Abstract
Aim
To assess the real-world healthcare resource utilization (HRU) and costs of patients with non-valvular atrial fibrillation (NVAF) and obesity newly initiated on rivaroxaban or warfarin in the US.
Methods
This retrospective study used IQVIA PharMetrics Plus data (01/2010–09/2019) to evaluate patients (≥18 years) with NVAF and obesity (body mass index ≥30 kg/m2) initiated on rivaroxaban or warfarin (on or after 01/2013). Inverse probability of treatment weighting (IPTW) was used to adjust for confounding between cohorts. HRU and costs were assessed post-treatment initiation. Weighted cohorts were compared using Poisson regression models and cost differences, with 95% confidence intervals (CIs) and p values generated using non-parametric bootstrap procedures.
Results
After IPTW, 10,555 and 5,080 patients were initiated on rivaroxaban and warfarin, respectively (mean age: 59 years). At 12 months follow-up, the rivaroxaban cohort had lower all-cause HRU, including fewer hospitalizations (rate ratio [RR]: 0.80, 95% CI: 0.74, 0.87), emergency room visits (RR: 0.89, 95% CI: 0.83, 0.97), and outpatient visits (RR: 0.72, 95% CI: 0.69, 0.77; all p < .05). Medical costs were also reduced in the rivaroxaban cohort (mean difference: −$6,759, 95% CI: −$9,814, −$3,311) due to reduced hospitalization costs (mean difference: −$5,967, 95% CI: −$8,721, −$3,327), resulting in lower total all-cause healthcare costs compared to the warfarin cohort (mean difference: −$4,579, 95% CI: −$7,609, −$1,052; all p < .05). The rivaroxaban cohort also had lower NVAF-related HRU and medical costs driven by lower hospitalization at 12 months post-treatment initiation. HRU and cost reductions associated with rivaroxaban persisted up to 36 months of follow-up.
Limitations
Claims data may have contained inaccuracies and obesity was classified based on ICD diagnosis codes given that patient BMI values were not available.
Conclusions
Rivaroxaban was associated with reduced HRU and costs compared to warfarin among NVAF patients with obesity in a real-world US setting.
Introduction
Atrial fibrillation (AF), the most common form of cardiac arrhythmiaCitation1, is associated with an increased likelihood of thromboembolic events such as stroke and systemic embolism (SE)Citation2–4. Obesity (body mass index [BMI] ≥30 kg/m2) is a strong independent risk factor for developing AFCitation5–7, and is associated with higher morbidity, severity, and persistence of AFCitation8–10. Moreover, obesity may indirectly increase the risk of AF through its association with other diseases such as obstructive sleep apnea, diabetes mellitus, and hypertensionCitation8,Citation11. According to the CDC National Center for Health Statistics, the prevalence of obesity is 42.4% in 2017–2018 among adults in the USCitation12. Given the rising rates of obesity and AF in the US, the societal and economic burden of disease is likely to increase in coming yearsCitation12,Citation13.
Non-valvular AF (NVAF) is the most common type of AF in the US, with NVAF-related strokes accounting for over 15% of all strokesCitation14. Chronic anticoagulation therapies are instrumental in reducing the risk of stroke/SE and mortality among patients with NVAFCitation15. In the past decade, direct-acting oral anticoagulants (DOAC) such as rivaroxaban have been approved by the US Food and Drug Administration (FDA)Citation16–18 and are being increasingly preferred over vitamin K antagonists (VKA) such as warfarinCitation19,Citation20. Several randomized clinical trials (RCT) have demonstrated that DOAC are at least as effective as VKA for stroke prevention in patients with NVAFCitation21,Citation22,Citation23,Citation24 while maintaining a similar, if not improved, safety profile without requiring laboratory monitoring and dosage adjustmentsCitation25,Citation26.
Although obesity may potentially alter the pharmacological properties of multiple drug classesCitation27, including some anticoagulantsCitation28, the clinical pharmacology of rivaroxaban is not significantly impacted by body weightCitation29–31. In 2016 guidance statements published by the International Society of Thrombosis and Haemostasis (ISTH)Citation32, DOACs including rivaroxaban were deemed safe and effective for patients with a BMI ≤40 kg/m2 and body weight ≤120 kg based on the results of subgroup analyses of efficacy by weight from Phase III trialsCitation23,Citation33–36, pooled analyses of Phase III trialsCitation37–39, and pharmacokinetic and pharmacodynamics studiesCitation29,Citation40–43. The 2016 ISTH guidance statements did not recommend the use of rivaroxaban, or any of the other currently marketed DOACs, for morbidly obese patients (BMI >40 kg/m2 or body weight >120 kg) due to insufficient evidence at the timeCitation32. Nonetheless, the results of recent real-world studiesCitation44–48 and post-hoc subgroup analyses of ROCKET AF clinical trialCitation49 suggest that rivaroxaban is also safe and effective in NVAF patients who are morbidly obese.
