Abstract
Aims
Although the benefit of first-line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) over chemotherapy in EGFR mutation-positive (EGFRm) non-small-cell lung cancer (NSCLC) has been demonstrated in clinical trials, the optimal treatment sequence remains unclear. The objective of our study was to evaluate the cost-effectiveness of dacomitinib in Sweden vs afatinib and osimertinib in first-line treatment of EGFRm NSCLC.
Materials and methods
A partitioned survival model was developed with three health states: progression-free, post-progression, and death. Progression-free and overall survival curves were used to inform movements between states. Clinical data were taken from randomized trials, compared via a network meta-analysis (NMA). Utility data were taken from published studies and costs from national Swedish sources. The model used a 15-year time horizon and a Swedish healthcare payer perspective. Sensitivity and scenario analyses were performed.
Results
The base-case analysis showed that dacomitinib accrued a total of 2.10 quality-adjusted life-years (QALYs) at a total cost of Swedish krona (SEK) 874,615. The incremental cost-effectiveness ratio (ICER) for dacomitinib vs afatinib was SEK 461,556 per QALY gained. The ICER of osimertinib vs dacomitinib, where the small QALY gains of the former came at a high additional cost, was SEK 11,444,709. Deterministic and probabilistic sensitivity analyses confirmed the robustness of these results; changes to drug and medical resource use costs and overall survival had the greatest impact on ICER estimates.
Limitations
This model is subject to uncertainty associated with extrapolating long-term treatment effects from shorter trial follow-up periods, although this would also be a limitation when using direct comparison or time-dependent hazard ratios. The NMA was limited by the use of indirect comparison, although sensitivity analyses supported the robustness of our findings.
Conclusions
Our model demonstrated that dacomitinib is cost-effective for first-line EGFRm NSCLC treatment in Sweden vs afatinib and osimertinib.
Introduction
Lung carcinoma is subdivided into small-cell and non-small-cell lung carcinoma (NSCLC)Citation1,Citation2. NSCLC is a heterogeneous group of carcinomas and accounts for 70–80% of lung cancer casesCitation1,Citation2. Activating somatic mutations of the tyrosine kinase domain of epidermal growth factor receptor (EGFR) define a further subset of patients with NSCLC and are observed in about 20% of non-Asian patients with NSCLCCitation1,Citation3.
Patients with activating somatic EGFR mutations have better clinical outcomes when treated with EGFR tyrosine kinase inhibitors (TKI) over chemotherapyCitation4,Citation5. Three generations of EGFR TKIs have been developed (first-generation: gefitinib [AstraZeneca]; erlotinib [Roche]; second-generation: afatinib [Boehringer Ingelheim]; dacomitinib [Pfizer]; and third-generation: osimertinib [AstraZeneca])Citation6. There has been considerable evidence of resistance to first- and second-generation EGFR TKIs, which may be linked to their mode of action and the secondary EGFR mutation T790MCitation7–10, which can be overcome by third-generation EGFR TKIsCitation11,Citation12. European Society for Medical Oncology (ESMO) guidelinesCitation13 recommend first-line treatment with an EGFR TKI for patients with advanced EGFR mutation-positive (EGFRm) NSCLC; second-line osimertinib is recommended for patients who initiate treatment with first- or second-generation EGFR TKI and develop T790M mutation upon progression. For patients who initiate first-line treatment with osimertinib, chemotherapy is the main option after progressionCitation13.
Dacomitinib is approved by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the “the first-line treatment of patients with metastatic NSCLC with EGFR exon 19 deletion or exon 21 L858R substitution mutations as detected by an FDA-approved test” and the “first-line treatment of adult patients with locally advanced or metastatic NSCLC with EGFR-activating mutations”, respectivelyCitation14,Citation15. It was granted full reimbursement by the Tandvårds- och läkemedelsförmånsverket (TLV) for use in Sweden in June 2019Citation16 and is now among the four currently available first- or second-generation EGFR TKIs (gefitinib, erlotinib, afatinib, and dacomitinib) recommended by ESMO for use in patients with active sensitizing EGFR mutations, although there is no consensus for the preference of any of these treatments over othersCitation13. The third-generation EGFR TKI osimertinib is considered the preferred option for first-line treatment of this populationCitation13. The recently updated Swedish guidelinesCitation17, however, do not recommend first-generation EGFR TKIs in this first-line setting, instead listing only second- or third-generation EGFR TKIs (osimertinib, dacomitinib, or afatinib) as the first-line choice for patients with EGFR-activating mutations.
The benefit of first-line EGFR TKIs over chemotherapy has been demonstrated in several clinical trialsCitation18. Although further work is needed to determine the optimal EGFR TKI sequence to be used, the position of osimertinib may be dictated by resistance mechanisms identified in the first-line settingCitation19. Real-world data indicate encouraging overall survival (OS) with sequential afatinib and osimertinib in patients with T790M mutations, especially Del-19-positive patientsCitation20.
In 2016, Sweden recorded 4,108 new lung cancer cases, while 3,677 patients died from the disease during the same yearCitation21,Citation22. As noted in a longitudinal study (2010–2016) in Sweden, the use of EGFR TKIs in patients with NSCLC improved survival, and accessibility of targeted therapies increased over this periodCitation23.
The objective of our study was to evaluate the cost-effectiveness of dacomitinib in the first-line treatment of EGFRm NSCLC in Sweden, compared with afatinib and osimertinib, the other recommended EGFR TKIs in this settingCitation17.
Methods
Model structure and settings
The model was created in Excel and structured as a partitioned survival model (PSM), which is a standard model structure in oncology and follows the best modeling practices described by the International Society of Pharmacoeconomics and Outcomes Research (ISPOR)Citation24 and the National Institute for Health and Clinical Excellence (NICE) Decision Support Unit (DSU)Citation25. In a PSM, the progression-free survival (PFS) and OS curves are used directly, and the share of patients with progressed disease is calculated as the difference between the PFS and OS curves. Three health states were included in the model: progression-free (PF), post-progression (PP), and death. PFS and OS curves were used to inform movement between the aforementioned states. Transition between the health states was one-directional.
