Abstract
Aims
Peripheral artery disease (PAD), often treated with lower extremity revascularization, is associated with risk of major atherothrombotic vascular events (acute limb ischemia [ALI], major non-traumatic lower-limb amputation, myocardial infarction [MI], ischemic stroke, cardiovascular death). This study aims to assess healthcare resource utilization and costs of such events among patients with PAD after revascularization.
Materials and methods
Patients aged ≥50 years with PAD who were treated with lower-extremity revascularization were identified from Optum Clinformatics Data Mart claims database (01/2014–06/2019). The first lower extremity revascularization after PAD diagnosis was defined as the index date. Patients had ≥6 months of health plan enrollment before the index date. Patients were followed until the earliest of 1) end of enrollment or data; 2) diagnosis of atrial fibrillation or venous thromboembolism; or 3) oral anticoagulant use. All-cause healthcare resource use per-patient-year was compared before and after a major atherothrombotic vascular event post-revascularization among those with an event. Additionally, event-related healthcare costs per-patient-year were reported for each event type.
Results
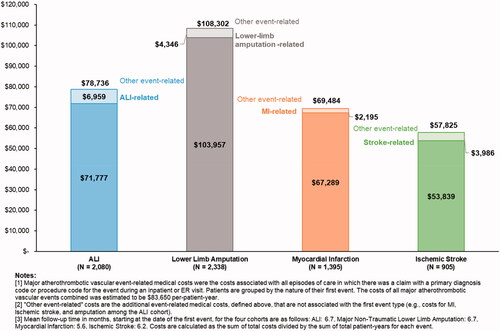
Of the 38,439 PAD patients meeting the study criteria, 6,675 (17.4%) had a major atherothrombotic vascular event. On average, patients were observed for 7.3 months before an event and 6.2 months after an event. Patients with an event had significantly higher all-cause healthcare resource use versus similar metrics pre-event (e.g. inpatient visits among those with ALI: 3.5 ± 5.8 post-event vs. 2.0 ± 8.1 pre-event, p < .05). Event-related costs ranged from $57,825±$131,810 per-patient-year for ischemic stroke to $108,302±$150,168 for major non-traumatic lower-limb amputation.
Limitations
Data do not contain clinical information. Additionally, results are limited to commercially insured and Medicare Advantage beneficiaries.
Conclusion
Patients with PAD who experience major atherothrombotic vascular events post-revascularization have considerably higher healthcare resource use and costs compared with similar metrics pre-event. Therefore, reducing the rate of such events could reduce overall healthcare costs for this population.
JEL CLASSIFICATION CODE:
Introduction
Peripheral artery disease (PAD) is characterized by the atherosclerotic occlusion of vessels in the lower limbs, leading to lower-extremity symptoms and functional impairmentCitation1–3 Patients with PAD are also at a high risk of experiencing other lower-limb complications such as intermittent claudication, acute limb ischemia (ALI) and amputationsCitation3,Citation4; as well as cardiovascular (CV) complications, including myocardial infarction (MI) and strokeCitation3,Citation5–7. Accordingly, PAD is associated with a substantial economic burden, with total costs for vascular-related hospitalizations in patients with PAD estimated to be more than $21 billion in the US in 2008Citation5. Another recent study reported that total healthcare costs were three times higher among PAD patients versus those without the conditionCitation8.
As PAD progresses, endovascular or surgical revascularization procedures may be required to improve symptoms and prevent tissue loss in severe casesCitation4, which in turn could improve patients’ quality of lifeCitation9,Citation10. Despite improvements in clinical management for PAD, evidence suggests that patients undergoing revascularization may face increased risk of major atherothrombotic vascular events after the procedure, specifically ALI, MI, amputation, ischemic stroke, and CV-related deathCitation11–16. For example, in a post hoc analysis of the EUCLID randomized trial for treatment for PAD, patients enrolled based on previous revascularization had four times the risk of ALI and a 29% increased risk of MI compared to those enrolled based on other diagnostic metrics (all p < .01)Citation12. Additionally, in a real-world study using administrative claims data for patients with PAD in the US, Bonaca et al. found that one in six PAD patients who underwent lower extremity revascularization experienced a major atherothrombotic vascular event within one year of the procedureCitation14.