AF is associated with substantial economic burden in the US, with annual costs estimated at $6 billion and rising to $26 billion when other cardiovascular and non-cardiovascular costs are includedCitation3. Previous real-world studies have shown that patients with NVAF initiated on rivaroxaban have reduced healthcare resource utilization (HRU) and healthcare costs compared to those initiated on warfarin irrespective of body weight or BMICitation50,Citation51. In a retrospective study of NVAF patients with morbid obesity (i.e. BMI ≥40 kg/m2), Peterson et al.Citation45 found that rivaroxaban was associated with reduced HRU and healthcare costs compared to warfarin in this population. However, uncertainty remains regarding the HRU and costs of rivaroxaban versus warfarin among patients with NVAF and all obesity classes (i.e. BMI ≥30 kg/m2) including, but not limited to, those with morbid obesity (i.e. BMI ≥40 kg/m2). Moreover, the long-term economic impact of rivaroxaban versus warfarin among NVAF populations with obesity is not well understood due to the relatively short mean follow-up period of prior studies (∼10 monthsCitation45).
In light of growing evidence that rivaroxaban is an effective treatment for NVAF patients with obesityCitation46, the present real-world study evaluated the HRU and healthcare costs of this patient population following the initiation of rivaroxaban versus warfarin.
Methods
Data source
IQVIA PharMetricsFootnotei Plus data spanning from January 1, 2010 to September 30, 2019 were used to meet the study objectives. The IQVIA PharMetrics Plus data offers a diverse representation of employers, payers and providers, and geographic zones covering all 50 states. It contains around 40 million patients with both medical and pharmacy benefits in any given recent year, with the average length of health plan enrollment of approximately 39 months. The enrollee population in the IQVIA PharMetrics Plus data is generally representative of the less-than 65 years of age, commercially insured population in the US with respect to both age and gender. The database contains historical information on patient demographics, plan enrollment, and claims for inpatient, outpatient (OP) and pharmacy as well as their associated costs. Data were de-identified and compliant with the Health Insurance Portability and Accountability Act (HIPAA).
Study design and population
A retrospective weighted-cohort design was used to evaluate outcomes among patients who were newly initiated on rivaroxaban or warfarin. Eligible patients had ≥1 pharmacy dispensings for rivaroxaban or warfarin between November 4, 2011 and September 30, 2019 (identification period). The date of the initiation of rivaroxaban or warfarin was defined as the index date. The baseline period was defined as the 12 months prior to the index date. As it may take a certain amount of time for recently approved medications to be prescribed and early adopters may have differing characteristics, only patients with an index date on or after January 1, 2013 were included in the study population. Further, eligible patients were required to meet the following inclusion criteria: ≥1 medical claim with a diagnosis of AF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]: 427.31 or International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM]: I48.0–148.2x, 148.91) during the baseline period or on the index date, ≥1 medical claim with a diagnosis code for obesity or BMI ≥30 kg/m2 (see Supplemental Table 1 for a list of ICD-9-CM and ICD-10-CM codes used to define obesity/BMI ≥30 kg/m2) during the baseline period or on the index date, ≥12 months of continuous health plan enrollment before the index date (i.e. baseline period), and ≥18 years of age in the index year. A prior study has validated the use of diagnosis codes for identifying obesity among NVAF patients with high positive predictive value (PPV: 89.8%) and high specificity (95.2%)Citation52.
Patients were excluded from the analysis if they had pharmacy dispensings for >1 oral anticoagulant (i.e. rivaroxaban, warfarin, apixaban, edoxaban, betrixaban, or dabigatran) at the index date or if they met any of the following exclusion criteria during the baseline period: ≥1 pharmacy dispensing for an oral anticoagulant, ≥1 medical claim for VTE or knee or hip replacement surgery, ≥1 medical claim with a diagnosis of mitral-stenosis, or ≥1 medical claim for a mechanical heart-valve procedure.
Patients’ demographics and clinical characteristics were evaluated during the baseline period. An intention-to-treat (ITT) approach was used to evaluate HRU and healthcare cost outcomes during the observation (follow-up) period, which spanned from the index date until the earliest of health plan disenrollment or end of data availability (including the index date). In this ITT design, patients were observed beyond the point at which they discontinued or switched to another similar medication, which reflects the real-world impact associated with the use of these agents. Study outcomes were evaluated over two distinct follow-up periods, censored at 12 months and 36 months of observation, separately. As a sensitivity analysis, the follow-up of patients was censored if they switched to another oral anticoagulant agent to evaluate HRU and cost only when patients were treated with the index agent.
Study outcomes
All-cause and NVAF-related HRU were assessed during the 12-month and 36-month follow-up periods and included the number of hospitalizations and the number of days of hospital stay, emergency room (ER) visits, and OP visits. OP visits were stratified by office visits, OP hospital visits, and other visits (including patient home and other unlisted facilities). All-cause and NVAF-related healthcare costs were assessed during follow-up up to 12 and 36 months and included medical (i.e. hospitalization, ER, and OP stratified by office, OP hospital, and other) and pharmacy costs. NVAF-related HRU and costs included all visits associated with a primary or a secondary diagnosis (i.e. identified in any other diagnosis fields) of AFCitation53.