All patients entered the model in the PF health state. At the end of each 28-day period (the model cycle length), patients in the PF state could either remain in the PF state or proceed to the PP state (having experienced disease progression) and stop first-line treatment; patients who died proceeded to the death state (with or without having proceeded to the PP state first).
Patients in the PP state received PP treatment (second- and third-line treatments) or best supportive care (BSC). Subsequent treatments contributed to costs and utility calculations in the model but did not impact OS. Patients who transitioned to the PP state could either remain in that state in the next model cycle (while alive) or move to the death state.
Outcomes were measured in life-years (LY) gained, progression-free life-years (PF-LY) gained, quality-adjusted life-years (QALYs) gained, and treatment costs. The incremental cost-effectiveness ratio (ICER) was then calculated as the difference in total costs between dacomitinib and a specific comparator divided by the difference in QALYs between dacomitinib and that comparator.
The model target population corresponded to patients with locally advanced or metastatic EGFRm NSCLC. The base-case time horizon used in the analysis was 15 years, i.e. a lifetime perspective for this patient population (based on the typical OS from published trialsCitation26,Citation27). This time horizon was chosen to capture all relevant clinical and economic outcomes that occur within the patient cohort in the economic model. Costs and outcomes were discounted at 3% per annum based on Swedish Health Technology Assessment (HTA) guidanceCitation28. The perspective of the model was that of the Swedish healthcare provider. A scenario analysis was included where the societal perspective was considered. Nearly all costs are presented in 2018 Swedish krona (SEK), with the exception of EGFR TKI drug costs. The reason is that there have been substantial changes in the prices of some EGFR TKIs recently, for erlotinib and gefitinib due to generic entry, and for dacomitinib there was a price change in April 2020. Therefore, all EGFR TKI costs have been updated to those published in 2020. There have been no meaningful price changes in other costs included in this analysis since 2018.
Data sources – clinical
To compare dacomitinib with afatinib and osimertinib, treatment efficacy was modeled through an indirect comparison using the gefitinib PFS and OS survival curves from ARCHER 1050 (NCT01774721) as the reference and applying estimated hazard ratios (HRs) obtained from Farris et al.Citation29 and Franek et al.Citation30.
To extrapolate the PFS and OS curves over the time horizon, first Kaplan-Meier curves for PFS and OS were generated using the patient-level data for the gefitinib arm from the ARCHER 1050 studyCitation27,Citation31. Standard parametric functions (exponential, Weibull, Gompertz, log-logistic, log-normal, and generalized gamma) were then fitted separately on each of these Kaplan-Meier curves using the streg procedure in STATA. Based on goodness-of-fit statistics (Akaike Information Criterion [AIC] and Bayesian Information Criterion [BIC]), visual inspection, and validation by external experts, the Weibull distribution was considered the most suitable. The distributions that had lower AIC and BIC than the Weibull distribution were judged by clinical experts to have unreasonably fat tails, thus leading to excessively positive long-run extrapolations. Goodness-of-fit statistics for the extrapolations with different distributions and graphs of Weibull distribution fitting compared with the observed Kaplan-Meier curves for PFS and OS for gefitinib are presented in Supplementary Table 1 and Supplementary Figure 1. The procedure followed the recommendations by NICE DSU 14Citation25.
Table 1. Second- and third-line treatment baskets composition per first-line treatment.
The HRs used in the comparison of dacomitinib with the other EGFR TKIs in the model were informed by a traditional network meta-analysis (NMA), and the detailed methods and results have been published previouslyCitation29,Citation30. Briefly, a NMA combines direct and indirect evidence to generate pairwise comparisons of every intervention against one another even in the absence of direct evidence. The NMA was based on the results from a systematic literature review (SLR). The SLR was performed according to the methodological principles of conduct for systematic reviews as detailed in the University of York Centre for Reviews and Dissemination guidance for undertaking systematic reviews in healthcareCitation32. A systematic search of 11 electronic databases and conference proceedings was conducted to identify randomized controlled trials of first-line EGFR TKI therapies and their comparators that molecularly selected or stratified patients with EGFRm-positive locally advanced or mestastatic NSCLC. The overall quality of evidence was evaluated using the National Institute for Clinical Excellence (NICE) Quality Appraisal Checklist for quantitative intervention studiesCitation33. Knowledge of clinical subject matter, data inspection, and network diagrams were used to assess the similarity of the trials and the feasibility of an NMA for the trials identified by the SLR. A traditional Bayesian NMA was deemed appropriate in accordance with NICE technical recommendations (NICE Technical Support Document 18)Citation34 and the International Society for Pharmacoeconomics and Outcomes Research Task Force on Indirect Treatment Comparison recommendationsCitation35.
In the NMA, the relative efficacy of dacomitinib and other first-line EGFR TKI therapies was assessed in terms of OS and PFS. A Bayesian fixed-effects NMACitation36 was conducted in R (v3.4.3 for the analysis of PFS and v3.6.1 for the update analysis of OS) using the package “GeMTC” (v0.8-2), which calls upon JAGS (using the rjags package)Citation37 for Markov Chain Monte Carlo simulations using a normal likelihood and identity linkCitation38. Models were assessed for convergence using the Brooks–Gelman–Rubin methodCitation39 and model fit using residual deviance, leverage, and the deviance information criterion (DIC)Citation40. As the network contained no closed loops, inconsistency was not assessed. The NMA used data from the ARCHER 1050 (dacomitinib vs gefitinib)Citation27,Citation31, LUX-Lung 7 (afatinib vs gefitinib; NCT01466660)Citation41,Citation42, and FLAURA (osimertinib vs gefitinib or erlotinib; NCT02296125)Citation26,Citation43 studies to derive constant HRs, expressed vs gefitinib, for PFS and OS. The HRs vs gefitinib for PFS and OS from the NMA are reported as part of the results in .
