Abstract
Aims
Hemophilia B (HB) is a rare congenital disorder characterized by bleeding-related complications which are managed by prophylactic or post-bleeding event (“on-demand”) replacement of clotting factor IX (FIX). The standard of care for severe HB is life-long prophylaxis with standard half-life (SHL) or extended half-life (EHL) products given every 2–3 or 7–14 days, respectively. FIX treatment costs in the US have been investigated, but the lifetime costs of HB treatment have not been well characterized, particularly related to the impact of joint health deterioration and associated health resource utilization. We developed a decision-analytic model to explore outcomes, costs and underlying cost drivers associated with FIX treatment options over the lifetime of an adult with severe or moderately severe HB.
Materials and methods
With participation from clinicians, health technology assessment specialists and patient advocates, a Markov model was constructed to estimate bleeding events and costs associated with health states including “bleed into joint”, “bleed not into joint”, “no bleed” and “death”. Sub-models of joint health were based on 0, 1, or ≥2 areas of chronic joint damage. US third-party payer and societal perspectives were considered with a lifetime horizon; sensitivity analyses tested the robustness of primary findings.
Results
Total adult lifetime costs per patient with severe and moderately severe HB were $21,086,607 for SHL FIX prophylaxis, $22,987,483 for EHL FIX prophylaxis, and $20,971,826 for on-demand FIX treatment. For FIX prophylaxis, the cost of FIX treatment accounts for >90% of the total HB treatment costs.
Conclusions
This decision analytic model demonstrated significant economic burden associated with the current HB treatment paradigm.
Keywords:
Introduction
Hemophilia B (HB) is a rare congenital blood disorder characterized by deficiency of clotting factor IX (FIX) with spontaneous bleeding episodes, most notably into joints, and delayed hemostasis in external bleeding eventsCitation1. There are approximately 6,000 people with HB in the US, with an estimated incidence of one per 20,000 live male birthsCitation2,Citation3. Recurrent bleeding into joints can cause long-term joint deterioration resulting in physical impairment, the need for joint replacements, chronic pain, and reduced quality of life (QoL)Citation4. The severity of HB is defined by the level of circulating FIX and management is based on FIX replacement therapy administered either prophylactically to prevent bleeding episodes or after a bleeding episode has occurred, known as “on-demand” treatmentCitation5,Citation6. The standard of care for patients with severe and moderately severe HB is FIX prophylaxisCitation7–10. FIX supplementation is given intravenously as using standard half-life (SHL) or extended half-life (EHL) treatments, which are given every 2–3 or 7–14 days, respectively. The frequent infusions required by FIX treatment incur a level of treatment burden that can compromise adherence and clinical effectivenessCitation11,Citation12. People with mild or moderate HB who tend to experience relatively infrequent bleeding episodes are often managed with on-demand FIX treatment in order to minimize treatment burden.
FIX prophylaxis is effective in reducing the frequency of bleeding events and improving morbidity and mortality for patients with HB. However, the cost of treatment is substantial. The mean annual cost of FIX prophylaxis for HB in the US has been reported to be $610,966, ranging from $397,491 to $788,861 for people with HB using SHL and EHL treatments, respectivelyCitation13. Despite regular use of FIX prophylaxis, patients with HB still experience breakthrough bleeding events, which require medical management (i.e. hospitalizations, office visits) in addition to FIX treatmentCitation13,Citation14. The CHESS USCitation13 burden of illness study found patients with severe hemophilia reported an annual mean of 0.18 hospitalizations with an average of 0.23 days spent in the intensive care unit each year due to bleed-related complications.
The reported costs of HB have been historically limited, or reported in per-patient terms over relatively short time periodsCitation15. The effects of joint health deterioration over longer time periods more closely approximating the disease course duration for this lifelong congenital condition have not been well characterized. Specifically, the ultimate costs related to medical management of HB, including office visits, hospitalizations and joint replacement, as well as the ongoing FIX treatment costs, have not been clarified from a population health management perspective.
