Abstract
Aims
With the advent of ICD-10-CM codes for PMBCL on 10/01/2015, assessment of treatment patterns and healthcare burden among US patients is possible. This study sought to describe the real-world treatment patterns and economic outcomes of patients with PMBCL.
Methods
Data from the Optum Clinformatics DataMart database was used (January 2013–March 2018). Patients with a first PMBCL ICD-10-CM diagnosis (with or without an antecedent ICD-10-CM diagnosis of DLBCL/other lymphoma, which may have been assigned before PMBCL confirmation) after 10/01/2015 (index date) and no ICD-9-CM diagnosis code for unspecified PMBCL/DLBCL were identified as incident patients. Those with PMBCL ICD-10-CM and unspecified ICD-9-CM diagnosis for PMBCL/DLBCL before 10/01/2015 (index date) were identified as prevalent patients. Patients were observed from the index date up to the earliest among death, end of data availability, or end of continuous health plan enrollment. An adapted algorithm was used to identify lines of therapy (LOT).
Results
Among 118 incident and 30 prevalent PMBCL patients, 14% and 20% of patients received ≥2 LOTs, respectively. In incident patients, 48% received ≥1 LOT, 14% ≥2, and 4% ≥3 LOTs. Among prevalent patients, 63% received ≥1 LOT and 20% ≥2 LOTs. The most frequently recorded 1 L therapy was R-CHOP both among incident and prevalent patients. Mean total healthcare costs for incident and prevalent patients were $149,340 and $92,799 per patient per year, respectively, with higher costs ≤12 months ($187,241 and $167,553). Outpatient costs were the main driver (accounting for 60.5% and 64.6% for incident and prevalent patients, respectively).
Limitations
Potential underuse of ICD-10-CM codes shortly after discontinuation of ICD-9-CM codes in 01/2015; regimens identified for each LOT using the claims-based algorithm may not reflect the regimen administered.
Conclusion
The multiple LOTs necessary for a sizeable minority of patients and the high costs of care highlight the significant unmet needs of PMBCL patients.
Introduction
Primary mediastinal B-cell lymphoma (PMBCL) is a rare but aggressive mature B-cell neoplasm that constitutes approximately 2–4% of all cases of non-Hodgkin lymphoma (NHL)Citation1. Although PMBCL was historically considered a subtype of diffuse large B-cell lymphoma (DLBCL), the World Health Organization (WHO) classification recognized PMBCL as a separate biological and clinical entity from DLBCL for the first time in 2001 based on their distinct molecular, pathological, and genetic featuresCitation2,Citation3. PMBCL has a slight female predominance and typically occurs in adolescents and younger adults with a median age of 35 years; the five-year survival rate ranges from 70% to 85% according to raceCitation4,Citation5. Due to the rarity of the disease and its recent re-classification, there is a paucity of evidence-based comparative trials and therefore no clear consensus regarding the optimal treatment for PMBCLCitation6–9.
While clinical management varies across centers, the most common first-line treatments in the US are rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), or rituximab plus dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (DA-EPOCH-R). The role of radiation therapy (RT) in first-line treatment is controversial given the adverse long-term toxicity effects in this predominantly young population, and may be omitted especially for patients after DA-EPOCH-R or in patients with complete metabolic remission postchemotherapyCitation5,Citation10,Citation11. If relapse occurs, which when it does most often happens within 12–18 monthsCitation12–14, relapsed/refractory PMBCL (rrPMBCL) is treated with salvage regimens commonly used for DLBCL which include rituximab-based chemoimmunotherapy regimens followed by autologous hematopoietic stem cell transplant (auto-HSCT). However, studies have shown a relatively poor response rate of rrPMBCL patients to salvage therapiesCitation12,Citation14–17. Beyond two or more lines of therapy, two new therapies have recently been approved: chimeric antigen receptor T-cell (CAR-T) or the anti-PD1 monoclonal antibody (mAb) pembrolizumab, with additional promising data from the combination of another PD-1 mAb, nivolumab, with the anti-CD30 antibody drug conjugate brentuximab vedotinCitation18.