Table 6. Resulting median PFS and OS for all included EGFR TKIs.
The NMA used in this studyCitation29,Citation30 differs from other previously published NMAs of EGFR TKIsCitation44–48 in several ways. The NMA used in this study focused only on EGFR TKI monotherapies, included more recently published data (e.g. mature OS data for osimertinib from FLAURACitation26 and updated OS data for dacomitinib from ARCHER 1050Citation27), and employed more stringent selection criteria regarding data specific to EGRFm-positive (namely it was restricted to randomized studies in which patients were molecularly selected for EGRFm-positive status and did not include non-randomized, post-hoc EGRFm-positive subgroup analyses).
It was assumed that in first-line therapy, patients would remain on treatment as long as they were PF and that all patients would discontinue first-line treatment upon progression. Subsequent treatments in the second- and third-line settings were modeled as baskets of treatments specific to the first-line treatment received, accounting for the resistance features of the EGFRm NSCLC population. The model specifically took into consideration that 50–60% of patients treated with first-line first- and second-generation EGFR TKIs develop the T790M mutationCitation49,Citation50, for which osimertinib is the only available targeted second-line therapy. It further considered that the resistance profile of osimertinib is largely unknown and that patients progressing on osimertinib in prior lines likely are left with chemotherapy treatment as the only subsequent option. Immunotherapy was not included as an option because current evidence does not support the use of immunotherapies in patients with EGFRm NSCLC ().
The time on treatment for subsequent lines of therapy is specific to the first-line treatment patients receive, and to the line of subsequent treatment (second or third). The times on treatment for second-line EGFR TKIs and chemotherapy (platinum-based and single-agent) were derived from data presented by Sequist et al.Citation51 for most alternatives. The reported durations of 5.7 months for first-generation EGFR TKIs and 2.9 months for platinum-based chemotherapy were converted to reflect the 28-day model cycle. For osimertinib, time on treatment was derived from the AURA3 trial which is directly pertinent to second-line osimertinib treatmentCitation52.
Third-line therapy durations were also derived from Sequist et al.Citation53 The reported durations of 2.9 months for first-generation EGFR TKIs and 2.5 months for single-agent chemotherapy were converted to reflect the 28-day model cycle. Third-line osimertinib was not reported, therefore it was assumed to be the same as in the second line. Platinum-based chemotherapy was assumed to be the same as the single-agent chemotherapy. The average treatment duration of subsequent treatment lines was calculated based on the respective shares and median duration of the specific subsequent treatments.
In the PP state, it was assumed that a decreasing share of patients would be treated in second- and third-line settings. Patients not proceeding to second- or third-line treatments were assumed to receive BSC. Upon discontinuation of third-line treatment, all living patients were given BSC. Patients were assumed to then receive BSC until death or the end of the modeled time horizon, whichever occurred earlier.
Data sources – utility
In the clinical study ARCHER 1050, patients reported their health-related quality of life using three well-established quality-of-life instruments: The European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire C-30 (EORTC QLQ-C30), the lung-cancer specific questionnaire QLQ-LC13, and the EuroQol 5-Dimension-3Level (EQ-5D-3L)Citation31. For the economic model, in line with previous TLV decisions, EQ-5D-3L utility scores for patients on first-line treatment were calculated using the British EQ-5D-3L tariff, and for a scenario analysis calculated using the Swedish tariffCitation54. The resulting utility value for dacomitinib-treated patients was 0.78. Afatinib-treated patients were assumed to have the same utility value (0.78) as dacomitinib-treated patients (since afatinib also is a second-generation irreversible EGFR TKI with a similar toxicity profile to dacomitinib). This utility value is in line with, but slightly higher than, the results from LUX-Lung 7Citation41, so may result in a conservative outcome. Because of the more benign adverse event (AE) profile of osimertinib, the utility value applied for patients treated with osimertinib was 0.83, and was assumed to be the same as for patients treated with gefitinib in ARCHER 1050. This is likely to be a highly conservative assumption, as according to a TLV evaluation of first-line osimertinibCitation55 based on FLAURACitation43, the utility value for patients treated with osimertinib was only 0.79. The corresponding utility values for the scenario analysis based on the Swedish tariff were 0.87 and 0.89. Quality of life during later treatment lines was based on utility values taken from two previously published studies for EGFR TKIsCitation56 and BSCCitation57. These values were 0.74 for all EFGR TKIs and chemotherapies in second-line treatment and 0.62 for all EFGR TKIs and chemotherapies in third-line treatment. BSC had a utility value of 0.47Citation57. These utility estimates in later treatment lines were applied independently of the treatments used in the first line.
Data sources – costs
Medical resource use (MRU) and treatment costs estimated in the model can be grouped into six categories:
First-line drug cost. The total drug acquisition cost was based on the calculated cycle treatment cost, accrued at each cycle for those patients who were still receiving first-line treatment. The per-cycle treatment acquisition cost was based on unit price and recommended dosing regimen described in the Summary of Product Characteristics (SPC) of each product. Unit costs were taken from the TLV database ()Citation58. The costs of wastage of first-line drugs was fully accounted for by having all first-line drug costs occurring at the beginning of each treatment cycle. As all the first-line treatments in the model are administrated orally and distributed through pharmacies, administration costs were assumed to correspond to the pharmacy dispensing fees, which were assumed to be included in the drug acquisition prices used in the model.
First-line treatment-associated AE costs. Treatment-related AE costs were based on the impact of AEs grade 3 or higher. An assumption was made that AEs occurred in the first cycle. Costs were taken from national Swedish estimates ()Citation59. The mean one-off costs of AEs were SEK 1,168 for dacomitinib, SEK 1,159 for afatinib, and SEK 359 for osimertinib.