CHESS US reported bleeding rates, healthcare resource use and costs available in a sample of medical charts, but was designed as a cross-sectional study using a focused sample of patientsCitation13. The Hemophilia Utilization Group Studies Part Vb (HUGS Vb) study reported overall bleeding events and costs but was limited to 1–2 years of medical and dispensing records from Hemophilia Treatment Centers (HTCs)Citation14. Recently published economic models for emicizumab, a bispecific antibody administered subcutaneously and indicated for the prevention of bleeding events in people with hemophilia A, and a hypothetical gene therapyCitation16,Citation17 have tried to estimate the effect of joint deterioration on lifelong costs and outcomes, but were focused on patients with hemophilia A and did not provide similar insights for HB. As such, we developed a decision-analytic model to explore outcomes, costs and cost drivers of HB management from both US third-party payer and societal perspectives on a lifetime horizon of adults with severe and moderately severe HB (IU/dL ≤2). In order to account for the breadth and depth of critical considerations in this research area, we invited an expert panel of clinicians, patient advocates, and health technology assessment (HTA) specialists to participate in the design and construction of the model.
Methods
Model overview
A Markov cohort model was constructed to estimate the adult lifelong cost of HB management. The initial model concept was informed by a targeted review of published economic modeling studies in hemophilia and presented to an expert panel consisting of hematologists, HTA specialists and patient advocacy representatives (more information provided in Appendix 1). The panel’s input on the model set-up, structure and key assumptions formed the basis of its final design. Panel input was incorporated in all aspects of the final model, including the structure, statistical approach, patient population, input parameters, perspective, timeframe, and specific sources and considerations related to each of the input parameters.
A hypothetical cohort of male adults (≥18 years old) with severe and moderately severe HB and no history of inhibitors entered the model. Three treatment options were included: SHL FIX prophylaxis, EHL FIX prophylaxis, and on-demand FIX treatment. SHL FIX prophylaxis was based on the use of nonacog alfaCitation7,Citation8,Citation18; other SHL products have limited real-world usage in the US and were excludedCitation19. EHL FIX prophylaxis included albutrepenonacog alfa, eftrenonacog alfa, and nonacog beta pegol. On-demand FIX treatment included both SHL and EHL products. For each treatment arm, the number of bleeding episodes and joint bleeds occurring within the time horizon of the model were recorded. Costs and benefits were discounted at an annual rate of 3%, which is standard in US economic models and in line with the Institute for Clinical and Economic Review (ICER) Value Assessment FrameworkCitation20. The model considered both US third-party payer and societal perspectives, where the payer perspective focused on direct medical costs and the societal perspective also included non-medical costs (resources supporting healthcare sector services) and indirect costs (e.g. productivity losses). The decision analytic model was developed in Microsoft Excel (Redmond, WA).
Model framework
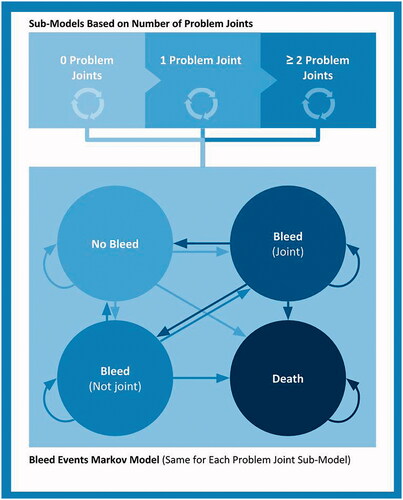
The Markov cohort model () was constructed with mutually exclusive health states based on naturally occurring events during the lifetime of people with HB. The health states included in the model were: “No bleed”, “Bleed (not joint)”, “Bleed (joint)” and “Death”. All patients began in the “No Bleed” health state and could transition over time to a “bleed” event or “dead” health state. Depending on the type of bleeding episode, patients transitioned to either “Bleed (joint)” or “Bleed (not joint)” based on the weekly transition probability, derived from the annual bleed rate (ABR) reported in clinical trials for each arm. The probability of death at the given point of time in the model was calculated based on age-specific male general population mortality in the USCitation21. A one-week cycle length with half-cycle correction was employed. As advised by the expert panel, a lifetime horizon was applied in the base case analysis. Shorter time horizons were tested in scenario analyses for 3, 5 and 10 years.
In order to quantify the impact of bleeding rate on joint damage over time, three sub-models were defined using the current number of problem joints (PJs) acquired. PJs are a measure of chronic joint damage and defined by symptoms such as limited range of motion, pain, and hemophilic arthropathyCitation22. According to the expert panel, this definition was deemed a better representation of long-term joint health than the target joint (TJ) definitionCitation22, which is defined as three bleeding events into a given joint during a 6-month period. It was recognized that the burden associated with repeated bleeding would be captured by the main model structure, as this was based on bleeding events. In each model cycle, to reflect progressing joint deterioration, a proportion of patients irreversibly moved from 0 PJ through 1 PJ to 2+ PJs sub-models (). The distribution of the cohort across joint damage sub-models at model entry was assumed to be 80% for 0 PJ, 10% for 1 PJ, and 10% for 2+ PJs. The probability of transition between PJ sub-models was based on Fischer et al.Citation23, where 12.6 joint bleeds on average generated an increase in Pettersson score and reflected progression in the deterioration of joint health. Based on this assumption, the weekly probability of transition to the next PJ sub-model was calculated considering annual joint bleed rate (AJBR) of each treatment arm.