PMBCL remains poorly characterized in the literature, especially with regards to treatment patterns, healthcare resource utilization (HRU), and costs in the real world. One reason may be that PMBCL was historically combined with DLBCL within the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). In the United States (US), PMBCL was administratively differentiated from DLBCL in October 2015 with the advent of International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) PMBCL-specific codes, allowing for specific querying of administrative data for patients with this diagnosis. Therefore, this study was conducted to describe the real-world demographic and clinical characteristics, treatment patterns, HRU, and costs among patients diagnosed with PMBCL in the United States.
Materials and methods
Data source
De-identified healthcare insurance claims from the Optum’s Clinformatics Data Mart Databases were used to conduct this study, with data spanning from January, 2013 to March, 2018. Optum Clinformatics covers 13 million annual enrolled UnitedHealth Group members in all census regions in the US. It contains more than 36 months of historical data on patient demographics, insurance coverage (i.e. commercial and Medicare), dates of eligibility and death, claims for inpatient [IP], outpatient [OP], and emergency room [ER] visits, OP pharmacy claims (including Medicare Advantage Part D), as well as the costs of services, and laboratory tests and results.
Study design and study population
A retrospective cohort design was used. Since PMBCL can only be administratively differentiated from DLBCL using ICD-10-CM diagnosis codes (available from October 2015), all study participants were identified and selected using ICD-10-CM codes for PMBCL. However, as the observation period for patients with newly diagnosed PMBCL since 2015 was limited, patients with a previous ICD-9-CM diagnosis code associated with unspecified PMBCL/DLBCL before October 2015 were also included in the study. Patients with PMBCL and no ICD-9-CM diagnosis prior to October 2015 were termed as incident patients, while patients with a previous ICD-9-CM diagnosis code (i.e. prior to October 2015) associated with unspecified PMBCL/DLBCL were termed as prevalent patients. These groups were mutually exclusive.
Both incident and prevalent patients may have had an antecedent ICD-10-CM diagnosis of DLBCL or other lymphoma, which may have been assigned before confirmation of PMBCL (i.e. the ICD-10-CM PMBCL diagnosis). For incident PMBCL patients, the index date was defined as the earliest of the following dates: the date of the first ICD-10-CM diagnosis of PMBCL, or the date of the first DLBCL/other lymphoma diagnosis (for those who had a DLBCL or other lymphoma diagnosis prior to PMBCL conformation). For prevalent PMBCL patients, the index date was defined as the date of the first ICD-9-CM diagnosis associated with unspecified PMBCL/DLBCL prior to October 1, 2015. The baseline period for all patients was defined as the 12-month period prior to the index date. The observation period (i.e. follow-up period) spanned from the index date up to the earliest date pertaining to death, end of data availability, or end of continuous health plan enrollment.
Patients were included in the study if they met the following criteria: ≥18 years old at the index date, ≥12 months of continuous enrollment prior to the index date (baseline period), ≥2 medical visits (hospitalization or OP) with a diagnosis of PMBCL whereby incident patients were required to have a first ICD-10-CM diagnosis (code: C85.2x) for PMBCL after October 1, 2015 and no ICD-9-CM diagnosis for unspecified PMBCL/DLBCL, and prevalent patients were required to have an ICD-10-CM diagnosis code for PMBCL and an unspecified ICD-9-CM diagnosis code for PMBCL/DLBCL before October 1, 2015.
Patients were excluded from the study if they had a prior diagnosis of Hodgkin lymphoma, follicular lymphoma, or multiple myeloma during the 12-month baseline period.
