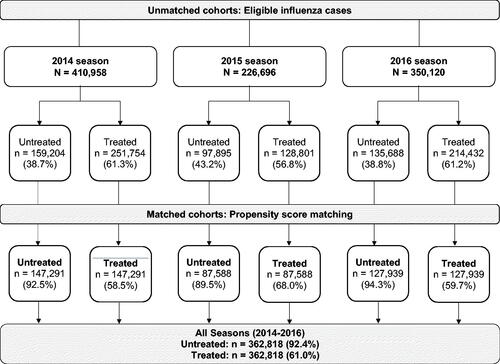
During the 2014, 2015, and 2016 influenza seasons, the MarketScan database covered a total of 19.8, 19.4, and 18.1 million individuals, respectively. Of these, 466,492 (2.4%), 241,826 (1.2%), and 407,674 (2.3%) cases had a claim with a diagnosis code for influenza during the 2014, 2015, and 2016 seasons, respectively. After exclusion of cases that had an index episode in the inpatient setting and those did not fulfill the continuous enrollment criteria, a total of 987,774 eligible cases were identified (). Overall, 60.2% of patients had received antiviral treatment, and the percentage of patients receiving antiviral treatment in each influenza season was similar and ranged between 56.8% and 61.3%. Using propensity score matching, a treated and an untreated cohort with 362,818 patients each was identified for the 2014, 2015, and 2016 influenza seasons combined ().
Baseline characteristics
No differences in baseline characteristics were observed across influenza seasons (). Of the identified influenza cases over three seasons, the mean age was 28.9 years, 10.1% were younger than 5 years, and 4.6% were older than 65 years; 4.3% of patients overall had been hospitalized and 20.9% visited the ED in the preceding year. The average CCI score was 0.28 in both cohorts. The proportion of patients with medical encounters for COPD (3.2%), asthma (8.4%), and obesity (5.8%) in the preceding year were similar in matched cohorts.
Table 1. Baseline demographic and clinical characteristics of matched cohorts for all influenza seasons combined (2014–2016) and individual influenza seasons (2014, 2015, 2016)a.
Health care resource utilization
All-cause HRU over the first 30 d for all three seasons combined was significantly lower in the treated than in the untreated cohort (hospitalizations: 0.6% vs. 0.8%; ED use: 4.1% vs. 4.9%; ICU/CCU admission: 0.2% vs. 0.4%; mechanical ventilation: <0.1% vs. 0.1%; p < .0001 for all comparisons) (). Lower HRU in the treated cohort for all seasons combined was maintained over the 90-d period (hospitalizations: 1.2% vs. 1.6%; ED use: 7.9% vs. 9.2%; ICU/CCU admission: 0.4% vs. 0.6%; mechanical ventilation: <0.1% vs. 0.1%; p < .0001 for all comparisons). For both follow-up periods, hospitalizations were 25% lower in the antiviral-treated cohort. Likewise, at both 30 and 90 d, data for individual influenza seasons also showed that significantly fewer patients in the treated cohort required use of health care services compared with the untreated cohort.
Table 2. Health care resource utilization up to follow-up days 30 and 90 in matched cohorts for all influenza seasons combined (2014–2016) and individual influenza seasons (2014, 2015, and 2016)a.
For all respiratory-related conditions and selected respiratory-related conditions, treated patients were significantly less likely than untreated patients to be hospitalized or have ED visits during both the first 30 and 90 d post-diagnosis for all seasons combined (p < .0001 for both follow-up periods) (). In general, HRU was lower in the treated cohort also during each individual influenza season.
In both the first 30 and 90 d, the mean number of hospitalizations, ED visits, and outpatient visits for any cause was significantly lower in the treated cohort than in the untreated cohort (p < .0001 for all comparisons, except for 90-d outpatient visits [p < .001]) (). However, the number of prescriptions filled was slightly higher in the treated group. A similar trend was noted for individual influenza seasons (Supplementary Table 1). ED and outpatient visits for all respiratory-related and selected respiratory-related complications were also lower in the treated cohort than in the untreated cohort at both 30 and 90 d after diagnosis ().
Table 3. Mean number of visits/stays/prescription fills up to follow-up days 30 and 90 in matched cohorts for all influenza seasons combined (2014–2016)a.