Statistical analysis
Rivaroxaban and warfarin cohorts were weighted using the inverse probability of treatment weighting (IPTW) approach based on the propensity score (PS)Citation54. Weights were calculated based on the probability of being treated with rivaroxaban, where the PS for each patient was estimated using a multivariable logistic regression adjusting for characteristics observed during the baseline period. IPTWs were defined as 1/PS and 1/(1 − PS) for the rivaroxaban and warfarin cohorts, respectively, and normalized within each cohort (i.e. dividing each weight by the mean of the weights per cohort). Baseline demographic characteristics used in the PS calculation included age, sex, year of index date, region, and type of insurance plan. Clinical characteristics used in the PS calculation included morbid obesity, baseline coronary artery disease or peripheral artery disease, baseline stroke/SE, baseline major bleeding, cardiovascular-related medications, cardiovascular procedures, use of non-oral anticoagulants (i.e. unfractionated heparin, fondaparinux, and low molecular weight heparin), number of different prescription drugs used during baseline, prior history of cancer diagnosis and treatment, baseline HRU and healthcare costs, and baseline risk factors for stroke and bleeding events (with ≥5% prevalence in either cohort). PS distributions are presented in Supplemental Figure S1.
Patient characteristics by treatment cohort were evaluated using descriptive statistics including mean, standard deviation (SD), and median values for the continuous variables, and relative frequencies and proportions for the categorical variables. Differences in baseline characteristics between patients in the two cohorts were assessed using standardized differences. A standardized difference of less than 10% was considered a negligible imbalanceCitation55.
HRU and healthcare costs during follow-up were evaluated per patient-years (PPY) to account for different lengths of observation periods among study patients. The mean rate of HRU, calculated as the number of events PPY, was compared between cohorts using rate ratios obtained from Poisson regression models. Mean healthcare costs PPY were inflated to 2019 US dollars based on the medical care component of the Consumer Price Index. To avoid overestimating costs by annualizing data for patients observed for a short period of time, each patient’s cost outcomes were weighted by length of follow-up. Cost differences between cohorts were obtained using multivariable linear regression models controlling for baseline healthcare costs, as some standardized differences were greater than 10% after weighting. Of note, the linear regression models were used only to obtain the point estimates (i.e. adjusted cost differences). P values and 95% confidence intervals (CI) were estimated using non-parametric bootstrap procedures with 499 repetitions, since HRU and cost data have positive values that follow a non-normal distribution and commonly include zero values. The non-parametric bootstrap method makes no assumptions about the underlying distribution of the data.
Results
A total of 137,512 adult patients with a diagnosis of AF initiated on an anticoagulant (rivaroxaban or warfarin) were identified. After applying all inclusion and exclusion criteria, a total of 15,635 patients with NVAF and obesity were included (10,555 patients initiating rivaroxaban and 5,080 patients initiating warfarin treatment; ).
Figure 1. Patient disposition – NVAF population. This figure has been reproduced with permission from Taylor and Francis of Berger, et al. Real-world effectiveness and safety of rivaroxaban versus warfarin among NVAF patients with obesity in a US population. Curr Med Res Opin. 2021. DOI:10.1080/03007995.2021.1901223. Data Source: IQVIA PharMetrics Plus data, consisting of AF patients with obesity, from January 1, 2010 to September 30, 2019. Abbreviations. AF, atrial fibrillation; BMI, body mass index; GPI, generic product identifier; ICD, international classification of disease; NVAF, non-valvular atrial fibrillation; VTE, venous thromboembolism. Notes: 1. GPI drug codes were used to identify pharmacy claims for rivaroxaban and warfarin. 2. A total of 326 rivaroxaban patients with >1 oral anticoagulant medications on the index date were excluded. 3. A total of 379 warfarin patients with >1 oral anticoagulant medications on the index date were excluded. 4. Continuous eligibility was defined as continuous health plan enrollment with medical and pharmacy coverage. 5. Baseline period was defined as the 12 months prior to the index date. 6. AF was identified with the following ICD-9-CM codes: 427.31, and ICD-10-CM: I48.0–148.2x, 148.91. 7. BMI-related ICD-9-CM and ICD-10-CM diagnosis codes were used to identify obesity (defined as BMI ≥30 kg/m2).

Baseline demographics and clinical characteristics
Baseline demographic and clinical characteristics of the unweighted and weighted rivaroxaban and warfarin cohorts are shown in . After IPTW, the rivaroxaban and warfarin cohorts were generally well-balanced (i.e. standardized difference <10%) with respect to demographic and clinical characteristics.
Table 1. Baseline demographics and clinical characteristics of NVAF patients treated with rivaroxaban or warfarin.
At the index date and after weighting, the mean age of the rivaroxaban and warfarin cohorts was 59 years and about a third of the patients in each cohort were female (). The mean (SD) total all-cause healthcare costs during the baseline period were $41,725 ($76,079) for the rivaroxaban cohort and $39,185 ($73,591) for the warfarin cohort (). During the baseline period and after weighting, the proportion of patients with morbid obesity (BMI ≥40 kg/m2) was 37.7% in the rivaroxaban cohort and 39.0% in the warfarin cohort (). Patients in the rivaroxaban and warfarin cohorts had a comparable mean Quan-Charlson comorbidity index (CCI) score (1.79 and 1.75, respectively), mean CHA2DS2-VASc [congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke or transient ischemic attack (TIA), vascular disease, age 65–74 years, sex category] score (2.65 and 2.70, respectively), and mean HAS-BLED [hypertension, abnormal renal and liver function, stroke, bleeding] score (1.80 and 1.79, respectively; ). During the baseline period, 5.8% and 5.1% of patients in the rivaroxaban and warfarin cohorts had a stroke/SE and 3.8% and 2.9% had major bleeding, respectively ().