Despite the evidence highlighting this high-risk subpopulation of PAD patients following revascularization, few studies have characterized the resource use and economic burden among these patients. For example, a recent claims-based, real-world study conducted by Berger et al. found that compared with PAD patients without major adverse cardiovascular events and major adverse limb events, the healthcare costs for patients with such events increased by $36,806 for critical limb ischemia to $60,852 for amputationCitation7. While these results highlight the high cost burden of these events among patients with PAD, they did not specifically evaluate this burden among the population of high-risk patients who underwent peripheral revascularization.
This study builds upon previous literature by evaluating the all-cause healthcare resource use burden among the subset of patients with PAD and lower-extremity revascularization who subsequently experienced a major atherothrombotic vascular event. Additionally, the healthcare costs for the individual major atherothrombotic vascular events were estimated for up to one year after the first observed event.
Methods
Data source
This analysis was conducted using health insurance claims from the Optum Clinformatics Data Mart database (Optum, Inc.) during the period from January 2014 to June 2019. This database includes 12–14 million annual covered lives in all census regions in the United States and comprises both commercial and Medicare Advantage health plan data, containing more than 36 months of historical data on patient demographics, dates of eligibility and death, claims for inpatient and outpatient visits, pharmacy claims, costs of services, and laboratory tests and results.
Data used in this study were de-identified and complied with the requirements of the Health Insurance Portability and Accountability Act (HIPAA); therefore, review by an institutional review board was not required.
Study design and sample selection
A retrospective design was used (). The study sample was derived from the patient population assessed in the study by Bonaca et al.Citation14.
Figure 1. Study design. Abbreviations. HCRU, healthcare resource use; OAC, oral anticoagulant; PAD, peripheral artery disease; VTE, venous thromboembolism.
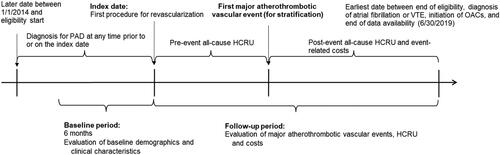
Briefly, patients meeting the following criteria were included in the study: (1) had ≥1 procedure for a peripheral revascularization in any setting, the first of which was defined as the index date; (2) had ≥6 months of continuous health insurance coverage prior to the index date, which was defined as the baseline period; (3) had ≥1 diagnosis of lower-extremity PAD in any setting at any time prior to or on the index date; and (4) were ≥50 years of age on the index date. Those with diagnoses of IS, atrial fibrillation, venous thromboembolism (VTE), intracranial hemorrhage, end stage renal disease (ESRD), or oral anticoagulant use during 6 months pre-index; or diagnoses of MI 30 days or ALI 14 days pre-index were excluded. Patients were followed until the earliest of 1) end of enrollment or data; 2) diagnosis of atrial fibrillation or VTE; or 3) oral anticoagulant use.
From this sample, patients experiencing a major atherothrombotic vascular event (i.e. ALI, MI, major non-traumatic lower-limb amputation, ischemic stroke, or CV-related death) post-revascularization were identified for the present analysis. ALI, MI, and ischemic stroke events were identified based on primary diagnosis codes listed on claims for inpatient or emergency room (ER) visits, whereas, major non-traumatic lower-limb amputation was identified based on procedure codes listed on claims for inpatient or ER visits. CV-related death was determined by a primary CV diagnosis on the last inpatient or ER visit prior to deathCitation7.
Baseline characteristics and outcome measures
Patient characteristics measured during the baseline period included demographic characteristics (i.e. age, gender, year of index date, region of residence, and insurance plan type) and clinical characteristics (i.e. revascularization procedure at index date, Charlson Comorbidity Index [CCI] scoreCitation17, comorbidities, all-cause healthcare resource use, and prescription drug use).
All-cause metrics of healthcare resource use per-patient-year before and after the first non-fatal major atherothrombotic vascular event, defined as the number of events divided by the patient-years of observation during the respective time period, were evaluated. Healthcare resource use outcomes were stratified into the following categories: inpatient visits, ER visits, outpatient visits, and skilled nursing facility (SNF) visits. The pre-event period spanned the time between the date of revascularization and occurrence of the first non-fatal major atherothrombotic vascular event post-revascularization. All-cause healthcare resource use was assessed for each non-fatal event type separately. Note, in the event patients experienced multiple types of major atherothrombotic vascular events, only the first event was considered for stratification of outcomes by event type.