Algorithm to identify lines of therapy with claims data
Incident and prevalent patients with PMBCL were further stratified by the following lines of therapy: First-line (1 L) patients treated with ≥1 L therapy; Second-line (2 L) patients treated with ≥2 L therapy; Third-line (3 L) patients treated with ≥3 L therapy (due to low sample size of prevalent patients [N < 6], 3 L was only reported for incident patients). A claims-based algorithm adapted from previously published algorithmsCitation19,Citation20,Citation21,Citation22 was used to identify lines of therapy in patients with PMBCL (Figure S1). All records of anti-cancer systemic agents were identified using Generic Product Identifier (GPI), Healthcare Common Procedure Coding System (HCPCS), Current Procedural Terminology (CPT), and National Drug Code (NDC) codes. 1 L of therapy was defined by all unique agents received within the first 21 days following initiation of the first anti-cancer therapy, a period that corresponds to the recommended duration of an R-CHOP or DA-EPOCH-R cycleCitation5,Citation23.
A new line of therapy started with the initiation of a new agent not included in the 1 L of therapy, and each new line of therapy was defined by the unique agents used within the first 21-day period following the initiation of the new line of therapy (Figure S1, Scenario 4). Discontinuation of a line of therapy was defined as a 90-day discontinuation of all agents. Re-initiation of the same agents after a 90-day gap was considered a new line of therapy (Figure S1, Scenario 2). The discontinuation of a single agent or more than one agent from a combination therapy was not considered a new line of therapy (Figure S1, Scenario 5). RT was not considered as a new line of therapyCitation20–22. Moreover, if a patient received RT within 90 days of the end of a specific line of therapy (e.g. 1 L), RT was considered part of this line (except if a new line of therapy was started before 90 days; in this case, RT was considered part of the 1 L only for the period from the end of the 1 L to the start of the 2 L).
Outcome measures
Demographics and clinical characteristics of incident and prevalent patients were collected at the index date or during the 12-month baseline period and included age, gender, year of index date, region, insurance plan type, Charlson comorbidity index (CCI) score, comorbidities (nonpsychiatric and psychiatric), all-cause HRU (i.e. IP [including hospitalizations and skilled nursing and long-term care facilities], OP visits, ER visits, and other types [including home services and hospice] of visits), and all-cause healthcare costs (i.e. IP, OP, ER, and OP pharmacy costs) reported in 2018 US dollars based on the medical care component of the Consumer Price Index.
Treatment patterns of anti-cancer systemic agents were evaluated during the observation period for each line of therapy and several related factors were assessed including the proportion of patients who received a specific line of therapy (i.e. 1 L, 2 L, 3 L), the duration of therapy (DOT) for each line of treatment (defined as the number of days from the date of initiation of a new anti-cancer treatment up to the discontinuation of all agents in the line of therapy, a switch to another line, the addition of a new agent to the current line, or the end of the eligibility period, whichever occurred first), the time from index date to 1 L initiation, proportions of patients with a next line of therapy (defined as the number of patients in each line of therapy receiving the subsequent line of therapy), and any anti-cancer therapy initiated after the first PMBCL diagnosis, including monotherapy and combination therapies based on, but not limited to, the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®)Citation5.
HRU was evaluated for both incident and prevalent treated patients during the evaluation period, which spanned from the 1 L treatment initiation date up to the earliest of end of data availability, death, or end of insurance coverage. The analyses were repeated up to 12 months from the date of 1 L treatment initiation or only among patients treated with R-CHOP in the 1 L. HRU was reported per-patient-per-year (PPPY) and included the number of all-cause hospitalizations, ER visits, OP visits, and other visits.
Healthcare costs were evaluated for both incident and prevalent treated patients during the evaluation period. The analyses were repeated up to 12 months from the date of 1 L treatment initiation or only among patients treated with R-CHOP in the 1 L. Healthcare costs paid by the insurer were reported PPPY in 2018 US dollars and included all-cause total costs such as those associated with hospitalizations, ER visits, and OP visits (with or without drug administration procedures), as well as OP pharmacy costs. Costs were standardized by Optum using standard pricing algorithms to account for differences in pricing across health plans and provider contractsCitation24. Healthcare costs were then further stratified by commercial or Medicare Advantage coverage.
