Abstract
Objective
To examine direct and indirect economic burden associated with hypothyroidism in the United States.
Methods
Medical costs attributable to hypothyroidism were estimated for patients with hypothyroidism. Non-hypothyroid (euthyroid) controls were matched to patients with hypothyroidism based upon patient characteristics and availability of productivity data. Multivariable analyses examined resource utilization, annual medical costs, comorbidities, and productivity costs.
Results
Estimates of hypothyroidism-related total medical costs ranged from $460 to $2,555 per patient per year. Compared to euthyroid controls, patients with hypothyroidism had significantly higher all-cause medical costs and medical resource utilization. For the subset of patients with available productivity data, hypothyroidism was associated with significantly higher absenteeism and long- and short-term disability costs but significantly lower worker’s compensation costs.
Conclusions
Hypothyroidism is associated with significant direct and indirect economic burden among employed, commercially insured patients in the US. Clinical Significance: Despite the availability of relatively inexpensive generic therapies for hypothyroidism, this study found significant direct and indirect costs associated with the condition. The large number of patients diagnosed with hypothyroidism combined with increased costs associated with hypothyroidism result in a significant burden for patients, payers, and employers.
Introduction
According to National Health and Nutrition Examination Survey (NHANES) III research, an estimated 4.6% of the US population has low thyroid function (hypothyroidism)Citation1. In addition to being common, hypothyroidism is associated with significant morbidity, as it affects not only the endocrine and metabolic systems, but also cardiovascular, reproductive, neurological, and digestive systemsCitation2–7. Moreover, hypothyroidism commonly co-occurs with and may impactCitation8 a number of psychiatric illnesses, including schizophrenia, chronic and acute mania, bipolar disorder, anxiety, and depressionCitation9–11.
While less expensive to treat than some other chronic conditions, such as diabetes or asthmaCitation12, hypothyroidism may be associated with substantial costs, given the large number of individuals affected and the associated morbidity. However, little research has focused on the costs of the disease. A 1990 analysis looked at the cost of alternative thyroid function testing strategies in the USCitation13, while a 2010 study examined the differential in costs associated with switching from branded to generic levothyroxineCitation14. A more recent investigation showed that multiple dose adjustments of levothyroxine were associated with lost productivity and higher direct medical costsCitation15.
In response to the need for current economic information, we examined differences in medical and pharmacy costs, productivity costs, resource utilization, and comorbid diagnoses between hypothyroid patients and matched controls in the US.
Methods
The Truven Health Analytics MarketScan Commercial Claims and Encounters (CCAE) and Health and Productivity (HPM) databases were used for this study. The CCAE database contains information on diagnoses, costs, medical resource utilization, and prescription drug use for the inpatient and outpatient medical care of approximately 40 million commercially insured individuals who are covered by fully- or partially-capitated, fee-for-service health plansCitation16. The HPM database is provided by employers and contains information about absenteeism, short-term disability, long-term disability, and worker’s compensation. The CCAE database contains records from 1 July 2011 through 31 December 2015, while the HPM database had available data from 1 July 2011 through 31 December 2014. Both databases contain deidentified patient level data that are fully compliant with the Health Insurance Portability and Accountability Act (HIPAA).
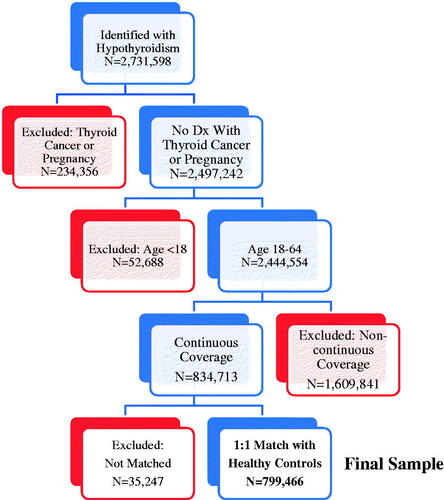
To be included in the hypothyroidism cohort, patients were required to have two separate claims for hypothyroidism (identified by ICD-9-CM of 243.xx or 244.xx, except 244.2x and 244.3x) in the calendar years 2012, 2013, or 2014, with the first such date identified as the index date. They were also required to have continuous insurance for medical care and prescription drugs from 6 months prior to the index date (i.e. pre-period) through 1-year after the index date (i.e. post-period). Individuals were excluded if they were younger than 18 years old as of the index date or diagnosed as pregnant (ICD-9-CM of 630.xx-679.xx, V22.xx, V23.xx, V30.xx-V39.xx, or V72.42) at any time during the study period. These criteria resulted in a sample of 834,713 individuals before matching with controls.