Table 2. Baseline comorbidities of NVAF patients treated with rivaroxaban or warfarin.
Healthcare resource utilization
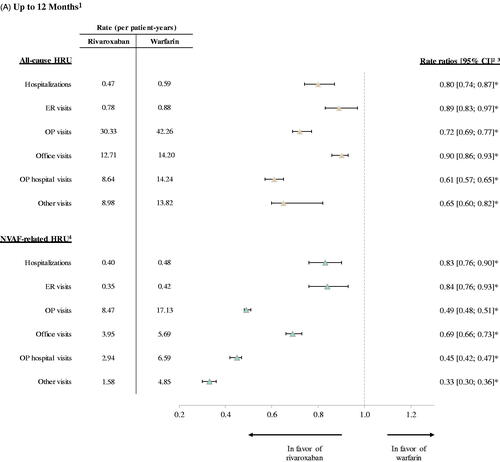
All-cause and NVAF-related HRU during follow-up among patients in the weighted rivaroxaban and warfarin cohorts is shown in . During 12 months of follow-up, the mean rate of all-cause HRU was significantly lower in the rivaroxaban cohort compared to the warfarin cohort across all observed components (). Over a mean (median) follow-up time of 10.0 (12) months and 9.8 (12) months for the weighted rivaroxaban and warfarin cohorts, respectively, patients in the rivaroxaban cohort had lower rates of all-cause hospitalizations (RR: 0.80, 95% CI: 0.74, 0.87; p < .001), ER visits (RR: 0.89, 95% CI: 0.83, 0.97; p = .020), and OP visits (RR: 0.72, 95% CI: 0.69, 0.77; p < .001) compared to those in the warfarin cohort. For all-cause hospitalizations, the median length of stay was 4 days in the rivaroxaban cohort and 5 days in the warfarin cohort. The significantly lower rate of all-cause OP visits in the rivaroxaban cohort compared to the warfarin cohort included lower mean rates of office visits (RR: 0.90, 95% CI: 0.86, 0.93; p < .001), OP hospital visits (RR: 0.61, 95% CI: 0.57, 0.65; p < .001), and other visits (RR: 0.65, 95% CI: 0.60, 0.82; p < .001) relative to patients in the warfarin cohort.
Figure 2. Healthcare Resource Utilization among Rivaroxaban vs. Warfarin Cohorts. (A) Up to 12 Months. (B) Up to 36 Months. *p value < .05. Abbreviations. CI, confidence interval; ER, emergency room; HRU, healthcare resource utilization; NVAF, non-valvular atrial fibrillation; OP, outpatient. Notes: 1. For the analysis up to 12 months, the mean (median) length of observation period was 10 (12) months for the rivaroxaban cohort and 9.8 (12) months for the warfarin cohort. For the analysis up to 36 months, the length of observation period was 20.3 (19) months for the rivaroxaban cohort and 19.6 (18) months for the warfarin cohort. 2. Rate ratios obtained from Poisson regression models. 3. Confidence intervals and p values were calculated using non-parametric bootstrap procedure (B = 499). 4. HRU was considered NVAF-related if it had been associated with a primary or secondary diagnosis of AF.
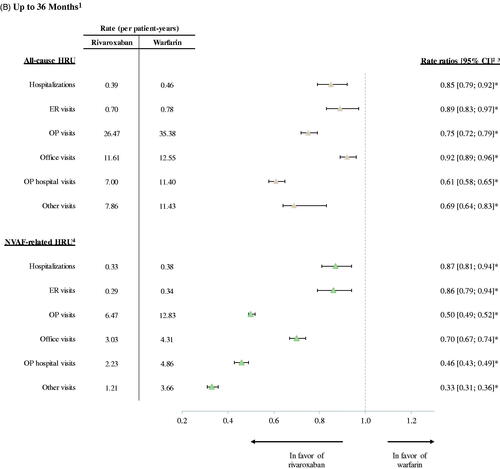
Similar results were observed for NVAF-related HRU during the 12-month follow-up (). In particular, patients in the rivaroxaban cohort had significantly lower rates of hospitalizations (RR: 0.83, 95% CI: 0.76, 0.90; p < .001), ER visits (RR: 0.84, 95% CI: 0.76, 0.93; p < .001), and OP visits (RR: 0.49, 95% CI: 0.48, 0.51; p < .001) compared to those in the warfarin cohort. Finally, a 36-month follow-up analysis of all-cause and NVAF-related HRU yielded similar results to those obtained in the 12-month analysis ().