Model inputs
The base case model inputs and ranges of model inputs used for sensitivity analyses are summarized in . All costs were translated to 2019 USD($) and adjusted to the length of the weekly treatment cycle as appropriate. Annual bleed rates and AJBRs were based on the pivotal trial results of the FIX products and used to calculate non-joint bleed rates. It was assumed that patients in the 1 PJ and 2+ PJs sub-models could undergo an orthopedic surgery once or twice per lifetime (between the ages of 45 and 89 years), respectivelyCitation16,Citation17.
Table 1. Key model input parameters.
Prophylaxis and on-demand dosing information was based on US prescribing information (US PI) for FIX products. The unit price for FIX products was based on the wholesale acquisition cost (WAC), as reported in Redbook. The dose per infusion of FIX therapy was calculated using national average weight in the US, using published weight tablesCitation28 for the age-matched US male population. Market research data on real-world usage were used to derive the treatment mix of people with HB using alternative EHL products in the EHL prophylaxis arm and of people with HB using SHL and EHL products in the on-demand armCitation19. Data from five clinical trialsCitation8,Citation9,Citation24,Citation29,Citation30 across SHL and EHL products were used to calculate an average of 1.2 FIX infusions needed to treat a bleeding event.
Non-drug costs of HB management included hospitalizations due to bleeding, orthopedic surgery, or intracranial hemorrhage, and outpatient visits. The frequency and average length of stay of bleed-related hospitalizations and the frequency of office visits were derived from the CHESS US studyCitation13. The unit cost for hospitalization and office visits were sourced from literature and the CMS Physician Fee Schedule. Non-medical costs included expenses incurred due to travelling to the HTC and were sourced from the CHESS US studyCitation13. The components of indirect cost included productivity losses and social benefits. The human capital approachCitation31 was used to estimate productivity losses. Data sourced from the CHESS US(+) studyCitation32 (a patient-centric follow-up study to CHESS US that gathered data on indirect costs of hemophilia) were used to estimate both productivity losses and social benefits cost.
Sensitivity analysis
Model inputs were tested in one-way sensitivity analysis (OWSA) primarily based on the 95% confidence interval for each parameter. For variables with no available estimates of certainty, 20% variation was assumed. Further testing was conducted for selected variables (discounting rates, on-demand FIX doses, duration of GTx treatment effect, GTx discount for partial responders and orthopedic surgery age) using specified ranges representing plausible ranges to inform the sensitivity of the outcomes.
Results
Base case results
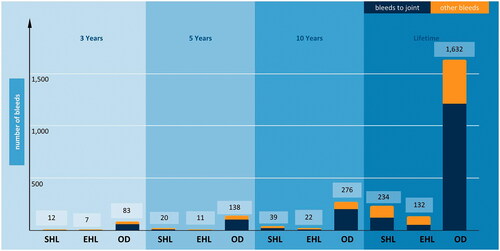
Model results showed substantial cost of severe and moderately severe HB management associated with all three treatment options (). From the societal perspective, the adult lifetime total cost per patient was $21,086,607 for SHL FIX prophylaxis, $22,987,483 for EHL FIX prophylaxis, and $20,971,826 for on-demand FIX treatment. From the payer perspective, the adult lifetime direct medical cost per patient was $21,032,332 for SHL FIX prophylaxis, $22,933,207 for EHL FIX prophylaxis, and $20,934,426 for on-demand FIX treatment. Most of the direct medical cost for HB management was driven by FIX treatment, estimated at $19,754,862 and $22,202,092 for prophylaxis with SHL and EHL, respectively (both accounted for more than 90% of direct medical costs). On-demand FIX treatment accounted for approximately 60% of direct medical costs, at $12,179,003. Non-medical direct and indirect costs constituted a relatively small proportion of the total cost of HB management (from 0.18% to 0.26% in lifetime horizon). When the model was run with shorter time horizons, the total cost per patient ranged from $2.2 to $2.4 million over 3 years, $3.6 to $3.9 million over 5 years, and $6.7 to $7.3 million over 10 years across all three treatment arms ().