Potential non-hypothyroid (euthyroid) controls were identified as individuals who did not have any claims associated with hypothyroidism at any time during the study period. These individuals were randomly assigned an index date assuming a uniform distribution. Controls were then matched to cases 1:1, without replacement, based upon age (in 5-year intervals), gender, region of residence, insurance type, and availability of post-period data on absenteeism, short-term disability (STD), long-term disability (LTD), and workers’ compensation (WC). This matching resulted in a 95.8% successful match rate, with a final sample of 799,466 individuals who had hypothyroidism and an equal number of controls. illustrates how the inclusion and exclusion criteria and matching of hypothyroid individuals to controls affected sample size.
Outcomes examined in these analyses included hypothyroidism-related and all-cause costs. Hypothyroidism-related costs were constructed once by identifying all costs associated with any accompanying diagnosis of hypothyroidism (broad) and again by identifying only costs associated with a primary or first diagnosis of hypothyroidism (narrow). The broad cost definition represented an upper bound for estimated hypothyroidism-related costs, while the narrow construction constituted a lower bound. Notably, costs could not be disaggregated for any visit where an individual was treated for multiple conditions. Both hypothyroidism-related and all-cause costs were subcategorized into inpatient, outpatient, emergency room (ER), laboratory, and drug components. All costs were converted to 2015 dollars using the medical component of the Consumer Price IndexCitation17.
In addition to examining costs, we also compared post-period resource utilization and comorbidities between patients with hypothyroidism and controls. All-cause resource utilization measures included the probability of hospitalization, number of hospitalizations, hospital length of stay (LOS), probability of an emergency room (ER) visit, and number of ER visits. We also assessed the likelihood of having certain post-period diagnoses, including Addison’s disease, bipolar disorder, celiac disease, depression, dyslipidemia, heart failure, hypertension, migraine, myasthenia gravis, obesity, pernicious anemia, rheumatoid arthritis, schizophrenia, systemic lupus erythematosus, type 1 diabetes, and type 2 diabetes. These conditions were identified as common comorbidities of hypothyroidism either by previous researchCitation18–24 or by guidelines of the American Thyroid Association and American Association of Clinical EndocrinolgistCitation25. Appendix A provides the ICD-9-CM codes used to identify each of these comorbidities.
The analyses also examined losses in productivity, as measured by short-term disability, long-term disability, or workers’ compensation. The absenteeism data in the HPM database is presented in hours and was converted to days by assuming an 8-hour workdayCitation26. Days of absenteeism were then converted to costs by using the Bureau of Labor Statistics data on average wage rates for 2015. Short-term disability, long-term disability, and workers’ compensation costs were priced at 60% of absenteeism costs, given that this percentage is a common reimbursement paymentCitation27.
To adjust for potential underlying confounders, we utilized multivariable analyses which controlled for patient age, gender, region, insurance type, Charlson Comorbidity IndexCitation28,Citation29, and, in hypothyroidism costs estimates, diagnosing physician type (endocrinologist or other). Cost analyses for total medical costs, outpatient costs, drug costs, and absenteeism were constructed using generalized linear models with gamma distribution and log linkCitation30. Inpatient costs, ER costs, laboratory costs, STD costs, LTD costs, and WC costs were estimated using a two-part model in which the predicted probability of a service estimated in the first part of the model was multiplied by estimated costs for individuals who used the service to obtain average cost estimatesCitation31. To examine the probability of being hospitalized, visiting the ER, or being diagnosed with a given comorbidity, logistic models were utilized. The number of hospitalizations, number of ER visits, hospital LOS, and hours of missed work, were examined using negative binomial modelsCitation32. Estimated mean outcomes were derived from the multivariable analyses.