Healthcare costs
Mean all-cause and NVAF-related healthcare costs PPY among patients in the weighted rivaroxaban and warfarin cohorts are shown in . In the up to 12 months follow-up (), patients in the rivaroxaban cohort had reduced total all-cause medical costs compared to those in the warfarin cohort (mean difference: −$6,759, 95% CI: −$9,814, −$3,311; p < .001) which was driven by lower hospitalization costs (mean difference: −$5,967, 95% CI: −$8,721, −$3,327; p < .001). These all-cause medical cost savings associated with rivaroxaban fully offset the higher pharmacy costs (mean difference: $2,180, 95% CI: $1,910, $2,486; p < .001), resulting in significantly lower total all-cause healthcare costs in the rivaroxaban cohort compared to the warfarin cohort (mean difference: −$4,579, 95% CI: −$7,609, −$1,052; p < .001). No significant differences were found in all-cause ER costs (mean difference: −$130, 95% CI: −$294, −60; p = .180) and all-cause OP costs (mean difference: −$662, 95% CI: −$1,867, $738; p = .393). Among OP costs, patients in the rivaroxaban cohort had significantly reduced office visit costs (mean difference: −$343, 95% CI: −$516, −$115; p = .008) and other visit costs (mean difference: −$722, 95% CI: −$1,035, −$413; p < .001), as well as similar OP hospital visit costs (mean difference: $403, 95% CI: −$684, $1,580; p = .413), compared to patients in the warfarin cohort. NVAF-related total medical costs were also lower among patients in the rivaroxaban cohort compared to those in the warfarin cohort (mean difference: −$3,352, 95% CI: −$5,959, −$743; p = .012) which was primarily driven by lower hospitalization costs (mean difference: −$4,504, 95% CI: −$7,040, −$2,111; p < .001; ).
Figure 3. Healthcare Costs among Rivaroxaban vs. Warfarin Cohorts1. (A) Up to 12 Months. (B) Up to 36 Months. *p value <.05. Abbreviations. ER: emergency room; NVAF: non-valvular atrial fibrillation; OP: outpatient; PPY: per person-years; US: United States. Notes: 1. Cost differences were calculated using a multivariable linear regression model controlling for the healthcare costs during baseline. Confidence intervals and p values were calculated using non-parametric bootstrap procedure (B = 499). 2. Healthcare costs were considered NVAF-related if they had been associated with a primary or secondary diagnosis of AF.
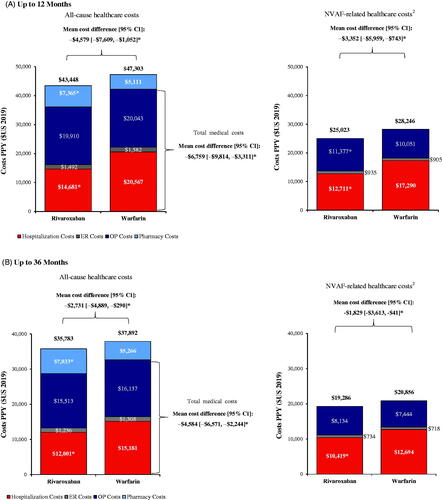
In the 36-month follow-up analysis, the total costs of care were slightly lower compared to those observed in the 12-month follow-up analysis, and the differences between cohorts were smaller but remained significant. Patients initiated on rivaroxaban had significantly lower total all-cause healthcare costs compared to patients initiated on warfarin (mean difference: −$2,731, 95% CI: −$4,889, −$290; p = .032). Similar to the 12-month analysis, the difference in total all-cause healthcare costs was primarily driven by significantly lower hospitalization costs (mean difference: −$3,401, 95% CI: −$5,037, −$1,556; p < .001; ).
Sensitivity analysis
Approximately 12% of patients in the rivaroxaban cohort and 26% of patients in the warfarin cohort switched from their index anticoagulant to another oral anticoagulant during the follow-up period. Based on the results of a sensitivity analysis, censoring at switch to another anticoagulant agent further increased the difference in all-cause and NVAF-related HRU and costs observed between rivaroxaban and warfarin users. Across all observed components, the mean rate of HRU was significantly lower in the rivaroxaban cohort compared with the warfarin cohort (Supplemental Table 2).
The sensitivity analysis of mean healthcare costs PPY also yielded similar results to the main analysis (Supplemental Table 3). In the 12-month follow-up analysis, total all-cause healthcare costs were significantly lower in the rivaroxaban cohort compared to the warfarin cohort (mean difference: −$5,485, 95% CI: −$8,943, −$1,730; p < .001; Supplemental Table 3). In the 36-month follow-up analysis, the difference in total all-cause healthcare costs remained significantly reduced in the rivaroxaban cohort (mean difference: −$3,426, 95% CI: −$5,712, −$608; p = .012). Corresponding NVAF-related costs in the 12-month follow-up analysis were significantly lower in the rivaroxaban cohort compared to the warfarin cohort (mean difference: −$4,210, 95% CI: −$7,190, −$1,451; p < .001; Supplemental Table 3). In the 36-month follow-up analysis, the difference in total NVAF-related total medical costs remained significantly lower in the rivaroxaban cohort (mean difference: −$2,419, 95% CI: −$4,190, −$518; p = .012).
Discussion
To our knowledge, this US-based retrospective cohort study is the first to compare the HRU and healthcare costs of rivaroxaban versus warfarin among newly initiated patients with NVAF and obesity, including, but not limited to, morbid obesity. Overall, rivaroxaban was associated with reduced HRU and cost burden compared to warfarin. All-cause and NVAF-related HRU and total healthcare costs were significantly lower for the rivaroxaban cohort compared to the warfarin cohort, with significant medical cost savings due to lower hospitalization fully offsetting higher pharmacy costs. This pattern of results remained robust upon analysis of patients with up to 36 months of follow-up, highlighting the long-term economic benefits of rivaroxaban over warfarin among NVAF patients with obesity.