Figure 2. Total costs associated with different time horizons. Abbreviations. EHL, Extended half-life; m, Million; OD, On-demand; SHL, Standard half-life.
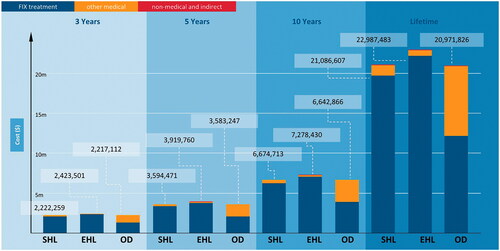
Table 2. Base case analysis results in US adults with hemophilia B (lifetime horizon).
Patients receiving EHL FIX prophylaxis had the fewest total bleeding events (132) and joint bleeds (52) over the adult lifetime horizon. Patients receiving SHL FIX prophylaxis had 234 total bleeding events and 121 joint bleeds, and patients receiving on-demand FIX treatment had 1,632 total bleeding events and 1,211 joint bleeds (). Similar trends were observed for total and joint bleed results within the shorter time horizon scenarios ().
Sensitivity analysis results
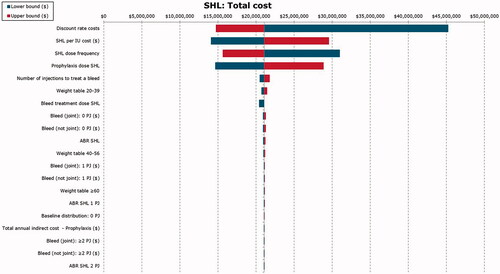
One-way sensitivity analysis results were generally consistent with the base case results. Total adult lifetime cost of HB management was most sensitive to variations in the unit cost of FIX treatment, discount rates, and the number of injections needed to treat a bleeding event, regardless of the treatment arm ( for SHL FIX prophylaxis, Supplemental Figures 1 and 2 for EHL FIX prophylaxis and on-demand FIX treatment).
Discussion
This decision analytic model showed substantial costs of managing severe and moderately severe HB across the adult lifetime in the US, exceeding $20 million in all scenarios. Occurrence of bleeding events including joint bleeds persisted despite FIX prophylaxis, which accounted for >90% of direct costs, but were markedly greater for patients receiving on-demand treatment only (10- to 20-times more bleeding events in some scenarios). As FIX treatment costs accounted for so much of the total costs, the model was most sensitive to the unit costs of FIX treatment, discount rates, and the number of FIX administrations required to treat a bleeding event. This model illustrated a tangible unmet need related to bleeding events and the need for lower costs to prevent and treat bleeding events, from both societal and third-party payer perspectives.
Our model findings are consistent with published real-world utilization studies that the cost of FIX prophylaxis accounts for >90% of direct medical costs of HB managementCitation14,Citation33,Citation34. Moreover, substantial lifetime cost of disease management can be also seen in other rare diseases such as Gaucher disease (€6 million)Citation35 and paroxysmal nocturnal hemoglobinuria ($9 million)Citation36.
Our study offers a lifetime perspective on treatment cost drivers, where FIX prophylaxis constituted approximately 95% of total medical costs, regardless of EHL or SHL products used. On-demand FIX treatment costs accounted for roughly 60% of total HB costs, but resulted in much higher rates of bleeding events and a similar overall lifetime cost ($21 million) as either SHL or EHL prophylaxis ($21 and $23 million, respectively). Across the adult lifetime horizon, SHL and EHL FIX prophylaxis were associated with 85–90% reductions in total bleeds (132–234 vs. 1,632 total bleeds) and 90–95% reduction in joint bleeds (52–121 vs. 1,211 joint bleeds) compared to on-demand FIX treatment. Taken together, the model suggested that an on-demand treatment strategy did not confer any meaningful direct cost savings compared to prophylaxis, with non-drug medical costs increasing over the long term likely attributable to poorer clinical outcomes. These findings were consistent with previous cost-effectiveness modelsCitation37–41 in hemophilia A comparing factor VIII prophylaxis with on-demand treatment. The broader view offered by these results also highlights that the residual burden of bleeding events with FIX prophylaxis is significant (134–234 total bleeds, 52–121 joint bleeds), particularly considering progressive joint damage. The limited motion and joint pain associated with hemophilic arthropathy are known to further worsen patients’ QoL and well-being, underscoring the persistent unmet medical need in this populationCitation42,Citation43. It is well documented that there is the clinical benefit of prophylaxis in bleed prevention, joint health, and improving QoLCitation5–7,Citation44. Recent cost-effectiveness analyses (CEAs) also showed prophylaxis is cost-effective compared to on-demand treatmentCitation39,Citation40,Citation45–47.