Additionally, total major atherothrombotic vascular event-related healthcare costs per-patient-year were estimated up to one year after the event. Results were stratified by the type of first non-fatal major atherothrombotic vascular event experienced. ALI, MI, ischemic stroke, and major non-traumatic lower-limb amputation-related costs were identified based on primary diagnosis (or procedure) codes listed on claims for inpatient or emergency room visits. Costs associated with subsequent events of the same type as the first event were assessed separately from costs associated with other types of major atherothrombotic vascular events. For example, among people who had MI as the first major atherothrombotic vascular event, the costs of subsequent MI events were reported separately from the costs of other non-fatal major atherothrombotic vascular events such as ischemic stroke, major non-traumatic lower-limb amputation, and ALI.
The costs reflect the amounts paid by the insurer to the provider. All costs were inflated to 2019 US dollars using the Consumer Price Index for Medical CareCitation18.
Statistical analysis
Baseline characteristics of the study sample were described using means and standard deviations (SDs) continuous variables, and relative frequencies and proportions for categorical variables. Frequencies of all-cause healthcare resource use before and after the major atherothrombotic vascular events were described using means, standard deviations, medians and interquartile ranges. Statistical significance of differences in outcomes were compared using Wilcoxon sign-rank test to account for repeated measurements (before and after the event) for the same patients. p values <.05 were considered statistically significant.
All analyses were conducted using SAS Enterprise Guide software Version 7.15 (SAS Institute, Cary, NC).
Results
Patient characteristics
Among 38,439 patients with PAD and lower extremity revascularization included in the study, 6,675 (17.4%) had a major atherothrombotic vascular event post revascularization, over a median follow-up period of 1.0 year (). Major non-traumatic lower-limb amputation was the most common type of first vascular event post-revascularization, followed by ALI, MI, and ischemic stroke. Less than 4% of those experiencing a major atherothrombotic vascular event post revascularization experienced CV-related death as the first event and were excluded from the analyses of healthcare resource use and costs.
Figure 2. Sample selection. Abbreviations. ALI, acute limb ischemia; ESRD, end-stage renal disease; MI, myocardial infarction; OAC, oral anticoagulant; PAD, peripheral artery disease; VTE, venous thromboembolism. Note: *Categories are not mutually exclusive; 208 patients with multiple types of events on the day of the first event contribute to counts for the individual components.

The average age of patients experiencing a major atherothrombotic vascular event post-revascularization was 71 years [SD = 9.0] and 39.1% were females (). The most frequent type of revascularization procedure done at the index date was endovascular (64.2%) and at the femur (62.3%). The mean baseline CCI score was 1.9 [SD =1.7], and approximately 30% of patients used antiplatelet drugs. The mean time from the revascularization procedure to the first major atherothrombotic vascular event was 7.3 months [SD = 10.6] and patients were further followed for 6.2 months on average [SD = 4.9] following the event.
Table 1. Baseline patient demographic and clinical characteristics.
All-cause healthcare resource use before and after non-fatal major atherothrombotic vascular events
Compared to the time period between revascularization and the first observed non-fatal major atherothrombotic vascular event, patients experienced significantly higher number of inpatient visits and skilled nursing facility visits after the event (). Results were similar for outpatient/physician office visits among patients experiencing ALI, MI or ischemic stroke as the first major atherothrombotic vascular event. However, among patients experiencing major non-traumatic lower-limb amputation as the first major atherothrombotic vascular event, the number of outpatient/physician office visits were significantly higher before the event.
Table 2. Healthcare resource utilization before and after first non-fatal major atherothrombotic vascular event – stratified by first event type.
Non-fatal major atherothrombotic vascular event-related costs
During the period after first non-fatal major atherothrombotic vascular event, the event-related costs ranged from $57,825±$131,810 per-patient-year for ischemic stroke to $108,302±$150,168 per-patient-year for major non-traumatic lower-limb amputation (). For each group, the majority of the event-related costs were attributable to the first event type, which was also the most common type of subsequent events observed among the MI and ischemic stroke groups (). For example, among people with MI as the first major atherothrombotic vascular event, 97% of the costs in the subsequent year were attributable to claims for MI. Following the initial MI, 9.5% of the patients had additional claims for MI, 7.5% experienced CV-related death, 2.9% had ALI and ischemic stroke, and 1.3% experienced major non-traumatic lower-limb amputations.
Discussion
In this study, 17.4% of patients with PAD who underwent revascularization experienced a major atherothrombotic vascular event within 1 year of follow-up. These patients generally had significantly higher healthcare resource use compared with their resource use during the time period before the non-fatal major atherothrombotic vascular event. Additionally, we find that these major atherothrombotic vascular events are associated with high annual cost burden, ranging on average from $57,825 per-patient-year for ischemic stroke to $108,302 per-patient-year for major non-traumatic lower-limb amputation.