These results are consistent with previous findings among NVAF patients with morbid obesity. In a prior retrospective study by Peterson et al.Citation45, NVAF patients with morbid obesity who were treated with rivaroxaban had significantly lower all-cause medical costs compared to matched warfarin-treated patients and these medical cost savings were driven primarily by reduced hospitalizations. Consistent with the present study findings, medical cost savings compensated for higher pharmacy costs resulting in mean total all-cause healthcare costs per patient per year that were $3,890 lower in the rivaroxaban versus warfarin cohort ($48,552 versus $52,418, respectively).
The results of the present study generally align with those reported among patients with NVAF irrespective of body weight/BMI. In prior studies comparing matched patients from the general NVAF population, those treated with rivaroxaban had significantly fewer hospitalization days and OP visitsCitation56, as well lower associated costs, compared to their warfarin-treated counterpartsCitation50,Citation51. In one study by Laliberté et al., rivaroxaban use was associated with significantly lower all-cause and NVAF-related costs for hospitalizations and OP visits, which compensated for higher pharmacy costs compared to warfarinCitation50. Whereas this prior study found that rivaroxaban was overall cost-neutral compared to warfarin, the present study observed significant total cost savings with rivaroxaban. These differences with respect to total healthcare costs may be partly explained by variations in patient characteristics among study populations, including differences in mean body weight/BMI. Additionally, the present study included younger patients from a commercially-insured population in the US, whereas the prior study included older patients with commercial and Medicare plans.
Prior evidence suggests that rivaroxaban and warfarin have comparable safety and efficacy among NVAF patients with obesityCitation23,Citation49, as well as those with morbid obesityCitation44,Citation45. More recent study findings suggest that rivaroxaban may be associated with a lower risk of stroke/SE among NVAF patients with obesity, with potential implications for HRU and cost outcomes. In particular, an electronic health records study of more than 71,000 NVAF patients with obesity with over 30 months of follow-up found that rivaroxaban was associated with significantly lower risk of stroke/SE and major bleeding compared to warfarinCitation46.
Factors contributing to the reduced HRU and costs among patients initiated of rivaroxaban versus warfarin may have included differences in routine safety monitoring. Among patients treated with warfarin, international normalized ratio (INR) monitoring is required to maintain drug responses within the therapeutic range needed to achieve the benefits of anticoagulation, while avoiding the risk of major bleedingCitation57. In real-world clinical practice, warfarin-treated patients undergo INR monitoring on a regular basis, although monitoring may become less frequent once patients have established a stable treatment regimenCitation45,Citation58. Routine INR monitoring is not required among DOAC users, which may have contributed to the lower rates of HRU, particularly OP visits in the present study.
The present study should be viewed in the context of certain limitations. We note that obesity was classified based on ICD diagnosis codes for high BMI and not a patient’s actual BMI value, since height and weight are not available in claims data. Therefore, it is possible that some patients with obesity were not captured in this analysis. Based on findings from multiple validation studies, it is evident that diagnostic obesity codes may underestimate the true prevalence of obesity. However, given the high specificity and modest to high positive predictive values, obese patients can be identified using diagnosis codesCitation52,Citation59–61. Of note, within the obesity definition (BMI ≥30 kg/m2) patients with different weight or BMI categories are not differentiated.
The present study may have been subject to additional limitations commonly associated with retrospective claims analyses. Analyses of administrative claims data depend on correct diagnosis, procedure, and drug codes, and coding inaccuracies may lead to misidentification. In addition, the data may not contain all prescription-related information, particularly medications administered in inpatient settings, and over-the-counter medications, such as aspirin. Despite the use of IPTW, the present study results may have also been influenced by unmeasured confounders not available in claims databases. Furthermore, the healthcare costs assessed in this study did not include indirect costs, such as lost productivity, and therefore the findings may under-represent the overall burden of NVAF and obesity. Finally, the present study population was broadly representative of patients with commercial insurance in the US; therefore, the results may not be generalizable to other populations.
Conclusion
Among NVAF patients with obesity initiated on anticoagulant therapy, rivaroxaban use was associated with significantly lower HRU, including lower rates of hospitalization, compared to warfarin use. Rivaroxaban use was also associated with significantly lower total all-cause and NVAF-related healthcare costs when compared to warfarin use, with significant medical cost savings due to lower rates hospitalization fully offsetting higher pharmacy costs.