Second- and third-line drug costs. Subsequent treatment costs were calculated assuming baskets of treatments that differed depending on which treatment was used in the first line. Drug acquisition costs were taken from the TLV database and apoteket.se, and the administration costs for chemotherapy from national estimates ()Citation59. These costs were applied as a one-off cost when patients started a subsequent treatment line. Dosing information was obtained from the FASS website (www.fass.se), which is based on the SPC for individual drugs.
Disease management and monitoring costs were based on each health state (i.e. PF, second- or third-line treatment with progressed disease, and BSC with progressed disease). These primarily consisted of outpatient visits to specialists, general practitioners, and nurses, in addition to investigations such as computerized tomography or x-ray, and palliative radiotherapy. The resource use per cycle was assumed to be the same across therapies. It was also assumed to be the same in each health state and in each line of therapy, with the exception of those patients receiving BSC. Resource use information was obtained through interviews with specialist Swedish clinicians. Costs were taken from regional Swedish cost data ()Citation60,Citation61. The aggregated MRU costs per cycle were SEK 3,016 on either initial treatment or subsequent treatment and SEK 8,373 for post-progression on BSC.
Terminal care costs that arise at the final stages of life were applied as a one-off cost upon death in the model. The list unit price for palliative care on a daily basis of SEK 7,899/day was usedCitation60. For the analysis and in line with previous TLV evaluations, 10 days of palliative care for end-of-life treatment were assumed, i.e. a total cost of SEK 78,986Citation60.
Indirect costs. Previous evidence shows that some patients with non-progressed NSCLC continue to work while on treatment [data on file]. Therefore, indirect costs were calculated as part of a scenario analysis in the model on the assumption that a fraction of PF patients continued to work because of treatment, while untreated patients would not be able to work (the model assumed no patients in the PP state continued to work). The average monthly salary in Sweden in 2018 was SEK 34,600, excluding compulsory employer contributionsCitation62. Adding the average employer contribution of 31.42%Citation63 resulted in an estimated value of a month’s production of SEK 45,471 per employee. The costs of productivity loss averted as a result of treatment were accounted for in the model after multiplying this estimated value of production by the proportion of patients capable of working in the PF state (25%, based on a chart review by Karolinska University Hospital [data on file], after also taking into account the employment percentage (81.8%) and average retirement age (65 years old) in Sweden [data on file]). These costs were only included in a scenario analysis.
Table 2. Unit costs of EFGR TKIs and costs per 28-day cycle.
Table 3. Frequency and cost of adverse events.
Table 4. Cost of subsequent therapies.
Table 5. Unit costs of disease management and monitoring.
Sensitivity and scenario analyses
Both deterministic and probabilistic sensitivity analyses were performed. Deterministic results involved varying key parameters either using their 95% confidence intervals (CI) (if known) or by ±20% (if CI not known). In the probabilistic analysis, 1,000 simulations were generated, varying key parameters simultaneously at each simulation. Scenario analyses included exploring the results from a societal perspective accounting for indirect costs related to production losses, and using Swedish tariffs for EQ-5D-3L utility scores.
Results
Base case
The resulting median PFS and OS for all the included EGFR TKIs are shown in , along with the underlying HRs. The median PFS ranged from 12.63 months for afatinib treatment to 17.42 months with osimertinib treatment. Median OS ranged from 34.01 months for afatinib treatment to 37.58 months for dacomitinib treatment.
The results of the base-case analysis () showed that LYs were highest with dacomitinib (3.38) and QALYs were highest with osimertinib (2.15); LYs and QALYs were both lowest with afatinib (3.08 and 1.91, respectively). Total costs were highest for osimertinib (SEK 1,488,712), followed by dacomitinib (SEK 874,615), and lowest for afatinib (SEK 785,767). The largest component in the total cost of each EGFR TKI was drug acquisition costs. The ICER for dacomitinib vs afatinib was SEK 461,556 per QALY. Dacomitinib was less costly and had lower QALY gain compared with osmertinib. The ICER of osimertinib vs dacomitinib was SEK 11,444,709.
Table 7. Base-case results.
Sensitivity and scenario analyses
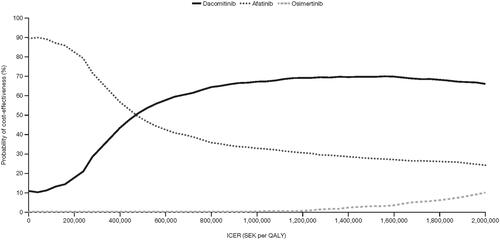
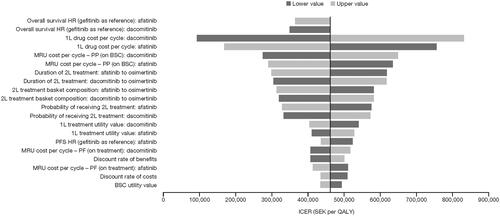
When indirect costs were included in the analyses, the ICER for dacomitinib changed to SEK 365,788 compared with afatinib, making dacomitinib increasingly cost-effective. Using the Swedish tariff for EQ-5D-3L utility scores also improved the ICER for dacomitinib to SEK 428,686 compared with afatinib. The deterministic sensitivity analysis (DSA) against afatinib can be seen in the tornado diagram in for the most influential variables. Changes to drug and medical resource use costs and OS had the most impact on ICERs. Dacomitinib remained less costly than osimertinib when indirect costs were included in the analysis, and the ICER of osimertinib vs dacomitinib changed marginally to SEK 10,949,221. A DSA was therefore not conducted for osimertinib vs dacomitinib. Results of the probabilistic sensitivity analyses in the form of cost-effectiveness acceptability curves are shown in .
Figure 1. Deterministic sensitivity analysis vs afatinib. Abbreviations. BSC, best supportive care; HR, hazard ratio; ICER, incremental cost-effectiveness ratio; MRU, medical resource use; PF, progression-free; PFS, progression-free survival; PP, post-progression; QALY, quality-adjusted life-year; SEK, Swedish krona; 1L, first line; 2L, second line.