Additionally, Supplemental Table 1 provides an overview of identified cost-effectiveness studies reporting costs of lifelong hemophilia A management in the USCitation17,Citation41,Citation48–50. The majority utilized Markov models and more recent studies included health states capturing joint deterioration, similar to the approach used for our study. Discrepancies in lifetime costs reported by those studies can be partially explained by differences in follow-up periods (due to patients’ ages when entering the model) and cost categories included in the analyses. Although the majority of costs were associated with FIX treatment costs, the considerable scope and impact of indirect costs and non-medical costs should not be overlooked when assessing the overall burden of HB on patients, their caregivers and society. Based on results from 112 patients across 10 HTCs, the HUGS VbCitation14 study captured the impact of HB on absenteeism, presenteeism, productivity levels and overall employment status, as well as unpaid hemophilia-related caregiver time. The study reported a significant impact of HB on employment status and work productivity in the US, indicating that indirect costs constitute 9% of the total HB costs. In contrast, our model focused primarily on direct costs and only managed to capture some non-medical and indirect costs, which accounted for less than 1% of total cost. This disparity might be partially explained by the different indirect cost components considered by both studies.
Cost-effectiveness analyses based on decision analytic models are commonly used by health policy makers to determine the value of novel treatments. Waters and KarpfCitation51 postulated that CEA could be used to inform the need for cost control, provision of efficient and effective care, as well as evaluation of alternative payment models. Increasingly, more payers and manufacturers use value-based pricing approaches to determine prices for pharmaceutical products, which allows them to determine a price that reflects health gains generated by the treatment of interest. Decision analytic models play a central role in estimating these parameters. Most payers during reimbursement decision-making focus on the evaluation or direct costs, but in some regions or countries indirect costs are also considered. As shown by this research, in hemophilia the total cost is primarily driven by direct medical costs, but inclusion of the societal perspective may be of paramount importance to the cost-effectiveness of therapies in other conditions that are also associated with substantial impairment of patients’ productivity.
Modern treatment advances to date have offered meaningful improvements over historical therapeutic options in terms of clinical outcomes, life expectancy, and QoL; however, this model has quantified that severe and moderately severe HB still poses a significant burden to payers and society, driven primarily by the high costs of FIX treatment. It should be noted that several novel treatments are in development for HB, including gene therapy, and may be considered in future iterations of this work. Based on available phase 1/2 clinical trialCitation52,Citation53 findings, HB gene therapy may provide >90% reduction in FIX usage together with further reductions in bleed rates compared to FIX prophylaxis among patients with severe and moderately severe HB, presenting an opportunity for substantial cost offsets in HB management.
Interpretation of this decision analytic model should consider certain strengths, limitations, and contextual factors. The model framework and assumptions combined published estimates with robust input from a panel of clinicians, HTA specialists, and patient advocates, and was aligned with the approach of a recent ICER model for the evaluation of emicizumab for patients with hemophilia A and inhibitorsCitation17. The panel input ensured that detailed model assumptions allowed for close approximation of a natural disease history including relevant clinical events and associated costs. Representatives from clinical, patient, and health policy stakeholders ensured that the perspective and model parameters accounted for their considerations in HB management.
Similarly, our model and the ICER model both attempted to simulate a natural disease progression, with emphasis on joint deterioration, and used similar approaches for transition probabilities across sub-models of joint health. We utilized a joint health definition of “problem joints” that considered published health outcomes research on the impact of TJs on patients’ lives and QoLCitation44, whereas the ICER model used a more clinical definition based on the presence of arthropathy. The ICER model also used a structure for bleed-related health states that differentiated between treated and untreated bleeding events. Published data sources used for our model inputs were consistent with other published economic evaluations in hemophiliaCitation16,Citation17,Citation38. The lack of appropriate information about the number of PJs accrued by people with HB at different ages was a limitation to the model. The solution to this problem was to assume a baseline distribution of patients with 0, 1 and 2+ PJs. This distribution was then modified within the scenario analysis and consistent estimates were still generated, indicating the robustness of the model findings. The model did not capture the impact of HB on caregivers, which would have increased the indirect cost estimates.