Statistical analysis
All analyses were performed using SAS Enterprise Guide Version 7.1 (SAS Institute, Cary, NC). Patient characteristics and treatment patterns were summarized using descriptive statistics and included means (± standard deviations [SDs]) and medians for continuous variables and frequencies and proportions for categorical variables. Rates of HRU PPPY were calculated as number of events (i.e. hospitalization, ER visits, OP visits) divided by patient-years of observation. Healthcare costs PPPY were calculated as the total cost divided by the total number of days of enrollment, multiplied by 365 days where costs were weighted by each patient’s length of follow-up to avoid overestimating costs by annualizing data for patients observed for less than 1 yearCitation25. Since this study is purely descriptive, no statistical comparisons were conducted between patient groups.
Results
Baseline characteristics
A total of 148 patients with PMBCL were identified, including 118 incident and 30 prevalent patients ().
Figure 1. Patient disposition. Abbreviations. DLBCL, diffuse large B-cell lymphoma; ICD-9-CM, International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision; PMBCL, primary mediastinal large B-cell lymphoma; US, United States. 1. PMBCL can only be identified using ICD-10-CM codes, which are available since October 1, 2015, in the US.
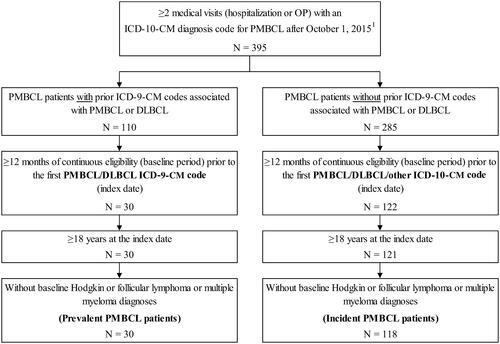
Among incident patients, the median age was 66 years and 59% were female (); the mean CCI score was 2.2. At baseline, the most prevalent non-psychiatric comorbidity was hypertension (49%) while the most prevalent psychiatric comorbidity was sleep-wake disorders (21%). Total baseline all-cause healthcare costs were on average $22,925, with OP visits accounting for approximately one-third ($8,148) of the costs. Overall, incident patients had a mean observation period of 405.8 days. The characteristics of prevalent patients are summarized in and were largely consistent with those of incident patients (). Prevalent patients had a mean observation period of 907.9 days.
Table 1. Baseline Demographic and Clinical Characteristics for Patients with PMBCL.
Treatment patterns
Among incident patients, almost half were recorded as receiving at least 1 line of therapy during the observation period (48%), while 14% and 4% of the treated patients were observed to receive ≥2 and ≥3 lines of therapy, respectively (). The mean time from the index date to the initiation of 1 L was 48 days, and the mean DOT for the 1 L among patients treated with ≥1 line of therapy was 86 days. DOT was progressively shorter for the 2 L among those treated with ≥2 lines of therapy and the 3 L among those treated with ≥3 lines of therapy. The most commonly used treatments among incident patients were R-CHOP in the 1 L (54%; ).
Figure 2. Most Frequent Treatments during the Observation Period for Patients with Incident (a) and Prevalent (b) PMBCL. Abbreviations. BR, bendamustine plus rituximab; EPOCH-R, etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin, plus rituximab; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; Ib + R, ibrutinib plus rituximab; PMBCL, primary mediastinal B-cell lymphoma; R-CEOP, rituximab plus cyclophosphamide, etoposide, vincristine, and prednisone; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab plus cyclophosphamide, vincristine, and prednisone; R-GemOx, rituximab plus gemcitabine and oxaliplatin; 1L, first line; 2L, second line; 3L, third line.
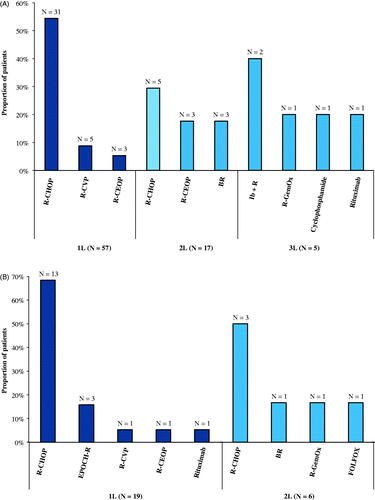
Table 2. Treatment patterns during the observation period for patients with PMBCL.