Costs
Influenza patients treated with antivirals during the three seasons combined had significantly lower mean all-cause total costs (including prescription costs) and medical costs (inpatient, ED, and outpatient) over the first 30 d in the treated cohort compared with the untreated cohort (mean standard error [SE] total costs: $633 [8] vs. $778 [11]), despite higher prescription costs (mean [SE]:$140 [2] vs. $122 [2]) (). Mean 90-d all-cause total and medical costs were also lower in the treated group than in the untreated group (mean [SE] total costs: $1778 [17] vs. $2119 [23]; mean [SE] medical costs: $1366 [16] vs. $1757 [23]); again, prescription costs were higher (mean [SE], $412 [5] vs. $362 [5]) in the treated group (). Lower total and medical costs at days 30 and 90 were also reported for the treated cohort for each influenza season (Supplementary Table 2). Mean [SE] medical costs for all respiratory-related complications were significantly lower in the treated group both at day 30 ($102 [2] vs. $160 [5]) and at day 90 ($197 [5] vs. $310 [10]) (). Mean [SE] medical costs for selected respiratory-related conditions for the three seasons combined were also significantly lower in the treated groups at both day 30 ($62 [2] vs. $93 [2]) and day 90 ($98 [2] vs. $151 [4]).
Table 4. Mean health care costs up to follow-up days 30 and 90 in matched cohorts for all influenza seasons combined (2014–2016)a,b.
Discussion
Influenza is a highly variable seasonal disease with far-reaching impact that extends beyond its most common symptoms. Although prevention of influenza through limiting exposure, limiting transmission, and, importantly, through yearly vaccination remains one of the primary methods of decreasing the impact of influenza, treating acute influenza infection with antivirals is also an essential component. This study used real-world claims data from the last three available influenza seasons to analyze the effect of antiviral treatment on the frequency of complications, HRU, and costs. The study included a large dataset of almost 1 million patients with a diagnosis of influenza who had continuous insurance coverage over the year preceding the first influenza diagnosis and up to 91 d after. Over the three influenza seasons analyzed, an average of only 2% of covered patients sought care for influenza each year based on a claim with an influenza diagnosis code. By comparison, the Centers for Disease Control and Prevention estimate for the annual incidence of influenza in the United States was 8%Citation24,Citation25. Our study was limited to commercially insured patients who sought professional care for influenza and clearly underrepresents the full burden of influenza because self-treatment for influenza is common in the general population, and any influenza-related complications or HRU arising from self-treated cases will not be reflected in administrative claims data.
The proinflammatory cascade triggered in response to influenza viral infection is well understood and often linked to significant morbidity and, in some cases, even mortality. A recently published study indicated that these effects can persist in COPD patients for as long as 1 year after acute influenza infectionCitation26. The primary objective of this study was to understand the burden of influenza-associated complications, HRU, and costs for a commercially insured population. The results show that compared with untreated patients, those treated with a neuraminidase inhibitor had significantly lower HRU over the first 30 d and up to at least 90 d after diagnosis. The results were similar for general respiratory-related complications and for specific respiratory-related conditions known to be affected by influenza, such as asthma, COPD, and respiratory infection (including pneumonia). The finding of increased HRU up to 90 d after diagnosis suggests that influenza continues to have longer-term deleterious effects even after the acute phase has resolved and that antiviral treatment may mitigate these effects. The reduction in HRU with antiviral use was reflected in significantly lower overall and medical all-cause costs at each time period, despite higher prescription costs. Additionally, this study found that among patients who sought care and were diagnosed with influenza, only ∼60% were treated with antivirals. A large segment of the influenza patient population thus remained untreated. Use of antivirals in this group thus has the potential not only to improve patient outcomes but also to confer cost savings from a health system perspective.
In our study, there were fewer complications and hospitalizations following anti-influenza treatment, findings that are broadly consistent with those of previous meta-analyses of randomized trials and database studies. A study that used pooled data from 10 placebo-controlled randomized trials showed that antiviral treatment of patients with confirmed influenza reduced lower respiratory tract complications by 55% and reduced any-cause hospitalization by 59% compared with placeboCitation19; the findings were subsequently confirmed in updated analysesCitation18,Citation27. Results of other claims-based analyses also support these findings. A recent study that included more than 1.5 million cases from four influenza seasons using the U.S. MarketScan Research Databases reported decreases of 11%, 29%, 24%, and 11% in complications, risk of hospitalization, ED visits, and need for two or more outpatient visits among patients receiving antiviral treatmentCitation17. In another retrospective analysis from six influenza seasons (2000–2006), antiviral treatment reduced secondary complications from influenza and hospitalization for any reason but not hospitalization for respiratory disease or health care expendituresCitation28. In children and adolescents with chronic medical conditions, a claim-based study using data from six influenza seasons showed that individuals prescribed a neuraminidase inhibitor at diagnosis had a reduced rate of respiratory complications and all-cause hospitalizationsCitation29. The cost impact of anti-influenza treatment was shown in another claims-based study for five influenza seasons (2001–2006) in which both total health care costs as well as costs related to hospitalizations (pneumonia-related and non-pneumonia-related) were significantly reduced in treated patientsCitation30.