All analyses were conducted using SAS, version 9.4. A p-value of <0.05 was determined, a priori, to be statistically significant.
Results
describes baseline demographics both before and after matching. Prior to matching, patients with hypothyroidism were older, more likely to be female, more likely to reside in the Northeast, and more likely to be insured by a preferred provider organization. After matching, all relevant covariates were the same between the cohorts with the exception that there remained a trivial but statistically significant difference in age (49.99 vs. 49.90 years; p < 0.0001) due to matching by age groups rather than exact age.
Table 1. Descriptive statistics pre- and post-matching.
Hypothyroidism-related costs
Hypothyroidism-related costs were estimated for the entire hypothyroidism cohort prior to matching (N = 834,713), since euthyroid controls, by construction, had no costs related to hypothyroidism. Estimates of total hypothyroidism-related costs per patient per year ranged from $460 when costs were defined narrowly and $2,555 when costs were defined broadly (). With the narrower identification of hypothyroidism-related costs, outpatient visits and prescription drug costs constituted the vast majority of hypothyroidism-related medical costs. With the broader construction of hypothyroidism-related costs, inpatient and outpatient visit costs were the largest estimated components of hypothyroidism-related total medical costs.
Table 2. Hypothyroidism-related costs per person per year in US dollars.
All-cause costs
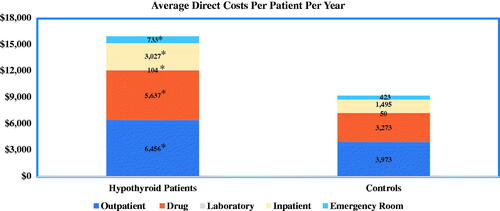
illustrates the direct all-cause costs associated with hypothyroidism. Outpatient, inpatient, emergency room, prescription drug, and laboratory costs were all significantly higher for patients with hypothyroidism than for euthyroid controls. For both the hypothyroid cohort and the control group, outpatient and prescription drugs were the largest cost components. All-cause inpatient and laboratory costs were over twice as much for patients with hypothyroidism compared to controls. Estimated total costs were also significantly higher for patients with hypothyroidism compared to controls ($15,737 vs. $8,809; p < 0.0001).
All-cause healthcare resource utilization and comorbidities
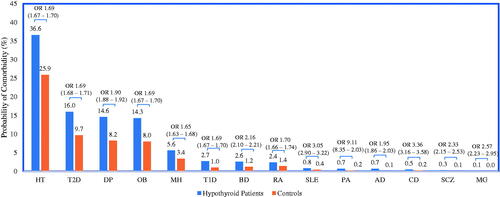
and illustrate differences in healthcare resource utilization and comorbidities between euthyroid controls and hypothyroidism-related patients. Adjusted results from the multivariable models show that, compared to controls, patients with hypothyroidism were significantly more likely to have an all-cause ER visit or hospitalization and to have more all-cause ER visits and hospitalizations. Furthermore, among those hospitalized, patients with hypothyroidism had a significantly longer hospital length of stay compared to euthyroid controls. Corresponding to this increase in medical resource utilization, patients with hypothyroidism were also more likely to be diagnosed with comorbid conditions in the 1-year post-period. The most frequently diagnosed comorbidities for both hypothyroid patients and controls were hypertension, type 2 diabetes, depression, and obesity and for each of these, patients with hypothyroidism were nearly twice as likely to have these comorbidities compared to controls (adjusted odds ratios 1.69, 1.90, 1.69, and 1.69, respectively). Adjusted odds ratio results also revealed that patients with hypothyroidism, compared to controls, were over 9-times as likely to be diagnosed with Addison’s disease, over 3-times as likely to be diagnosed with pernicious anemia or celiac disease, and over twice as likely to be diagnosed with myasthenia gravis, schizophrenia, or bipolar disorder.
Figure 3. Estimated all-cause health resource utilization. *Differences are statistically significant (p < 0.0001). Results from multivariable analyses that controlled for age, sex, region of residence, insurance type, and general health. OR, odds ratios. 95% confidence intervals given in parentheses. aHypothyroid patients, n = 70,236; Controls, n = 40,399.