Among prevalent patients, most were treated during the observation period with ≥1 line of therapy (63%), while 20% of the treated patients were observed to receive ≥2 lines of therapy (). The mean time from the index date to the initiation of 1 L was 76 days, and the mean DOT for the 1 L among patients treated with ≥1 line of therapy was 88 days. DOT was longer for the 2 L among those treated with ≥2 lines of therapy (143 days). The most commonly used treatments among prevalent patients were also R-CHOP in the 1 L (68%; ).
Healthcare resource utilization and costs
For incident patients treated with 1 L therapy, the mean duration of the evaluation period was 370 days. During this period, these patients had an average of 1.6 IP stays, 1.8 ER visits, and 45.5 OP visits PPPY (). The corresponding mean total healthcare costs PPPY was $149,340 and were mostly driven by medical costs (). OP visits – particularly those with drug administration – were the main cost driver, accounting for 60% of total healthcare costs ($90,329 PPPY). When restricting the evaluation period up to 12 months from the date of 1 L treatment initiation (mean evaluation period of 265 days), HRU PPPY for all treated incident patients was slightly higher than that in the overall population, with 1.8 IP stays, 2.1 ER visits, and 56.8 OP visits (). A similar trend was found for the mean total healthcare costs PPPY at $187,241 (). Among patients treated with R-CHOP in the 1 L, HRU PPPY was similar to that observed in the overall population, with 1.2 IP stays, 1.4 ER visits, and 41.3 OP visits (Table S1). The associated mean total healthcare costs PPPY were also similar at $129,881 (Table S2).
Table 3. HRU during the evaluation period for patients with PMBCL treated with ≥1 L therapy.
Table 4. Healthcare costs during the evaluation period for patients with PMBCL treated with ≥1 L therapy.
For prevalent patients, the mean evaluation period was 856 days and those treated had an average of 1.1 IP stays, 1.3 ER visits, and 42.1 OP visits PPPY (). Their corresponding mean total healthcare costs PPPY were $92,799 and were primarily driven by medical costs (). Specifically, OP costs accounted for 65% of total healthcare costs. When restricting the evaluation period to up to 12 months from the date of 1 L treatment initiation (mean evaluation period of 355 days), HRU PPPY was found to be higher for all treated prevalent patients (1.9 IP stays, 1.8 ER visits, and 63.2 OP visits; ). Similar trends were found for mean total healthcare costs PPPY at $167,553 (). Among those treated with R-CHOP in the 1 L, HRU PPPY and total healthcare costs were similar to those in the overall population (HRU: 1.2 IP stays, 1.2 ER visits, and 45.0 OP visits; mean total healthcare costs PPPY: $97,961; Tables S1 and S2).
When stratified by commercial or Medicare Advantage coverage, mean total healthcare costs PPPY were numerically higher among commercially insured incident ($179,772) and prevalent patients ($104,130) than Medicare Advantage insured incident ($139,624) and prevalent patients ($89,918; Table S3).
Discussion
In this retrospective cohort study, most incident and prevalent patients with PMBCL were treated with ≥1 line of therapy, and up to 20% of these patients were observed to require additional lines of therapy. The most commonly used treatment among both incident and prevalent patients with PMBCL was R-CHOP. HRU and healthcare expenditures were high among treated patients, with incident and prevalent patients incurring total healthcare costs of $149,340 and $92,799 PPPY, respectively. Most of these costs were incurred in the first 12 months following initiation of treatment.