Limitations
Some limitations of this study could affect the conclusions that can be drawn. As the study was retrospective, randomization was not used to allocate patients to the treated and untreated cohorts; although propensity score matching was used to reduce the risk of selection bias, the possibility of residual bias cannot be discounted. The database represents individuals enrolled in commercial health plans and some supplemental Medicare plans and may therefore not be representative of the entire U.S. population. Because of limitations with our data set, elderly individuals, who are at higher risk of complications, were underrepresented in this study (<5% of cases were ≥65 years); the geographic distribution was also unbalanced, with 57.4% of cases from the South (). Despite its importance in limiting the impact of influenza in both the community and individuals, vaccination status was not available in the claims data used in the present study and thus was not controlled for in matching. This could have impacted our results because vaccination can reduce the severity of influenza complications and may not have been evenly distributed among cohorts. In addition, while this study focused on respiratory complications of influenza, other serious complications or exacerbations of underlying diseases can also be triggered by influenza. Additionally, the influenza cases included in the study were identified by clinical diagnosis and were not necessarily confirmed by laboratory-based influenza testing; they may therefore have included cases with other viral illnesses identified as influenza. Further, patient selection was based on diagnosis codes in the claims databases that were not verified by review of medical records; this tactic could have resulted in unconfirmed events being included or conditions being missed because of coding errors, both of which would lead to inaccurate estimates of eventsCitation31–33. Patients were included in the treated group based solely on a prescription claim and, as with studies of this design, we were unable to verify that patients actually took the medication.
Conclusions
Data for the last three available influenza seasons (2014, 2015, and 2016) showed that influenza patients treated with antiviral therapy had significantly lower HRU and costs over the 30- and the 90-d periods after a diagnosis of influenza. Reduced HRU was observed across multiple health care settings, including hospitalizations, outpatient visits, ED use, and ICU/CCU admission, and for mechanical ventilation. Overall costs were also significantly lower in treated cohorts than in untreated cohorts, despite higher prescription costs. HRU and costs for complications, and specifically, respiratory-related conditions and selected respiratory-related conditions such as influenza, asthma, COPD, and infections, were also significantly reduced in the antiviral-treated cohort. These findings suggest that timely treatment of influenza may improve patient outcomes and lower costs to the health system.
Transparency
Declaration of funding
The study was sponsored and funded by Genentech, Inc., South San Francisco, CA.
Declaration of financial/other relationships
All authors are current or past employees of Genentech/Roche, Inc. and hold Roche stock. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved with all aspects of the manuscript – data collection and analysis as well as manuscript review and approval.
Previous presentations
This study was presented in poster format at IDWeek 2018, San Francisco, CA, October 3–7, 2018.
Supplemental Material
Download MS Word (18.1 KB)Supplemental Material
Download MS Word (19.2 KB)Acknowledgements
Meher M. Dustoor, PhD, Melissa L. Bogen, ELS, and Esther Tazartes, MS, of Global Outcomes Group provided editorial assistance; these services were funded by Genentech, Inc.
Data availability statement
The data that support the findings of this study are available from IBM MarketScan Research Databases, but these data are not publicly available. All relevant data are provided within the manuscript and supporting files.
References
- World Health Organization. Fact sheet: influenza (Seasonal); 2020. [cited 2020 March 22]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal)
- Garten R, Blanton L, Elal AIA, et al. Update: influenza activity in the United States during the 2017-18 season and composition of the 2018-19 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2018;67(22):634–642.
- Centers for Disease Control and Prevention. FluView: laboratory-confirmed influenza hospitalizations; 2020. [cited 2020 March 22]. Available from: https://gis.cdc.gov/grasp/fluview/fluhosprates.html
- GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7(1):69–89.
- Iuliano AD, Roguski KM, Chang HH, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. Lancet. 2018;391(10127):1285–1300.
- Centers for Disease Control and Prevention. People at high risk of developing serious flu-related complications. 2018. [cited 2020 March 22]. Available from: https://www.cdc.gov/flu/about/disease/high_risk.htm
- Mertz D, Kim TH, Johnstone J, et al. Populations at risk for severe or complicated influenza illness: systematic review and meta-analysis. BMJ. 2013;347:f5061.
- Putri W, Muscatello DJ, Stockwell MS, et al. Economic burden of seasonal influenza in the United States. Vaccine. 2018;36(27):3960–3966.