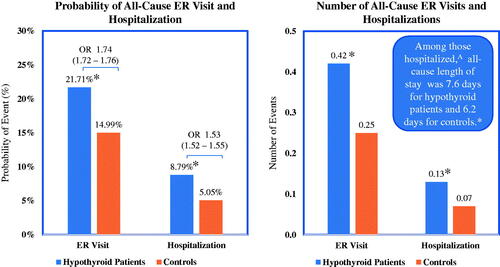
Figure 4. Estimated probability of comorbidity. All differences are statistically significant (p < 0.0001). Results from multivariable analyses that controlled for age, sex, region of residence, insurance type, and general health. Odds ratios (ORs) and 95% confidence intervals (in parentheses) presented above estimated probabilities. Abbreviations. AD, Addison’s disease; BD, bipolar disorder; CD, celiac disease; DP, depression; HT, hypertension; MG, myasthenia gravis; MH, migraine headache; OB, obesity; PA, pernicious anemia; RA, rheumatoid arthritis; SCZ, schizophrenia; T1D, type 1 diabetes; T2D, type 2 diabetes.
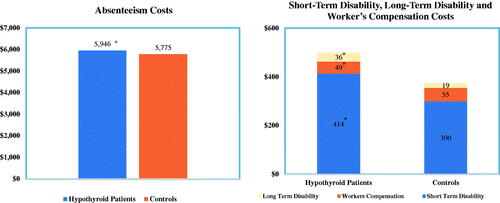
Productivity costs
While shows the direct medical costs, illustrates the estimated indirect costs associated with lost productivity for the subset of patients with available data for these productivity measures. Productivity costs were also generally higher for patients with hypothyroidism, although the differences between the hypothyroid and control cohorts were generally smaller when comparing indirect vs direct costs. The analyses revealed an estimated $171 additional absenteeism cost per year, as well as significantly higher long-term disability and short-term disability costs for patients with hypothyroidism compared to controls. However, hypothyroidism was associated with significantly lower workers’ compensation costs.
Figure 5. Estimated productivity costs. Sample sizes: Absenteeism, n = 17,830; STD, n = 151,762; LTD, n = 128,564; WC, n = 70,020. *Difference are statistically significant (p < 0.005). Results from multivariable analyses that controlled for age, sex, region of residence, insurance type, and general health.
Discussion
This study examined both the direct and indirect costs of patients with hypothyroidism in the US. The results revealed that direct costs, indirect costs, and resource utilization were all significantly higher for individuals with hypothyroidism compared to euthyroid controls. In addition, a large number of comorbidities were more common in the hypothyroid cohort than in the control group. These findings have implications for the US healthcare system, employers, and patients, as discussed below.
In this study, the annual, per-patient healthcare costs related directly to hypothyroidism amounted to a conservative estimate of $460 and a broad estimate of $2,555 (). Given the prevalence of hypothyroidism in the USCitation1, these numbers reveal a substantial economic burden of between $384 million and $2.1 billion annually (based on narrow or broad assessments, respectively). Disease-related costs accounted for only a fraction of the total direct and indirect costs for patients with hypothyroidism. Specifically, individuals with hypothyroidism in this study had nearly $7,000 higher total annual all-cause, per patient medical costs relative to euthyroid controls (), with the largest cost components for outpatient and drug costs. The higher direct medical costs associated with hypothyroidism is consistent with the findings in this study of significantly higher rates of comorbid conditions and all-cause medical resource utilization associated with hypothyroidism. In addition to being associated with higher direct medical costs, hypothyroidism was also associated with higher indirect costs. Individuals with hypothyroidism had an additional $171 in absenteeism costs relative to controls, an average excess cost that translates to nearly one additional day (6.7 hours) of lost work time per worker.