Patients included in this model represented those at greatest risk of spontaneous bleeds, a pool of patients also frequently represented in clinical trials of FIX treatment candidates, and the annual bleeding rate estimates were based on those from clinical trials. Considering the differences between trial participants and those encountered in regular clinical practice, rates of treatment adherence may have been overestimated, and overall bleeding events and joint bleeds may have been underestimated compared to “real-world” rates in a more heterogeneous population. The model may have underestimated the magnitude of clinical, humanistic, and economic burden both for patients entering the model and as they progressed over time with current standards of care.
To our knowledge, this is the first economic model to assess lifetime health outcomes and costs for adults with HB over the natural history of disease, with particular focus on the impact of long-term joint deterioration. This model demonstrated the significant economic burden of current treatment options that exceeded $20 million in any clinical and treatment scenario. Total direct costs were overwhelmingly driven by FIX treatment costs, yet bleeding events and long-term consequences of accumulated bleeds persisted, including joint deterioration and associated medical management. Indirect and non-medical costs appeared provincial in the shadow of FIX treatment costs, but should not be underrepresented in the holistic calculus of long-term burden of HB and the potential to offer patients long-term relief from the meaningful negative impact on employment and life. Despite advances in the available therapeutic approaches to prevent and treat breakthrough bleeding, notable unmet needs remain to further improve clinical, humanistic, and economic outcomes for patients with HB and society.
Transparency
Declaration of funding
This study was supported by research funding from uniQure, Inc.
Declaration of financial/other relationships
NL and EKS are uniQure Inc. employees and shareholders. HCD Economics ltd were funded by uniQure Inc. to conduct this research. KM, GG, MTS, TB, AM and JOH are employees of HCD Economics ltd. MS reports consulting fees from HCD Economics ltd. MR has received research support for his institutions from Bayer, BioMarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark, Takeda, and uniQure; reports consultant fees from Catalyst Biosciences, CSL Behring, Genentech, HCD Economics Ltd., Hema Biologics, Kedrion, Novo Nordisk, Pfizer, Sanofi, Takeda, and uniQure; is on the board of directors of the Foundation for Women and Girls with Blood Disorders and Partners in Bleeding Disorders. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
The study was presented at the World Federation of Hemophilia (WFH) Virtual Summit, June 14–19, 2020; Academy of Managed Care Pharmacy (AMCP) Nexus Virtual Meeting, October 19, 2020; and the National Association of Specialty Pharmacy (NASP) Annual Meeting and Expo Virtual Experience, September 14–18, 2020.
Supplemental Material
Download MS Word (11.6 KB)Supplemental Material
Download MS Word (24.5 KB)Supplemental Material
Download JPEG Image (116.6 KB)Supplemental Material
Download JPEG Image (103.1 KB)Acknowledgements
The authors thank Jeff Frimpter, MPH of Integrative Life Sciences for providing medical writing support.
References
- Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388(10040):187–197.
- Davis J, Yan S, Matsushita T, et al. Systematic review and analysis of efficacy of recombinant factor IX products for prophylactic treatment of hemophilia B in comparison with rIX-FP. J Med Econ. 2019;22(10):1014–1021.
- National Center for Health Statistics; [Internet]; 2020; [cited 2020 Feb 25]. Available from: https://www.cdc.gov/nchs/nvss/deaths.htm
- Fischer K, Steen Carlsson K, Petrini P, et al. Intermediate-dose versus high-dose prophylaxis for severe hemophilia: comparing outcome and costs since the 1970s. Blood. 2013;122(7):1129–1136.
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544.
- Gringeri A, Lundin B, Von Mackensen S, et al. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT study). J Thromb Haemost. 2011;9(4):700–710.
- Kavakli K, Smith L, Kuliczkowski K, et al. Once-weekly prophylactic treatment vs. on-demand treatment with nonacog alfa in patients with moderately severe to severe haemophilia B. Haemophilia. 2016;22(3):381–388.
- Lambert T, Recht M, Valentino LA, et al. Reformulated BeneFix: efficacy and safety in previously treated patients with moderately severe to severe haemophilia B. Haemophilia. 2007;13(3):233–243.
- Santagostino E, Martinowitz U, Lissitchkov T, et al. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood. 2016;127(14):1761–1769.
- Witmer C, Presley R, Kulkarni R, et al. Associations between intracranial haemorrhage and prescribed prophylaxis in a large cohort of haemophilia patients in the United States. Br J Haematol. 2011;152(2):211–216.