Discussion
Our study found that dacomitinib was under the Swedish cost-effectiveness threshold for severe disease when compared to afatinibCitation64. Osimertinib was shown to result in slightly more QALYs, but at a very high cost, resulting in an ICER of osimertinib to dacomitinib of over SEK 11 million. An ICER of that magnitude is clearly not cost-effective, implying that dacomitinib is cost-effective in relation to osimertinib. The actual price paid for osimertinib is confidential, but it is possible that a large rebate would enable osimertinib to be considered cost-effective compared with dacomitinib.
The findings from our Swedish study confirm the findings of other dacomitinib modeling, which showed cost-effectiveness vs afatinib, erlotinib, and gefitinib in England and Wales by NICECitation65, and in Scotland by the Scottish Medicines Consortium (SMC)Citation66. Our analysis focused only on the EGFR TKIs recommended for first-line treatment in Swedish guidelines, and currently no peer-reviewed studies of the cost-effectiveness of dacomitinib vs afatinib or osimertinib have been published. Dacomitinib was found to be cost-effective vs gefitinib based on a cost-effectiveness analysis in China and to be the dominant strategy vs gefitinib based on a cost-effectiveness analysis in PortugalCitation67,Citation68. Moreover, studies of the cost-effectiveness of the other EFGR TKIs are available. Multiple studies from a variety of countries have found that osimertinib would not be a cost-effective option as first-line therapy compared with first- and second-generation EGFR TKIsCitation69–77. These findings are consistent with our own analyses.
For afatinib, cost-effectiveness in the first-line setting varied more with country. Previous analyses out of France and China found that first-line afatinib appeared cost-effective compared with gefitinib in patients with EGFRm NSCLCCitation78,Citation79. It was also shown to be cost-effective compared with erlotinib and pemetrexed/cisplatin in ChinaCitation80. However, when compared with pemetrexed/cisplatin, afatinib was not cost-effective as a first-line treatment for advanced EGFRm NSCLC in Singapore or ChinaCitation81. In addition, erlotinib was shown to be more cost-effective than afatinib or pemetrexed/cisplatin in the first-line treatment of advanced EGFRm NSCLC in the USCitation82. Erlotinib was also the dominant strategy in Spain, Italy, and France when compared with standard chemotherapyCitation83, but gefitinib was shown to be more cost-effective than erlotinib or afatinib in Japan in an analysis of patients with advanced EGFRm NSCLCCitation83,Citation84.
Since dacomitinib is manufactured by the sponsor of this study, extensive measures have been taken to safeguard against any bias in favor of the sponsor’s product. First, the cornerstone of the analysis is the NMA. The development of the NMA followed standard procedures, the details of which have been published elsewhereCitation29,Citation30, making it possible to reproduce the results. Second, health economists working on model development routinely checked the internal validity and technical accuracy of the model through all stages of model development. The internal validity and technical accuracy of the model were also checked by an independent health economist (one that was not involved in the model development) using an extensive quality checklist. Any errors identified by the quality check were addressed in the final economic model. Finally, an earlier version of the analysis based on earlier data cuts, along with the health economic model, was scrutinized by the Swedish HTA agency TLVCitation16, which, therefore, functioned as an independent reviewer of the computerized model.
In the model development and evaluation processes, it was concluded that all variables that have, or would have, a significant impact on the results have been included. The two most important factors impacting the results were first-line drug treatment costs and the relative efficacy of treatments. Thus, only significant changes in those factors would potentially alter the results.
The strengths of our analyses are that the most recent clinical data on survival for each EGFR TKI were taken from large randomized studies and compared via a new NMA. In addition, utility data were drawn from a large clinical trial, supplemented by published evidence. Cost data were also taken from national and regional Swedish data, minimizing the number of assumptions required. There are, however, limitations in our analyses. Whilst the use of NMA is well established, its use does increase the element of uncertainty in the EGFR TKI comparison when compared with the use of direct long-term studies. Although assumptions based on long-term treatment effects which extrapolate effects from an observed trial follow-up period to a much longer model time horizon increase the element of uncertainty in cost-effectiveness comparisons, this would also be a limitation when using direct comparison or time-dependent HRs (e.g. a fractional polynomial approach). As an NMA relies on the proportional hazards assumption (that is, it assumes that hazard rates are proportional between comparator arms in each individual study in the network, and thus also that each indirect comparison satisfies the same assumption), we performed supporting deterministic and probabilistic sensitivity analyses, which showed the findings to be robust. The results of our analyses are also consistent with the findings of HTAs in the UK. Moreover, as noted earlier, the NMA used in this studyCitation29,Citation30 differs from other previously published NMAs of EGFR TKIsCitation44–48 because it included more recently published data for OS (e.g. mature OS data for osimertinib from FLAURACitation26 and updated OS data for dacomitinib from ARCHER 1050Citation27), and employed more stringent selection criteria regarding data specific to EGFRm patients. However, the results of the NMA used in this study are directionally consistent with previously published NMAs for indirect comparisons of PFS, while the differences in indirect comparisons of OS are due to previously published NMAsCitation46,Citation48, relying on the immature OS data for osimertinib from FLAURACitation43.
Conclusions
Based on our economic model, dacomitinib is a cost-effective alternative for the first-line treatment of EGFRm NSCLC in Sweden compared with afatinib and osimertinib.
Transparency
Declaration of funding
This study was sponsored by Pfizer Inc.
Declaration of financial/other interests
FOLN and STA are employees of Pfizer Innovation AB and own stocks in Pfizer Inc. JI is an employee of Pfizer Inc and owns stock in Pfizer Inc. PG and IH are employees of Evidera, and this analysis was developed in collaboration with Evidera and sponsored by Pfizer.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (64.5 KB)Acknowledgements
This study was sponsored by Pfizer Inc. Editorial support was provided by Keith Evans and Jade Drummond of inScience Communications, Springer Healthcare (Chester, UK), and Claire Lavin, PhD, on behalf of CMC AFFINITY, McCann Health Medical Communications, with funding from Pfizer Inc.