Before discussing the results, it is important to consider the limitations of this study. First, the analysis period of this study included data immediately following the discontinuation of ICD-9-CM in October 2015, and the use of ICD-10-CM codes for PMBCL may yet change over time. Second, the proportion of patients treated and DOT might be under-estimated since medication used during hospitalization (such as chemotherapy) are not fully captured into the database. While revenue codes may indicate the administration of chemotherapy for some patients (if data are available), these codes are nonspecific and thus do not specify the specific chemotherapy drug used. Additionally, as with all claims-based analyses, the events observed during the study are limited by the length of health plan enrolment of each patient. This potentially explains why only 48–63% of PMBCL patients in the current study were treated with 1 L therapy. Moreover, some patients may have been considered too old/frail for treatment with anti-cancer therapy (i.e. 16.1% of incident and 20.0% of prevalent patients were ≥80 years). Third, since a claims-based algorithm was used to identify lines of therapy, the classification of anti-cancer agents within each line of therapy may not have completely reflected the actual treatment regimens of the patients. For example, the algorithm may have re-classified patients in 1 L of treatment with R-CHOP who have additional drugs added to their primary line of treatment at a later date as 2 L patients, therefore overestimating the use of R-CHOP in 2 L. Lastly, coding inaccuracies or omissions in procedures and diagnoses could have occurred due to the nature of claims databases. Accordingly, the apparent under-coding of HSCT in the database led to the inclusion of anti-cancer systemic agents only in our analysis of treatment patterns.
Currently, the NCCN Guidelines recommend DA-EPOCH-R or R-CHOP–containing regimens for 1 L therapyCitation5. According to the available data, R-CHOP was the most commonly used treatment regimen in the 1 L among incident and prevalent patients. The evidence for DA-EPOCH-R is relatively newer and the regimen is associated with increased toxicity compared to R-CHOPCitation10,Citation26,Citation27 which may explain why it was not commonly used in the current study. Its use may grow in the future, which could affect HRU and costs. However, a previous study evaluating PMBCL patients treated with DA-EPOCH-R showed high event-free survival and overall survival rates without the need for mediastinal RTCitation10,Citation11. Therefore, this treatment option warrants further investigation to fully assess its risks, substantiate claims of its benefits, and determine the associated economic impact.
Despite high response rates after 1 L therapy in the literatureCitation6,Citation10,Citation26, a significant number of PMBCL patients require additional therapy after 1 L, presumably due to relapse or refractory disease after standard R-CHOP or similar treatmentCitation28–31. Previous publications have demonstrated that these patients tend to have a poor response to salvage treatment with a low overall survival rateCitation12,Citation14–17 and typically have a worse prognosis regardless of subsequent treatmentCitation14. Additionally, therapy options for rrPMBCL are not well establishedCitation5 as reflected by the heterogeneity in treatment patterns observed in the 2 L and 3 L. The non-negligible number of patients requiring additional therapy after 1 L in the current study highlights the unmet need for more effective PMBCL therapies. Furthermore, targeted therapies were either recently approved (pembrolizumab; CAR-T axicabtagene ciloleucel) or are currently in development (nivolumab + brentuximab) for the treatment of relapsed or refractory PMBCLCitation15,Citation32; however, further studies are required to assess their impact on real-world treatment patterns among patients with PMBCL.
The economic burden of PMBCL in the real world has thus far not been assessed in the literature. As seen in the current study, healthcare costs were highest within the first year following initiation of treatment. The highest proportion of healthcare costs were incurred from OP visits, likely associated with treatment and follow-up visits. Although not explored in the current study, supportive care is important in the management of PMBCL and may significantly contribute to these costs. For example, the steroid component of a regimen (i.e. prednisone in R-CHOP) can lead to bone demineralization and fractures, which may require monitoring, concomitant treatments (e.g. bisphosphonates)Citation5, and increased healthcare costs. Additionally, there is strong clinical evidence that hepatitis C infection contributes to the pathogenesis of B-cell lymphomas; antiviral treatment is recommended in patients with hepatitis C virus, which might increase healthcare costs as wellCitation5. Additionally, recurrences usually occur during initial treatment or within 6 to 12 months of initial treatmentCitation33; therefore, 2 L treatment may have also contributed to the high costs observed in the first year. As the treatment landscape continues to change, the associated costs may also change along with guideline recommendations.
To the best of our knowledge, this study represents the first real-world evaluation of treatment patterns, HRU, and healthcare costs of patients diagnosed with PMBCL in the United States. The real-world outcomes of patients with PMBCL remained understudied in the existing literature due to the lack of specific ICD-10-CM codes prior to 2015, which historically hindered the conduct of health insurance claims database analysis. Taken together, the results of the present study shed light on the significant unmet medical needs of patients with PMBCL and should motivate clinical efforts to further improve the outcomes of these patients.