Transparency
Declaration of funding
Financial support for this research was provided by Janssen Scientific Affairs, LLC. The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Declaration of financial/other relationships
JSB received consultancy fees from Janssen Scientific Affairs, LLC. VA and AK are employees of Janssen Scientific Affairs, LLC and KTM is an employee at Janssen Pharmaceuticals Inc. who may own stock or stock options. FL, DL, YJ, and PL are employees of Analysis Group, Inc., a consulting firm that received consulting fees from Janssen Scientific Affairs, LLC for the conduct of this study.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed no interests specific to this manuscript but potential conflicts including consulting fees from Abbott, AbbVie, ACI Clinical, Alexion Pharmaceuticals, Aplagon Ltd, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, BTG, Daiichi-Sankyo, EmstoPA Ltd, EPG Health, Guidepoint Global, Gulf Coast Developments, Jannsen, Johnson & Johnson, Leo Pharma, LifeSciences Consulting, Medscape, McKinsey, Navigant, North Star Communications, ONO Pharmaceuticals, Pfizer, Portola Pharmaceuticals, Sanofi, Takeda, Total CME, Windrose Consulting Group; advisory board membership with Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Johnson and Johnson, Pfizer, Portola, Sanofi; payments for lectures, payments for preparation of reports and payment for development of educational presentations from Aspen, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Johnson & Johnson, Medscape, Pfizer and Portola. The reviewer has also advised the UK Government Health Select Committee, the all-party working group on thrombosis, the Department of Health, and the NHS, on the prevention of VTE. He is also an advisor to Lifeblood: the thrombosis charity and is the founder of the European educational charity the Coalition to Prevent Venous Thromboembolism. The reviewers have no other relevant financial relationships or otherwise to disclose.
Author contributions
JSB, AK, KTM, and VA contributed to study conception and design, and data analysis and interpretation. FL, DL, YJ, and PL contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Supplemental Material
Download MS Word (63.2 KB)Acknowledgements
Medical writing assistance was provided by Mona Lisa Chanda, PhD, an employee at Analysis Group, Inc. and funded by Janssen Scientific Affairs, LLC.
Notes
i IQVIA PharMetrics is a registered trademark of IQVIA Inc. in the United States, the European Union, and various other countries.
References
- Centers for Disease Control and Prevention. Atrial Fibrillation. 2020 [cited 2020 Sep 9]. Available from: https://www.cdc.gov/heartdisease/atrial_fibrillation.htm
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988.
- Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4:313–320.
- Lim GB. Atrial fibrillation. Risk of systemic emboli in AF. Nat Rev Cardiol. 2015;12:561.
- Freeman AL, Pendleton RC, Rondina MT. Prevention of venous thromboembolism in obesity. Expert Rev Cardiovasc Ther. 2010;8:1711–1721.
- Wong CX, Sullivan T, Sun MT, et al. Obesity and the risk of incident, post-operative, and post-ablation atrial fibrillation: a meta-analysis of 626,603 individuals in 51 studies. JACC Clin Electrophysiol. 2015;1:139–152.
- Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. Jama. 2004;292:2471–2477.
- Nalliah CJ, Sanders P, Kottkamp H, et al. The role of obesity in atrial fibrillation. Eur Heart J. 2016;37:1565–1572.
- Pandey A, Gersh BJ, McGuire DK, et al. Association of body mass index with care and outcomes in patients with atrial fibrillation: results from the ORBIT-AF registry. JACC Clin Electrophysiol. 2016;2:355–363.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375.
- Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2011;123:1501–1508.
- Centers for Disease Control and Prevention. Adult obesity facts. 2020 [cited 2020 Sep 9]. Available from: https://www.cdc.gov/obesity/data/adult.html
- Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112:1142–1147.
- Reiffel JA. Atrial fibrillation and stroke: epidemiology. Am J Med. 2014;127:e15–e16.
- American College of Cardiology F, American Heart A, European Society of C, et al. Management of patients with atrial fibrillation (compilation of 2006 ACCF/AHA/ESC and 2011 ACCF/AHA/HRS recommendations): a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2013;127:1916–1926.
- United States Food and Drug Administration. ELIQUIS® (apixaban) – prescribing information. 2019 [cited 2020 Sep 9]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202155s024lbl.pdf
- United States Food and Drug Administration. PRADAXA® (dabigatran etexilate) – prescribing information. 2020 [cited 2020 Sep 9]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022512s039lbl.pdf
- United States Food and Drug Administration. XARELTO® (rivaroxaban) – Prescribing Information. 2021 [cited 2021 Feb 26]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/022406s036,202439s036lbl.pdf
- Saraiva JFK. Stroke prevention with oral anticoagulants: summary of the evidence and efficacy measures as an aid to treatment choices. Cardiol Ther. 2018;7:15–24.
- Dzeshka MS, Lip GY. Non-vitamin K oral anticoagulants in atrial fibrillation: where are we now? Trends Cardiovasc Med. 2015;25:315–336.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50:e1–e88.
- Compendium EM. SPC. Warfarin 0.5 mg tablets. 2017 [cited 2019 Nov 22]. Available from: https://www.medicines.org.uk/emc/medicine/27651
- Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet. 2010;49:71–87.
- Domienik-Karlowicz J, Pruszczyk P. The use of anticoagulants in morbidly obese patients. Cardiol J. 2016;23:12–16.
- Kubitza D, Becka M, Zuehlsdorf M, et al. Body weight has limited influence on the safety, tolerability, pharmacokinetics, or pharmacodynamics of rivaroxaban (BAY 59-7939) in healthy subjects. J Clin Pharmacol. 2007;47:218–244.
- Willmann S, Zhang L, Frede M, et al. Integrated population pharmacokinetic analysis of rivaroxaban across multiple patient populations. CPT Pharmacometrics Syst Pharmacol. 2018;7:309–320.