Recent studies have assessed the economic implications of PAD patients experiencing major CV or limb complicationsCitation7,Citation8. The present study focuses specifically on the subgroup of PAD patients who experience major atherothrombotic vascular events after peripheral revascularization and finds that the economic burden associated with these complications may be even greater among these high risk patients. For example, using patients’ own data from before and after an incident MI, we find that patients experience 2.6 more inpatient visits after the event compared to the number of inpatient visits during the period between the revascularization and the complication. Similarly, Berger et al. estimated that PAD patients who had a major lower-limb amputation incurred $70,417 higher costs compared to similar patients who did not have an amputationCitation7. By comparison, we estimate the costs associated with major non-traumatic lower-limb amputation among PAD patients with peripheral revascularization to be $108,302. It should be noted that the two studies used different methods to assess the event-related costs. Specifically, in their study, Berger et al. estimated the event-related costs by comparing the all-cause costs among all stable/chronic PAD patients with the event to those without, whereas we only included costs associated with the medical claims that were used to identify the event of interest among the high-risk subset with lower extremity revascularization. Nonetheless, the higher healthcare resource use and costs observed in our study are consistent with the more severe disease profile of PAD patients treated with peripheral revascularizationCitation4. Future studies should assess the similarities and differences in the incidence of, and economic outcomes associated with major atherothrombotic vascular events among PAD patients with and without peripheral revascularization. Additional research is also warranted to assess the similarities and differences in outcomes among PAD patients with surgical vs. endovascular revascularization.
The study findings highlight the need for more effective therapeutic options to help reduce the risk of major atherothrombotic vascular events among these patients. Of note, rivaroxaban plus aspirin was recently evaluated in the VOYAGER PAD clinical trial, where the regimen led to a significantly lower incidence of the composite outcome of ALI, major amputation, MI, ischemic stroke, or CV death compared to aspirin alone in patients with PAD who underwent peripheral revascularizationCitation19. Further research is required to determine if this clinical benefit translates to real-world economic benefits among this subset of the PAD population that is particularly at a high-risk of experiencing major atherothrombotic vascular events and consequently incur high healthcare resource use and costs.
Limitations
The results of this study should be interpreted in light of some limitations. As with all claims-based studies, coding inaccuracies in the claims data may have led to case misidentification. Relatedly, although the study was limited to only patients with incident major atherothrombotic vascular events post-revascularization, it is not possible to draw causal inferences between the revascularization procedure and the incidence of these events due to the claims data-based retrospective study design. Additionally, the data lacked clinical information and thus limited the ability to assess certain outcomes, such as disease severity, reasons for death, and deaths occurring in non-hospital settings (data only available in certain states). To this end, CV-related death was determined using a claims-based algorithm and was not clinically validatedCitation7. The data also did not contain information on over-the-counter medications such as aspirin, which may have led to an underestimation of antiplatelet use. Furthermore, our results may be an underestimate of the total costs of these events as they do not include costs related to ambulatory care provided in the outpatient/physician offices. Relatedly, the rates and intensity of major atherothrombotic vascular events among patients included in the study may be lower than those excluded from the analyses. For example, people with pre-existing conditions such as atrial fibrillation may be at a higher risk of experiencing serious major atherothrombotic vascular events and require more intensive care compared to the patients included in this studyCitation20–22. Lastly, the results may not be generalizable to other patients not evaluated in this study, such as those enrolled in Medicaid or those without health insurance.
Conclusions
This real-world study demonstrated that nearly one in six patients with PAD who undergo peripheral revascularization experience complications such as ALI, major non-traumatic lower-limb amputation, MI, ischemic stroke, and CV-related death leading to significantly high healthcare resource use and cost. These findings highlight the need for more effective therapeutic and preventative options that may improve the outcomes among PAD patients, which in turn could help reduce the economic burden.
Transparency
Declaration of funding
This study was supported by Janssen Scientific Affairs, LLC. The sponsor was involved in the study design, data collection, data analysis, manuscript preparation, and publication decisions.