Conclusions
This real-world assessment of treatment patterns and costs for patients with PMBCL shows that HRU and healthcare costs of these patients were high, especially in the first 12 months following initiation of treatment. Taken together, the multiple lines of therapy necessary for a sizeable minority of patients and the high costs of care highlight the significant unmet need of patients with PMBCL. With the advent of novel treatment options for PMBCL, further research is warranted to evaluate the impact on HRU and costs in this population.
Transparency
Declaration of funding
This research was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Declaration of financial/other relationships
Four of the authors (FL, GG, MSD, and DL) are employees of Analysis Group, Inc., a consulting company that has received consultancy fees from Merck & Co. Four of the authors (XY, MR, SSS, and KD) are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. P.A. has received research grants from Merck & Co. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception and design, or analysis and interpretation of the data; the drafting of the paper or revising it critically for intellectual content; and the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Acknowledgements
Medical writing assistance was provided by Loraine Georgy, PhD, and Christine Tam, employees of Groupe d’analyse, Ltée, which provided paid consulting services to Merck & Co., Inc., Kenilworth, NJ, USA, for the conduct of this study.
Third party agreement
The data that support the findings of this study are available from Optum, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Any researchers interested in obtaining the data used in this study can access database through Optum, under a license agreement, including the payment of appropriate license fee.
Previous presentations
Parts of this manuscript were presented at the 2019 International Society for Pharmacoeconomics and Outcomes Research (ISPOR); May 18–22, 2019; New Orleans, LA as a poster presentation. Parts of this manuscript were also published in the 2019 European Hematology Association (EHA) Abstract Book and Library as well as online publication by the 2019 American Society of Clinical Oncology (ASCO) annual meeting.
Supplemental Material
Download MS Word (502.5 KB)References
- National Cancer Institute (NCI) Surveillance Epidemiology and End Results Program (SEER). Primary mediastinal (thymic) large B-cell lymphoma 2019. [cited 2019 June 18]. Available from: https://seer.cancer.gov/seertools/hemelymph/51f6cf56e3e27c3994bd5318/.
- Jaffe E, Harris N, Stein H, et al. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Vol. 3. Lyon, France: World Health Organization Press; 2001.
- Steidl C, Gascoyne RD. The molecular pathogenesis of primary mediastinal large B-cell lymphoma. Blood. 2011;118(10):2659–2669.
- Liu PP, Wang KF, Xia Y, et al. Racial patterns of patients with primary mediastinal large B-cell lymphoma: SEER analysis. Medicine (Baltimore). 2016;95(27):e4054.
- © National Comprehensive Cancer Network Inc. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Guideline Name V.32019. All rights reserved. 2019 [cited February 13, 2020]. Available from: to view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
- Rieger M, Osterborg A, Pettengell R, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol. 2011; Mar22(3):664–670.
- Zinzani PL, Martelli M, Bendandi M, et al. Primary mediastinal large B-cell lymphoma with sclerosis: a clinical study of 89 patients treated with MACOP-B chemotherapy and radiation therapy. Haematologica. 2001;86(2):187–191.
- Savage KJ, Al-Rajhi N, Voss N, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol. 2006;17(1):123–130.
- Zinzani PL, Martelli M, Bertini M, et al. Induction chemotherapy strategies for primary mediastinal large B-cell lymphoma with sclerosis: a retrospective multinational study on 426 previously untreated patients. Haematologica. 2002;87(12):1258–1264.
- Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;368(15):1408–1416.
- Dunleavy K, Pittaluga S, Janik J, et al. Primary Mediastinal Large B-Cell Lymphoma (PMBL) Outcome Is Significantly Improved by the Addition of Rituximab to Dose Adjusted (DA)-EPOCH and Overcomes the Need for Radiation. Blood. 2005;106(11):929–929.