- Khayat CD. Once-weekly prophylactic dosing of recombinant factor IX improves adherence in hemophilia B. J Blood Med. 2016;7:275–282.
- Thornburg CD, Duncan NA. Treatment adherence in hemophilia. Patient Prefer Adherence. 2017;11:1677–1686.
- Noone D, Pedra G, Asghar S, et al. Prophylactic treatment in people with severe hemophilia B in the US: an analysis of real-world healthcare system costs and clinical outcomes. Blood. 2019;134(Suppl. 1):2118.
- Chen CX, Baker JR, Nichol MB. Economic burden of illness among persons with hemophilia B from HUGS Vb: examining the association of severity and treatment regimens with costs and annual bleed rates. Value Heal. 2017;20(8):1074–1082.
- Li N, Sawyer EK, Maruszczyk K, et al. Economic burden of hemophilia B in the US: a systematic literature review. J Drug Assess. 2019;8(Suppl. 1):28.
- Machin N, Ragni MV, Smith KJ. Gene therapy in hemophilia A: a cost-effectiveness analysis. Blood Adv. 2018;2(14):1792–1798.
- Institute for Clinical and Economic Review. Emicizumab for hemophilia A with inhibitors: effectiveness and value final evidence report [Internet]; 2018; [cited 2020 Aug 19]. Available from: http://icer-review.org/programs/new-england-cepac/
- Roth DA, Kessler CM, John Pasi K, et al. Human recombinant factor IX: safety and efficacy studies in hemophilia B patients previously treated with plasma-derived factor IX concentrates. Blood. 2001;98(13):3600–3606.
- Wave # 26. Survey on hemophilia care & price monitoring United States; 2019; [cited 2020 Jan 27]. p. 1–215. Available from: http://www.dph.gla.ac.uk/heat
- Institute for Clinical and Economic Review. 2020–2023 value assessment framework: final framework [Internet]; 2020; [cited 2020 Aug 19]. Available from: https://icer-review.org/material/2020-value-assessment-framework-final-framework/
- NVSS – mortality data [Internet]; 2020; [cited 2020 Feb 25]. Available from: https://www.cdc.gov/nchs/nvss/deaths.htm
- Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935–1939.
- Fischer K, van Hout BA, van der Bom JG, et al. Association between joint bleeds and Pettersson scores in severe haemophilia. Acta Radiol. 2002;43(5):528–532.
- Collins PW, Young G, Knobe K, et al. Recombinant long-acting glycoPEGylated factor IX in hemophilia B: a multinational randomized phase 3 trial. Blood. 2014;124(26):3880–3886.
- Valentino LA, Rusen L, Elezovic I, et al. Multicentre, randomized, open-label study of on-demand treatment with two prophylaxis regimens of recombinant coagulation factor IX in haemophilia B subjects. Haemophilia. 2014;20(3):398–406.
- HCUPnet: a tool for identifying, tracking, and analyzing national hospital statistics [Internet]; 2020; [cited 2020 Apr 26]. Available from: https://hcupnet.ahrq.gov/#setup
- Patel AA, Ogden K, Veerman M, et al. The economic burden to medicare of stroke events in atrial fibrillation populations with and without thromboprophylaxis. Popul Health Manag. 2014;17(3):159–165.
- Fryar CD, Kruszon-Moran D, Gu Q, et al. National health statistics reports, Number 122, December 20, 2018; 2018.
- Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313–2323.
- Windyga J, Abbuehl BE, Hafeman AE. BAX326 (recombinant coagulation factor IX) for the treatment and prophylaxis of hemophilia B. Expert Rev Hematol. 2014;7(3):333–342.
- Lensberg BR, Drummond MF, Danchenko N, et al. Challenges in measuring and valuing productivity costs, and their relevance in mood disorders. Clin Outcomes Res. 2013;5:565–573.
- Burke T, Asghar S, Pedra G, et al. An introduction to the CHESS US and CHESS US + studies (The ‘Cost of Hemophilia across the United States – a Socio-Economic Survey’). World Fed Hemoph Virtual Summit 2020. Haemophilia. 2020;26:3–140.
- Tortella BJ, Alvir J, McDonald M, et al. Real-world analysis of dispensed IUs of coagulation factor IX and resultant expenditures in hemophilia B patients receiving standard half-life versus extended half-life products and those switching from standard half-life to extended half-life products. J Manag Care Spec Pharm. 2018;24(7):643–653.