Declaration of financial/other relationships
UD and JB are employees of Analysis Group, Inc., a consulting company that received research funding for this study from Janssen Scientific Affairs, LLC. FL and PL are employees of Groupe d’analyse, Ltée, a consulting company that has received research funding for this study from Janssen Scientific Affairs, LLC. AK and DM are employees of Janssen Scientific Affairs, LLC and stockholders of Johnson & Johnson. MPB, CNH, and WRH have received research grants from Janssen Scientific Affairs. PZ was an employee of Analysis Group, Inc. at the time of this study.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
UD, FL, PZ, JB, and PL contributed to study conception and design, data analysis and interpretation. MPB, CNH, AK, DM, and WRH contributed to study conception and design, and data interpretation. All authors reviewed and approved the final content of this manuscript.
Previous presentations
Part of the material in this manuscript was presented at the American Heart Association Scientific Sessions, November 14-16, 2020, Dallas, Texas.
Acknowledgements
None reported.
References
- Campia U, Gerhard-Herman M, Piazza G, et al. Peripheral artery disease: past, present, and future. Am J Med. 2019;132:1133–1141.
- Centers for Disease Control and Prevention (CDC). Peripheral Arterial Disease (PAD) Fact Sheet. 2019 [accessed 2020 May 26]. Available from: https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_pad.htm
- Kohlman-Trigoboff D. Update: diagnosis and management of peripheral arterial disease. J Nurs Pract. 2019;15:87–95.
- Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725.
- Hess CN, Norgren L, Ansel GM, et al. A structured review of antithrombotic therapy in peripheral artery disease with a focus on revascularization: a TASC (InterSociety Consensus for the Management of Peripheral Artery Disease) Initiative. Circulation. 2017;135:2534–2555.
- Grenon SM, Vittinghoff E, Owens CD, et al. Peripheral artery disease and risk of cardiovascular events in patients with coronary artery disease: insights from the Heart and Soul Study. Vasc Med. 2013;18:176–184.
- Berger A, Simpson A, Bhagnani T, et al. Incidence and cost of major adverse cardiovascular events and major adverse limb events in patients with chronic coronary artery disease or peripheral artery disease. Am J Cardiol. 2019;123:1893–1899.
- Scully RE, Arnaoutakis DJ, Smith AD, et al. Estimated annual healthcare expenditures in individuals with peripheral arterial disease. J Vasc Surg. 2018;67:558–567.
- Devine EB, Alfonso-Cristancho R, Yanez ND, et al., for the Comparative Effectiveness Research Translation Network (CERTAIN) Collaborative. Effectiveness of a medical vs revascularization intervention for intermittent leg claudication based on patient-reported outcomes. JAMA Surg. 2016;151:e162024.
- Safley DM, House JA, Laster SB, et al. Quantifying improvement in symptoms, functioning, and quality of life after peripheral endovascular revascularization. Circulation. 2007;115:569–575.
- Bonaca MP, Gutierrez JA, Creager MA, et al. Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the trial to assess the effects of vorapaxar in preventing heart attack and stroke in patients with atherosclerosis-thrombolysis in myocardial infarction 50 (TRA2°P-TIMI 50). Circulation. 2016;133:997–1005.
- Jones WS, Baumgartner I, Hiatt WR, et al. Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. 2017;135:241–250.
- Sigvant B, Kragsterman B, Falkenberg M, et al. Contemporary cardiovascular risk and secondary preventive drug treatment patterns in peripheral artery disease patients undergoing revascularization. J Vasc Surg. 2016;64:1009–1017 e1003.
- Bonaca MP, Hess CN, Kharat A, et al. Incidence and costs of major atherothrombotic vascular events among patients with peripheral artery disease after revascularization. Circulation. 2020;142:A17116.
- Hess CN, Wang TY, Weleski Fu J, et al. Long-term outcomes and associations with major adverse limb events after peripheral artery revascularization. J Am Coll Cardiol. 2020;75:498–508.
- Hess CN, Rogers RK, Wang TY, et al. Major adverse limb events and 1-year outcomes after peripheral artery revascularization. J Am Coll Cardiol. 2018;72:999–1011.
- Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and Score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–682.
- United States Bureau of Labor Statistics. Consumer Price Index for Medical Care. Available from: https://www.bls.gov/cpi/factsheets/medical-care.htm
- Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382:1994–2004.
- Ruddox V, Sandven I, Munkhaugen J, et al. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24:1555–1566. Sep;
- Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174:107–114. Jan
- Bruggenjurgen B, Rossnagel K, Roll S, et al. The impact of atrial fibrillation on the cost of stroke: the Berlin Acute Stroke Study. Value in Health. 2007;10:137–143.