- Lazzarino M, Orlandi E, Paulli M, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol. 1997;15(4):1646–1653.
- Zinzani PL, Martelli M, Magagnoli M, et al. Treatment and clinical management of primary mediastinal large B-cell lymphoma with sclerosis: MACOP-B regimen and mediastinal radiotherapy monitored by (67)Gallium scan in 50 patients. Blood. 1999;94(10):3289–3293.
- Kuruvilla J, Pintilie M, Tsang R, et al. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma. 2008;49(7):1329–1336.
- Tomassetti S, Chen R, Dandapani S. The role of pembrolizumab in relapsed/refractory primary mediastinal large B-cell lymphoma. Ther Adv Hematol. 2019;10:2040620719841591.
- Sehn LH, Antin JH, Shulman LN, et al. Primary diffuse large B-cell lymphoma of the mediastinum: outcome following high-dose chemotherapy and autologous hematopoietic cell transplantation. Blood. 1998;91(2):717–723.
- Poire X, van Besien K. Autologous transplant for primary mediastinal B-cell lymphoma. Expert Rev Hematol. 2009;2(1):31–36.
- Zinzani PL. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large b-cell lymphoma: efficacy and safety from the phase 2 CheckMate 436 study. 15th International Conference on Malignant Lymphoma; June 18-22, 2019; Lugano, Switzerland 2019.
- Hurvitz S, Guerin A, Brammer M, et al. Investigation of adverse-event-related costs for patients with metastatic breast cancer in a real-world setting. Oncologist. 2014;19(9):901–908.
- Ramsey S, Henk H, Smith G, et al. First-, second- and third-line lung cancer treatment patterns and associated costs in a US healthcare claims database. Lung Cancer Management. 2015;4(3):131–143.
- Seal BS, Sullivan SD, Ramsey S, et al. Medical costs associated with use of systemic therapy in adults with colorectal cancer. J Manag Care Pharm. 2013;19(6):461–467.
- Wong W, Yim YM, Kim A, et al. Assessment of costs associated with adverse events in patients with cancer. PLoS One. 2018;13(4):e0196007.
- Wilson WH, Grossbard ML, Pittaluga S, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99(8):2685–2693.
- Optum I. Clinformatics® Data Mart User Manual. 2018;1–41.
- Diehr P, Yanez D, Ash A, et al. Methods for analyzing health care utilization and costs. Annu Rev Public Health. 1999;20:125–144.
- Shah NN, Szabo A, Huntington SF, et al. R-CHOP versus dose-adjusted R-EPOCH in frontline management of primary mediastinal B-cell lymphoma: a multi-centre analysis. Br J Haematol. 2018;180(4):534–544.
- Wilson WH, Grant C, Dunleavy K. Therapy in primary mediastinal B-cell lymphoma. N Engl J Med. 2013;369(3):283–284.
- Todeschini G, Ambrosetti A, Meneghini V, et al. Mediastinal large-B-cell lymphoma with sclerosis: a clinical study of 21 patients. J Clin Oncol. 1990;8(5):804–808.
- Kirn D, Mauch P, Shaffer K, et al. Large-cell and immunoblastic lymphoma of the mediastinum: prognostic features and treatment outcome in 57 patients. J Clin Oncol. 1993;11(7):1336–1343.
- Hamlin PA, Portlock CS, Straus DJ, et al. Primary mediastinal large B-cell lymphoma: optimal therapy and prognostic factor analysis in 141 consecutive patients treated at Memorial Sloan Kettering from 1980 to 1999. Br J Haematol. 2005;130(5):691–699.
- Haioun C, Gaulard P, Roudot-Thoraval F, et al. Mediastinal diffuse large-cell lymphoma with sclerosis: a condition with a poor prognosis. Am J Clin Oncol. 1989;12(5):425–429.
- YESCARTA® (axicabtagene ciloleucel) highlights of prescribing information. Kite Pharma, Inc.; 2019.
- Martelli M, Ferreri A, Di Rocco A, et al. Primary mediastinal large B-cell lymphoma. Crit Rev Oncol Hematol. 2017;113:318–327.