- Chhabra A, Spurden D, Fogarty PF, et al. Real-world expenditures associated with prophylactic factor IX replacement in severe hemophilia B patients in the US: a comparison between standard and extended half-life products. Blood Coagul Fibrinolysis. 2018;31(3):186–192.
- Van Dussen L, Biegstraaten M, Hollak CEM, et al. Cost-effectiveness of enzyme replacement therapy for type 1 Gaucher disease. Orphanet J Rare Dis. 2014;9:51.
- O'Connell T, Buessing M, Johnson S, et al. Cost–utility analysis of ravulizumab compared with eculizumab in adult patients with paroxysmal nocturnal hemoglobinuria. Pharmacoeconomics. 2020;38(9):981–994.
- Miners AH, Sabin CA, Tolley KH, et al. Cost–utility analysis of primary prophylaxis versus treatment on-demand for individuals with severe haemophilia. Pharmacoeconomics. 2002;20(11):759–774.
- Miners A. Revisiting the cost-effectiveness of primary prophylaxis with clotting factor for the treatment of severe haemophilia A. Haemophilia. 2009;15(4):881–887.
- Colombo GL, Di Matteo S, Mancuso ME, et al. Cost–utility analysis of prophylaxis versus treatment on demand in severe hemophilia A. Clinicoecon Outcomes Res. 2011;3:55–61.
- Castro Jaramillo HE, Moreno Viscaya M, Mejia AE. Cost–utility analysis of primary prophylaxis, compared with on-demand treatment, for patients with severe hemophilia type A in Colombia. Int J Technol Assess Health Care. 2016;32(5):337–347.
- Farrugia A, Cassar J, Kimber MC, et al. Treatment for life for severe haemophilia A – a cost–utility model for prophylaxis vs. on-demand treatment. Haemophilia. 2013;19(4):e228–e238.
- Instructions for Companies | Single technology appraisal: user guide for company evidence submission template | Guidance | NICE [Internet]; 2015; [cited 2020 Jan 7]. Available from: https://www.nice.org.uk/process/pmg24/chapter/instructions-for-companies
- O’Hara J, Walsh S, Camp C, et al. The relationship between target joints and direct resource use in severe haemophilia. Health Econ Rev. 2018;8:1.
- O’Hara J, Walsh S, Camp C, et al. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes. 2018;16(1):84.
- Risebrough N, Oh P, Blanchette V, et al. Cost–utility analysis of Canadian tailored prophylaxis, primary prophylaxis and on-demand therapy in young children with severe haemophilia A. Haemophilia. 2008;14(4):743–752.
- Fang H, Hen C, Han L, et al. Cost-effectiveness analysis of standard prophylaxis versus on-demand treatment in severe hemophilia A in China. ISPOR 22nd Annual International Meeting; Boston, MA, USA; 2017.
- Gharibnaseri Z, Davari M, Cheraghali A, et al. Cost–utility of prophylaxis vs. on-demand treatment in severe haemophilia A patients. ISPOR 19th Annual European Congress; Vienna, Austria; 2016.
- Earnshaw SR, Graham CN, McDade CL, et al. Factor VIII alloantibody inhibitors: cost analysis of immune tolerance induction vs. prophylaxis and on-demand with bypass treatment. Haemophilia. 2015;21(3):310–319.
- Li N, Bullement A, McMordie S, et al. Cost-effectiveness analysis of RFVIIIFC, pegylated RFVIII, and emicizumab for the prophylactic treatment of severe hemophilia a patients without inhibitors in the United States. New Orleans: ISPOR; 2019.
- Zhou Z-Y, Raimundo K, Patel AM, et al. Model of short- and long-term outcomes of emicizumab prophylaxis treatment for person with hemophilia A. ASH Annual Meeting [Internet]; 2018; [cited 2019 Apr 18]. Available from: https://www.mendeley.com/library/
- Waters TM, Karpf M. Healthcare reform in the U.S. must be driven by policy and data, not P. [Internet]; 2021; [cited 2021 Feb 3]. Available from: https://uknowledge.uky.edu/hsm_facpub/16/
- Drygalski A, Von Giermasz A, Castaman G, et al. Etranacogene dezaparvovec (AMT-061 phase 2b): normal/near normal FIX activity and bleed cessation in hemophilia B. Blood Adv. 2019;3(21):3241–3247.
- George LA, Sullivan SK, Rasko JEJ, et al. Efficacy and safety in 15 hemophilia B patients treated with the AAV gene therapy vector fidanacogene elaparvovec and followed for at least 1 year. Blood. 2019;134(Suppl. 1):3347.