- Yan S, Weycker D, Sokolowski S. US healthcare costs attributable to type A and type B influenza. Hum Vaccin Immunother. 2017;13(9):2041–2047.
- Lu PJ, Singleton JA, Euler GL, et al. Seasonal influenza vaccination coverage among adult populations in the United States, 2005–2011. Am J Epidemiol. 2013;178(9):1478–1487.
- Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1–e47.
- Heneghan CJ, Onakpoya I, Jones MA, et al. Neuraminidase inhibitors for influenza: a systematic review and meta-analysis of regulatory and mortality data. Health Technol Assess. 2016;20(42):1–242.
- Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014; 4:CD008965.
- Burch J, Paulden M, Conti S, et al. Antiviral drugs for the treatment of influenza: a systematic review and economic evaluation. Health Technol Assess. 2009;13(58):1–265.
- Muthuri SG, Venkatesan S, Myles PR, et al. Impact of neuraminidase inhibitors on influenza A(H1N1)pdm09-related pneumonia: an individual participant data meta-analysis. Influenza Other Respir Viruses. 2016;10(3):192–204.
- Doll MK, Winters N, Boikos C, et al. Safety and effectiveness of neuraminidase inhibitors for influenza treatment, prophylaxis, and outbreak control: a systematic review of systematic reviews and/or meta-analyses. J Antimicrob Chemother. 2017;72(11):2990–3007.
- Spagnuolo PJ, Zhang M, Xu Y, et al. Effects of antiviral treatment on influenza-related complications over four influenza seasons: 2006-2010. Curr Med Res Opin. 2016;32(8):1399–1407.
- Hernan MA, Lipsitch M. Oseltamivir and risk of lower respiratory tract complications in patients with flu symptoms: a meta-analysis of eleven randomized clinical trials. Clin Infect Dis. 2011;53(3):277–279.
- Kaiser L, Wat C, Mills T, et al. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163(14):1667–1672.
- Cooper NJ, Sutton AJ, Abrams KR, et al. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ. 2003;326(7401):1235.
- Dobson J, Whitley RJ, Pocock S, et al. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385(9979):1729–1737.
- Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices-United States, 2018–2019 Influenza Season. MMWR Recomm Rep. 2018;67(3):1–20.
- U.S. Bureau of Labor Statistics. Measuring price change in the CPI: medical care; 2020. [cited 2020 November 24]. Available from: https://www.bls.gov/cpi/factsheets/medical-care.htm.
- Centers for Disease Control and Prevention. Background document for "Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2018-19 Influenza Season”. August 23, 2018. [cited 2020 March 22]. Available from: https://www.cdc.gov/vaccines/acip/index.html.
- Tokars JI, Olsen SJ, Reed C. Seasonal incidence of symptomatic influenza in the United States. Clin Infect Dis. 2018;66(10):1511–1518.
- Wallick C, To TM, Korom S, et al. Impact of influenza on the short- and long-term health of patients with chronic obstructive pulmonary disease. Poster session presented at: 6th International Society for Influenza and other Respiratory Virus Diseases (ISIRV)-Antiviral Group (AVG) conference. Nov 13–15; Rockville, MD: 2018.
- Lipsitch M, Hernan MA. Oseltamivir effect on antibiotic-treated lower respiratory tract complications in virologically positive randomized trial participants. Clin Infect Dis. 2013;57(9):1368–1369.
- Blumentals WA, Schulman KL. Impact of oseltamivir on the incidence of secondary complications of influenza in adolescent and adult patients: results from a retrospective population-based study. Curr Med Res Opin. 2007;23(12):2961–2970.
- Piedra PA, Schulman KL, Blumentals WA. Effects of oseltamivir on influenza-related complications in children with chronic medical conditions. Pediatrics. 2009;124(1):170–178.
- Gums JG, Pelletier EM, Blumentals WA. Oseltamivir and influenza-related complications, hospitalization and healthcare expenditure in healthy adults and children. Expert Opin Pharmacother. 2008;9(2):151–161.
- Keren R, Wheeler A, Coffin SE, et al. ICD-9 codes for identifying influenza hospitalizations in children. Emerg Infect Dis. 2006;12(10):1603–1604.
- Feemster KA, Leckerman KH, Middleton M, et al. Use of administrative data for the identification of laboratory-confirmed influenza infection: the validity of influenza-specific ICD-9 codes. J Pediatric Infect Dis Soc. 2013;2(1):63–66.
- Amodio E, Tramuto F, Costantino C, et al. Diagnosis of influenza: only a problem of coding? Med Princ Pract. 2014;23(6):568–573.