Results from this study suggest that treatment of hypothyroidism may be more complex given the comorbidities and additional costs associated with the condition. These findings support previous research which found that, among postmenopausal hypothyroid women treated with levothyroxine, increases in thyroid stimulating hormone (TSH) were associated with worse laboratory test results, including higher total cholesterol, glycemia, and systolic and diastolic blood pressureCitation33. Additionally, research has found that the use of levothyroxine in combination with medications for the treatment of the comorbidities of metabolic syndrome, impaired fasting glycemia, diabetes mellitus, dyslipidemia, hypertension, coronary heart disease, or cerebrovascular disease was associated with greater TSH levels over a mean treatment period of 32.1 monthsCitation34. Similarly, other research has shown that a substantial proportion of patients taking thyroid medication continue to have abnormal levels of TSH at follow-upCitation35 and that multiple switches or dose adjustments or levothyroxine are associated with increased healthcare costs and productivity lossesCitation14,Citation15. Such sub-optimal patterns of disease management may have also affected the resource utilization and costs in our study population.
The present analyses had several strengths, including a large sample size and the examination of not only hypothyroidism but also a large number of comorbidities associated with the condition. In addition, hypothyroid patients and controls were well matched, which controlled for many potential confounders that could also impact outcomes. Notwithstanding these strengths, the analyses must be interpreted within the context of the study limitations, which stem, in large part, from the use of observational health insurance claims data from a commercially insured population. For instance, this well-insured population may not be generalizable to all hypothyroid patients. Moreover, the use of diagnostic codes is not as rigorous as formal assessment, as it relies on the assumption that the patients were coded correctly at the time of diagnosis. Third, the use of claims data precluded the analyses from directly controlling for non-documented factors, such as race and socioeconomic class, which may be associated with patient outcomes. In addition, the analyses did not distinguish between incident and prevalent comorbidities and did not examine the impact of subclinical hypothyroidism on healthcare utilization and comorbidities. Furthermore, all analyses focused on statistical significance and were unable to determine whether differences in outcomes represented clinically important changes. Finally, this study did not explore the role of potential factors such as non-treatment, sub-optimal treatment, non-adherence, and switching on patient costs and resource utilization.
In conclusion, the findings of this study illustrate that people with hypothyroidism have significantly higher direct costs, indirect costs, and resource utilization. In addition, individuals with hypothyroidism were found to be significantly more likely, relative to controls, to be diagnosed with a number of comorbid conditions. Future work should focus on the role of different treatments and explore potential drivers of this economic burden.
Transparency
Declaration of funding
Financial support for this study was provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
Declaration of financial/other relationships
Ramon Espaillat and Zsolt Hepp are employees of AbbVie and may own AbbVie stocks or options. Maureen J. Lage is the Managing Member of HealthMetrics Outcomes Research, which was compensated for work on this project. Ved V. Gossain is a consultant for AbbVie and has received consultancy fees from AbbVie in this role. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
The authors would also like to acknowledge Patricia Platt and Michael Treglia of HealthMetrics Outcomes Research provided medical writing and editing services in the development of this publication. AbbVie provided funding for this work.
References
- Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499.
- Han C, He X, Xia X, et al. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2015;10(8):e0135233.
- Chang Y-J, Hwu C-M, Yeh C-C, et al. Effects of subacute hypothyroidism on metabolism and growth-related molecules. Molecules. 2014;19(8):11178–11195.
- Liu H, Shan Z, Li C, et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid. 2014;24(11):1642–1649.
- Collet T-H, Gussekloo J, Bauer DC, et al. Subclinical hyperthyroidism and the risk of coronary heart disease and mortality. Arch Intern Med. 2012;172(10):799–809.
- Singh S, Trivedi R, Singh K, et al. Diffusion tensor tractography in hypothyroidism and its correlation with memory function. J Neuroendocrinol. 2014;26(11):825–833.
- Collins D, Wilcox R, Nathan M, et al. Celiac disease and hypothyroidism. Am J Med. 2012;125(3):278–282.
- Duntas LH, Maillis A. Hypothyroidism and depression: salient aspects of pathogenesis and management. Minerva Endocrinol. 2013;38(4):365–377.
- Radhakrishnan R, Calvin S, Singh JK, et al. Thyroid dysfunction in major psychiatric disorders in a hospital based sample. Indian J Med Res. 2013;138(6):888–893.
- Pattanayak RD, Pattanayak S. Chronic manic episode associated with hypothyroidism due to Hashimoto's disease: a case report. J Neuropsychiatry Clin Neurosci. 2014;26(4):E27.
- Khemka D, Ali JA, Koch CA. Primary hypothyroidism associated with acute mania: case series and literature review. Exp Clin Endocrinol Diabetes. 2011;119(8):513–517.