References
- Al Dayel F. EGFR mutation testing in non-small cell lung cancer (NSCLC). J Infect Public Health. 2012;5(Suppl 1):S31–S34.
- Dolesova L, Konecny M, Markus J, et al. Molecular analysis of EGFR gene in different types of tumor material from NSCLC patients. Neoplasma. 2015;62(3):439–448.
- Gelatti ACZ, Drilon A, Santini FC. Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in epidermal growth factor receptor (EGFR) mutation-positive non-small cell lung cancer (NSCLC). Lung Cancer. 2019;137:113–122.
- Aguiar F, Fernandes G, Queiroga H, et al. Overall survival analysis and characterization of an EGFR mutated non-small cell lung cancer (NSCLC) population. Arch Bronconeumol. 2018;54(1):10–17.
- Hsu WH, Yang JC, Mok TS, et al. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 2018;29(Suppl 1):i3–i9.
- Ghafoor Q, Baijal S, Taniere P, et al. Epidermal Growth Factor Receptor (EGFR) kinase inhibitors and non-small cell lung cancer (NSCLC) - advances in molecular diagnostic techniques to facilitate targeted therapy. Pathol Oncol Res. 2018;24(4):723–731.
- Brugger W, Thomas M. EGFR-TKI resistant non-small cell lung cancer (NSCLC): new developments and implications for future treatment. Lung Cancer. 2012;77(1):2–8.
- Deng Q, Xie B, Wu L, et al. Competitive evolution of NSCLC tumor clones and the drug resistance mechanism of first-generation EGFR-TKIs in Chinese NSCLC patients. Heliyon. 2018;4(12):e01031.
- Elamin YY, Gomez DR, Antonoff MB, et al. Local consolidation therapy (LCT) after first line tyrosine kinase inhibitor (TKI) for patients with EGFR mutant metastatic non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2019;20(1):43–47.
- Gaut D, Sim MS, Yue Y, et al. Clinical implications of the T790M mutation in disease characteristics and treatment response in patients with epidermal growth factor receptor (EGFR)-mutated non-small-cell lung cancer (NSCLC). Clin Lung Cancer. 2018;19(1):e19–e28.
- Lovly CM, Horn L. Strategies for overcoming EGFR resistance in the treatment of advanced-stage NSCLC. Curr Treat Options Oncol. 2012;13(4):516–526.
- Maione P, Sacco PC, Casaluce F, et al. Overcoming resistance to EGFR inhibitors in NSCLC. Rev Recent Clin Trials. 2016;11(2):99–105.
- ESMO. Clinical practice living guidelines – metastatic non-small-cell lung cancer; 2019 [cited 2020 Jun 2]. Available from: https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer
- European Medicines Agency (EMA). Vizimpro (dacomitinib) summary of product characteristics; 2019 [cited 2020 Aug 4]. Available from: https://www.ema.europa.eu/en/documents/product-information/vizimpro-epar-product-information_en.pdf.
- Food and Drug Administration (FDA). VIZIMPRO (dacomitinib) Prescribing Information; 2018 [cited 2020 Aug 04]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211288s000lbl.pdf.
- Tandvårds- och läkemedelsförmånsverket. Vizimpro ingår i högkostnadsskyddet; 2019 [cited 2019 Jul 24]. Available from: https://www.tlv.se/beslut/beslut-lakemedel/generell-subvention/arkiv/2019-06-14-vizimpro-ingar-i-hogkostnadsskyddet.html.
- Regionala Cancercentrum. Lungcancer Nationellt vårdprogram; 2020 [cited 2020 Jul 28]. Available from: https://www.cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/vardprogram/nationellt-vardprogram-lungcancer.pdf.
- Bria E, Milella M, Cuppone F, et al. Outcome of advanced NSCLC patients harboring sensitizing EGFR mutations randomized to EGFR tyrosine kinase inhibitors or chemotherapy as first-line treatment: a meta-analysis. Ann Oncol. 2011;22(10):2277–2285.
- Arriola E, Garcia Gomez R, Diz P, et al. Clinical management and outcome of patients with advanced NSCLC carrying EGFR mutations in Spain. BMC Cancer. 2018;18(1):106.
- Hochmair MJ, Morabito A, Hao D, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: updated analysis of the observational GioTag study. Future Oncol. 2019;15(25):2905–2914.
- Regionala Cancercentrum I Samverkan. Regionala Cancercentrum i samverkan. Lungcancer nationell kvalitetsrapport för 2018; 2019 [cited 2020 Jun 28]. Available from: https://www.cancercentrum.se/globalassets/cancerdiagnoser/lunga-och-lungsack/kvalitetsregister/rapport/20191015_nlcr_nationell_rapport_2018.pdf.
- Cancerfonden. Cancerfondsrapporten 2018; 2018 [cited 2020 Jul 28]. Available from: https://static-files.cancerfonden.se/Cancerfondsrapporten2018_webb_(2)_1521607903.pdf.
- Bergqvist M, Christensen HN, Wiklund F, et al. Real world utilization of EGFR TKIs and prognostic factors for survival in NSCLC during 2010–2016 in Sweden: a nationwide observational study. Int J Cancer. 2019;149(9):2510–2517.
- Caro JJ, Briggs AH, Siebert U, et al. Modeling good research practices-overview: a report of the ISPOR-SMDM modeling good research practices task force-1. Value Health. 2012;15(6):796–803.
- Latimer N. NICE DSU technical support document 14: survival analysis for economic evaluations alongside clinical trials - extrapolation with patient-level data. Report by the decision support unit June 2011; 2013 [cited 2020 Jul 24]. Available from: http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf.