- Barsam SJ, Patel JP, Roberts LN, et al. The impact of body weight on rivaroxaban pharmacokinetics. Res Pract Thromb Haemost. 2017;1:180–187.
- Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308–1313.
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808.
- Hokusai VTEI, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415.
- Investigators E, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510.
- Investigators E-P, Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297.
- Eriksson BI, Dahl OE, Feuring M, et al. Dabigatran is effective with a favourable safety profile in normal and overweight patients undergoing major orthopaedic surgery: a pooled analysis. Thromb Res. 2012;130:818–820.
- Pineo G, Gallus A, Raskob G, et al. Apixaban after hip or knee arthroplasty versus enoxaparin: efficacy and safety in key clinical subgroups. J Thromb Haemost. 2013;11:444–451.
- Turpie AG, Lassen MR, Eriksson BI, et al. Rivaroxaban for the prevention of venous thromboembolism after hip or knee arthroplasty. Thromb Haemost. 2011;105:444–453.
- Reilly PA, Lehr T, Haertter S, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol. 2014;63:321–328.
- Upreti VV, Wang J, Barrett YC, et al. Effect of extremes of body weight on the pharmacokinetics, pharmacodynamics, safety and tolerability of apixaban in healthy subjects. Br J Clin Pharmacol. 2013;76:908–916.
- Yin OQ, Tetsuya K, Miller R. Edoxaban population pharmacokinetics and exposure–response analysis in patients with non-valvular atrial fibrillation. European Journal of Clinical Pharmacology. 2014;70:1339–1351.
- Mueck W, Lensing AW, Agnelli G, et al. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50:675–686.
- Kido K, Ngorsuraches S. Comparing the efficacy and safety of direct oral anticoagulants with warfarin in the morbidly obese population with atrial fibrillation. Ann Pharmacother. 2019;53:165–170.
- Peterson ED, Ashton V, Chen YW, et al. Comparative effectiveness, safety, and costs of rivaroxaban and warfarin among morbidly obese patients with atrial fibrillation. Am Heart J. 2019;212:113–119.
- Costa OS, Beyer-Westendorf J, Ashton V, et al. Effectiveness and safety of rivaroxaban versus warfarin in obese nonvalvular atrial fibrillation patients: analysis of electronic health record data. Curr Med Res Opin. 2020;36:1081–1088.
- Kushnir M, Choi Y, Eisenberg R, et al. Efficacy and safety of direct oral factor Xa inhibitors compared with warfarin in patients with morbid obesity: a single-centre, retrospective analysis of chart data. Lancet Haematol. 2019;6:e359–e365.
- Perales IJ, San Agustin K, DeAngelo J, et al. Rivaroxaban versus warfarin for stroke prevention and venous thromboembolism treatment in extreme obesity and high body weight. Ann Pharmacother. 2020;54:344–350.
- Balla SR, Cyr DD, Lokhnygina Y, et al. Relation of risk of stroke in patients with atrial fibrillation to body mass index (from patients treated with Rivaroxaban and Warfarin in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation Trial). Am J Cardiol. 2017;119:1989–1996.
- Laliberte F, Cloutier M, Crivera C, et al. Effect of rivaroxaban versus warfarin on health care costs among nonvalvular atrial fibrillation patients: observations from rivaroxaban users and matched warfarin users. Adv Ther. 2015;32:216–227.
- Laliberte F, Pilon D, Raut MK, et al. Is rivaroxaban associated with lower inpatient costs compared to warfarin among patients with non-valvular atrial fibrillation? Curr Med Res Opin. 2014;30:1521–1528.
- Jain R, Watzker A, Luo X, et al. Validation of obesity coding among newly treated nonvalvular atrial fibrillation patients using an integrated electronic medical record and claims database. Curr Med Res Opin. 2020;36:189–197.
- Schroeder KM, Gelwicks S, Naegeli AN, et al. Comparison of methods to estimate disease-related cost and healthcare resource utilization for autoimmune diseases in administrative claims databases. Clinicoecon Outcomes Res. 2019;11:713–727.
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679.
- Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234.
- Laliberte F, Cloutier M, Crivera C, et al. Effects of rivaroxaban versus warfarin on hospitalization days and other health care resource utilization in patients with nonvalvular atrial fibrillation: an observational study from a cohort of matched users. Clin Ther. 2015;37:554–562.
- Dlott JS, George RA, Huang X, et al. National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation. 2014;129:1407–1414.
- Barnes GD, Misirliyan S, Kaatz S, et al. Barriers and facilitators to reducing frequent laboratory testing for patients who are stable on warfarin: a mixed methods study of de-implementation in five anticoagulation clinics. Implement Sci. 2017;12:87.
- Martin BJ, Chen G, Graham M, et al. Coding of obesity in administrative hospital discharge abstract data: accuracy and impact for future research studies. BMC Health Serv Res. 2014;14:70.
- Lloyd JT, Blackwell SA, Wei II, et al. Validity of a claims-based diagnosis of obesity among medicare beneficiaries. Eval Health Prof. 2015;38:508–517.
- Ammann EM, Kalsekar I, Yoo A, et al. Validation of body mass index (BMI)-related ICD-9-CM and ICD-10-CM administrative diagnosis codes recorded in US claims data. Pharmacoepidemiol Drug Saf. 2018;27:1092–1100.