- Druss BG, Marcus SC, Olfson M, et al. The most expensive medical conditions in America. Health Aff (Millwood). 2002;21(4):105–111.
- Schectman JM, Pawlson LG. The cost-effectiveness of three thyroid function testing strategies for suspicion of hypothyroidism in a primary care-setting. J Gen Intern Med. 1990;5(1):9–15.
- Katz M, Scherger J, Conard S, et al. Healthcare costs associated with switching from brand to generic levothyroxine. Am Health Drug Benefits. 2010;3(2):127–134.
- Ernst FR, Barr P, Elmor R, et al. The economic impact of levothyroxine dose adjustments: the CONTROL HE study. Clin Drug Investig. 2017;37(1):71–83.
- Hansen LG, Chang S. Health research data for the real world: the MarketScan databases; 2011 [accessed 2015 Oct 15]. Available from: http://truvenhealth.com/portals/0/assets/PH_11238_0612_TEMP_MarketScan_WP_FINAL.pdf.
- United States Department of Labor. Bureau of labor statistics data; 2017 [accessed 2017 Jun 5]. Available from: https://data.bls.gov/cgi-bin/dsrv.
- Lisotto C, Mainardi F, Maggioni F, et al. The comorbidity between migraine and hypothyroidism. J Headache Pain. 2013;14(S1):138.
- McElroy SL. Diagnosing and treating comorbid (complicated) bipolar disorder. J Clin Psychiatry. 2004;65 (Suppl 15):35–44.
- Bunevicius R, Prange AJ. Thyroid disease and mental disorders: cause and effect or only comorbidity? Curr Opin Psychiatry. 2010;23(4):363–368.
- Stabouli S, Papakatsika S, Kotsis V. Hypothyroidism and hypertension. Expert Rev Cardiovasc Ther. 2010;8(11):1559–1565.
- Schmidt-Ott UM, Ascheim DD. Thyroid hormone and heart failure. Curr Heart Fail Rep. 2006;3(3):114–119.
- Chaker L, Ligthart S, Korevaar TIM, et al. Thyroid function and risk of type 2 diabetes: a population-based prospective cohort study. BMC Med. 2016;14(1):150.
- Rizos C, Elisaf M, Liberopoulos E. Effects of thyroid dysfunction on lipid profile. Open Cardiovasc Med J. 2011;5:76–84.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18(6):988–1028.
- Birnbaum HG, Cremieux PY, Greenberg PE, et al. Using healthcare claims data for outcomes research and pharmacoeconomic analyses. Pharmacoeconomics. 1999;16(1):1–8.
- Kamal-Bahl SJ, Pantely S, Pyenson B, et al. Employer-paid nonmedical costs for patients with diabetes and end-stage renal disease. Prev Chronic Dis. 2006;3(3):1–10.
- D'Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. 1993;32(5):382–387.
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139.
- Malehi AS, Pourmotahari F, Angali KA. Statistical models for the analysis of skewed healthcare cost data: a simulation study. Health Econ Rev. 2015;5:11.
- Geue C, Lorgelly P, Lewsey J, et al. Hospital expenditure at the end-of-life: what are the impacts of health status and health risks? PLoS One. 2015;10(3):e0119035.
- Shafrin J. What is the negative binomial model? Healthcare Economist; 2013 [accessed 2015 Aug 26]. Available from http://healthcare-economist.com/2013/02/21/what-is-the-negative-binomial-model/.
- Morini E, Catalano A, Lasco A, et al. L-thyroxine malabsorption due to calcium carbonate impairs blood pressure, total cholesterolemia, and fasting glycemia. Endocrine. 2019;64(2):284–292.
- Benvenga S, Pantano R, Saraceno G, et al. A minimum of two years of undertreated primary hypothyroidism, as a result of drug-induced malabsorption of l-thyroxine, may have metabolic and cardiovascular consequences. J Clin Transl Endocrinol. 2019;16:100189.
- Scavone C, Sportiello L, Cimmaruta D, et al. Medication adherence and the use of new pharmaceutical formulations: the case of levothyroxine. Minerva Endocrinol. 2016;41(2):279–289.