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50.
- Mok T, Cheng Y, Zhou X, et al. Updated overall survival from extended follow up in ARCHER 1050: a randomized phase III study comparing dacomitinib with gefitinib as first-line therapy for patients with EGFR mutation. Annal Oncol. 2019;30:ix200–ix201.
- Pharmaceutical Benefits Board. General guidelines for economic evaluations from the Pharmaceutical Benefits Board (LFNAR 2003:2); 2003 [cited 2020 Jul 28]. Available from: https://www.tlv.se/download/18.2e53241415e842ce95514e9/1510316396792/Guidelines-for-economic-evaluations-LFNAR-2003-2.pdf.
- Farris M, Larkin-Kaiser K, Scory T, et al. Network meta-analysis of first-line therapy for advanced EGFR mutation positive non-small cell lung cancer: updated overall survival. Future Oncol. 2020;16(36):3107–3116.
- Franek J, Cappelleri JC, Larkin-Kaiser KA, et al. Systematic review and network meta-analysis of first-line therapy for advanced EGFR-positive non-small-cell lung cancer. Future Oncol. 2019;15(24):2857–2871.
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466.
- Centre for Reviews and Dissemination, University of York. Systematic reviews: CRD’s guidance for undertaking reviews in health care; 2009 [cited 2020 Jul 24]. Available from: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
- Ades AE, Caldwell DM, Reken S, et al. NICE decision support unit technical support documents. Evidence synthesis of treatment efficacy in decision making: a reviewer's checklist. London: National Institute for Health and Care Excellence (NICE); 2012.
- Phillippo D. NICE DSU technical support document 18: methods for population-adjusted indirect comparisons in submissions to NICE; 2016 [cited 2021 Jan]. Available from: https://research-information.bris.ac.uk/ws/portalfiles/portal/94868463/Population_adjustment_TSD_FINAL.pdf.
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428.
- Dias S, Ades AE, Welton NJ, et al. Network meta-analysis for decision-making. Hoboken (NJ): Wiley; 2018.
- Plummer M. rjags: Bayesian Graphical Models using MCMC. R package version. 4-10; 2019. Available from: https://cran.r-project.org/web/packages/rjags/rjags.pdf
- Spiegelhalter D, Thomas A, Best N, et al. BUGS 0.5: Bayesian inference using gibbs sampling–manual (version ii). UK: MRC Biostatistics Unit; 1996.
- Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7(4):434–455.
- Dias S, Welton NJ, Sutton AJ, et al. NICE decision support unit technical support documents. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. London: National Institute for Health and Care Excellence (NICE); 2014.
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589.
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28(2):270–277.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125.
- Popat S, Mok T, Yang JC, et al. Afatinib in the treatment of EGFR mutation-positive NSCLC-a network meta-analysis. Lung Cancer. 2014;85(2):230–238.
- Batson S, Mitchell SA, Windisch R, et al. Tyrosine kinase inhibitor combination therapy in first-line treatment of non-small-cell lung cancer: systematic review and network meta-analysis. Onco Targets Ther. 2017;10:2473–2482.
- Li XY, Lin JZ, Yu SH. Front-line therapy in advanced non-small cell lung cancer with sensitive epidermal growth factor receptor mutations: a network meta-analysis. Clin Ther. 2020;42(2):338.e4–350.e4.
- Lin JZ, Ma SK, Wu SX, et al. A network meta-analysis of nonsmall-cell lung cancer patients with an activating EGFR mutation: should osimertinib be the first-line treatment? Medicine (Baltimore). 2018;97(30):e11569.
- Holleman MS, van Tinteren H, Groen HJ, et al. First-line tyrosine kinase inhibitors in EGFR mutation-positive non-small-cell lung cancer: a network meta-analysis. Onco Targets Ther. 2019;12:1413–1421.
- Cabanero M, Sangha R, Sheffield BS, et al. Management of EGFR-mutated non-small-cell lung cancer: practical implications from a clinical and pathology perspective. Curr Oncol. 2017;24(2):111–119.
- Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34.
- Sequist LV, Wu YL, Schuler M, et al., editors. Subsequent therapies post-afatinib among patients with EGFR mutation-positive NSCLC in LUX-Lung (LL) 3, 6 and 7. Proceedings of the European Society For Medical Oncology (ESMO) Congress; 2017; Madrid, Spain, September 8–12.
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640.
- Sequist LV, Wu Y, Schuler M, et al. PS02.20 subsequent therapies post-afatinib among patients with EGFR mutation-positive (EGFRm+) NSCLC in LUX-Lung 3, 6 and 7: topic: medical oncology. J Thorac Oncol. 2017;12(11):S1572.
- Burström K, Sun S, Gerdtham U-G, et al. Swedish experience-based value sets for EQ-5D health states. Qual Life Res. 2014;23(2):431–442.
- AstraZeneca Pharmaceuticals LP. TAGRISSO (osimertinib) tablets, for oral use. Wilmington, DE; 2020. [cited 2021 Apr 6] Available from: https://www.azpicentral.com/tagrisso/tagrisso.pdf#page=1
- Chouaid C, Agulnik J, Goker E, et al. Health-related quality of life and utility in patients with advanced non-small-cell lung cancer: a prospective cross-sectional patient survey in a real-world setting. J Thorac Oncol. 2013;8(8):997–1003.
- Nafees B, Stafford M, Gavriel S, et al. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6(1):84.
- Tandvårds- och läkemedelsförmånsverket. Medicines and consumables database; 2020 [cited 2020 Aug 4]. Available from: https://www.tlv.se/beslut/sok-i-databasen.html.
- Sveriges Kommuner och Landsting. Kostnad per patient, KPP; 2018 [cited 2020 Jul 24]. Available from: https://skl.se/ekonomijuridikstatistik/statistik/kostnadperpatientkpp/kppdatabas.1079.html.
- Sodraregionsvardsnamnden. Regionala priser och ersättningar för Södra sjukvårdsregionen; 2018 [cited 2020 Jul 28]. Available from: https://sodrasjukvardsregionen.se/avtal-priser/regionala-priser-och-ersattningar/.
- Vardgivare Skane. Bild- och funktionsmedicin prislista; 2018 [cited 2020 Jul 24]. Available from: https://vardgivare.skane.se/patientadministration/avgifter-och-prislistor/prislistor-bild-funktionsmedicin/.
- Statistikmyndigheten SCB. Average monthly salary, SEK by sector, occupation (SSYK 2012. sex, level of education and year 2018 [cited 2020 Jul 28]. Available from: http://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__AM__AM0110/.
- Ekonomifakta. Arbetsmarknad; 2017 [cited 2020 Jul 24]. Available from: https://www.ekonomifakta.se/Fakta/Arbetsmarknad/.
- Svensson M, Nilsson FOL, Arnberg K. Reimbursement decisions for pharmaceuticals in Sweden: the impact of disease severity and cost effectiveness. Pharmacoeconomics. 2015;33(11):1229–1236.
- National Institute for Health and Care Excellence. Dacomitinib for untreated EGFR mutation-positive non-small-cell lung cancer; 2019 [cited 2020 Jul 24]. Available from: https://www.nice.org.uk/guidance/ta595/documents/final-appraisal-determination-document.
- Scottish Medicines Consortium. dacomitinib 15mg, 30mg and 45mg film-coated tablets (Vizimpro); 2019 [cited 2020 Jul 24]. Available from: https://www.scottishmedicines.org.uk/media/4706/dacomitinib-vizimpro-final-august-2019-for-website.pdf.
- Yu Y, Luan L, Zhu F, et al. PCN164 cost-effectiveness of dacomitinib vs. gefitinib as first-line treatment for egfr mutation positive advanced non-small-cell lung cancer in China. Value Health. 2019;22:S467–S468.
- Silva ML, Paquete AT, Alarcão J, et al. Cost-effectiveness analysis of dacomitinib versus gefitinib for the first-line treatment of locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor (EGFR)-activating mutations in Portugal. Value Health. 2020;23(S2):S447.
- Aguiar PN, Jr., Haaland B, Park W, et al. Cost-effectiveness of osimertinib in the first-line treatment of patients with EGFR-mutated advanced non-small cell lung cancer. JAMA Oncol. 2018;4(8):1080–1084.
- Aguilar-Serra J, Gimeno-Ballester V, Pastor-Clerigues A, et al. Osimertinib in first-line treatment of advanced EGFR-mutated non-small-cell lung cancer: a cost-effectiveness analysis. J Comp Eff Res. 2019;8(11):853–863.
- Bertranou E, Bodnar C, Dansk V, et al. Cost-effectiveness of osimertinib in the UK for advanced EGFR-T790M non-small cell lung cancer. J Med Econ. 2018;21(2):113–121.
- National Institute for Health and Care Excellence. Osimertinib for treating EGFR T790M mutation-positive advanced non-small-cell lung cancer; 2016 [cited 2020 Dec 7]. Available from: https://www.nice.org.uk/guidance/ta653.
- National Institute for Health and Care Excellence. Osimertinib for untreated EGFR mutation-positive non-small-cell lung cancer: Technology appraisal guidance; 2020 [cited 2020 Jan 22; 2020 Aug 4]. Available from: https://www.nice.org.uk/guidance/ta621/resources/osimertinib-for-untreated-egfr-mutationpositive-nonsmallcell-lung-cancer-pdf-82609012217797.
- Holleman MS, Al MJ, Zaim R, et al. Cost-effectiveness analysis of the first-line EGFR-TKIs in patients with non-small cell lung cancer harbouring EGFR mutations. Eur J Health Econ. 2020;21(1):153–164.
- Cai H, Zhang L, Li N, et al. Cost-effectiveness of osimertinib as first-line treatment and sequential therapy for EGFR mutation-positive non-small cell lung cancer in China. Clin Ther. 2019;41(2):280–290.
- Wu B, Gu X, Zhang Q. Cost-effectiveness of osimertinib for EGFR mutation-positive non-small cell lung cancer after progression following first-line EGFR TKI therapy. J Thorac Oncol. 2018;13(2):184–193.
- Wu B, Gu X, Zhang Q, et al. Cost-effectiveness of osimertinib in treating newly diagnosed, advanced EGFR-mutation-positive non-small cell lung cancer. Oncologist. 2019;24(3):349–357.
- Chouaid C, Luciani L, LeLay K, et al. Cost-effectiveness analysis of afatinib versus gefitinib for first-line treatment of advanced EGFR-mutated advanced non-small cell lung cancers. J Thorac Oncol. 2017;12(10):1496–1502.
- Wang H, Zeng C, Li X, et al. Cost-utility of afatinib and gefitinib as first-line treatment for EGFR-mutated advanced non-small-cell lung cancer. Future Oncol. 2019;15(2):181–191.
- Gu X, Zhang Q, Chu YB, et al. Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Lung Cancer. 2019;127:84–89.
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092.
- Ting J, Tien Ho P, Xiang P, et al. Cost-effectiveness and value of information of erlotinib, afatinib, and cisplatin-pemetrexed for first-line treatment of advanced EGFR mutation-positive non-small-cell lung cancer in the United States. Value Health. 2015;18(6):774–782.
- Vergnenegre A, Massuti B, de Marinis F, et al. Economic Analysis of First-Line Treatment with Erlotinib in an EGFR-Mutated Population with Advanced NSCLC. J Thorac Oncol. 2016;11(6):801–807.
- Kimura M, Yasue F, Usami E, et al. Cost-effectiveness and safety of the molecular targeted drugs afatinib, gefitinib and erlotinib as first-line treatments for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Mol Clin Onc. 2018;9(2):201–206.