Abstract
Aims
This study evaluated cost-effectiveness of defibrotide vs best supportive care (BSC) for the treatment of hepatic veno-occlusive disease/sinusoidal obstructive syndrome (VOD/SOS) with multiorgan dysfunction (MOD) post-hematopoietic cell transplantation (HCT) in Spain.
Materials and methods
A two-phase Markov model, comprising a 1-year acute phase with daily cycles and a lifetime long-term phase with annual cycles, was adapted to the Spanish setting. The model included a cohort of patients with severe VOD/SOS (defined as VOD/SOS with MOD) post-HCT. For the acute phase, efficacy and VOD/SOS-related length of stay were obtained from a phase 3 defibrotide study (NCT00358501). VOD/SOS-related hospital stays were 7.5 and 23.2 days in defibrotide-treated and BSC patients, respectively. Defibrotide-treated patients spent 30% of their stay in the intensive care unit vs 60% in BSC patients. Assumptions for the long-term phase and utility values were obtained from the literature. Costs were from the Spanish Health System perspective (€2019). Defibrotide cost was based on 25 mg/kg/day over 17.5 days, using local expert opinion. Life-years (LYs), quality-adjusted LYs (QALYs), and costs were estimated over a lifetime horizon, applying a 3% discount rate for costs and outcomes. Sensitivity analyses assessed the robustness of the results.
Results
Defibrotide produced an additional 1.214 QALYs and 1.348 LYs vs BSC, with a total cost of €33,708 more than BSC alone. However, defibrotide resulted in savings up to €16,644/patient for cost of hospital stay. Difference between costs and effective measures led to ratios of €27,757/QALY and €25,007/LY gained. Additional hospital stays had the greatest influence on base-case results. Probabilistic analysis confirmed the robustness of the deterministic results.
Limitations
Limitations include use of historical controls and assumptions extrapolated from the literature.
Conclusions
This cost-effectiveness model, adapted to the Spanish setting, showed that defibrotide is a cost-effective alternative to BSC alone in patients with severe VOD/SOS post-HCT.
Introduction
According to the World Health Organization, >50,000 hematopoietic cell transplantation (HCT) procedures are performed annually, worldwide, with the number increasing each yearCitation1. HCT has become a standard treatment for a variety of nonmalignant and malignant diseases, but it is expensive and can also result in significant complicationsCitation2,Citation3. Post-HCT complications, such as veno-occlusive disease or sinusoidal obstructive syndrome (VOD/SOS) or graft-versus-host disease, are major contributors to the high costs of HCT following transplantationCitation4. VOD/SOS, an unpredictable and potentially life-threatening condition, is one of the primary causes of HCT-related mortalityCitation5,Citation6. Without treatment, patients with severe VOD/SOS may die within days or weeks of an expensive transplant procedure, often in an intensive care settingCitation7. VOD/SOS is estimated to have an average incidence of 14%, but incidence of VOD/SOS has been reported to be as high as 40% in high-risk patientsCitation6,Citation8. A variety of factors can impact the risk of developing VOD/SOS, including conditioning regimen, type of HCT, age, and preexisting liver diseaseCitation9.
Although VOD/SOS pathophysiology is not fully understood, pathogenesis is initiated by activation of sinusoidal endothelial cells and damage to hepatocytes caused by the buildup of toxic metabolites generated by HCT conditioning regimensCitation10. These insults induce a procoagulant, proinflammatory, and antifibrinolytic environment that results in loss of endothelial cell structure and subsequent sinusoidal narrowing and obstructionCitation5.
Clinically, VOD/SOS is characterized by hepatomegaly, jaundice, weight gain, and ascites. Severity of VOD/SOS ranges from mild disease to severe disease associated with multiorgan dysfunction (MOD)Citation6. Severe VOD/SOS with MOD has been associated with a mortality rate >80% if treated with supportive care alone. Therefore, there is a particular interest in effective approaches to prevent and/or treat severe VOD/SOSCitation5.
Several symptomatic measures, such as oxygen therapy, analgesia, paracentesis, and thoracentesis, as appropriate, can be used to reduce discomfort caused by massive ascites or pleural effusionsCitation11. In cases of suspected VOD/SOS, therapeutic interventions, such as optimizing hydroelectrolytic balance and restricting the supply of sodium by diuretics or using hemodialysis or hemofiltration when fluid accumulation and renal failure cannot be controlled, may be used. In some cases, VOD/SOS has been resolved with an intrahepatic portosystemic shunt or, more rarely, with liver transplantationCitation12. In addition, defibrotide, a mixture of predominantly single-stranded polydeoxyribonucleotides, is approved in Europe for the treatment of severe hepatic VOD/SOS following HCT in adults and pediatric patients aged >1 monthCitation13. In vitro studies suggest that the mechanism of action of defibrotide involves two distinct elements: the protection of endothelial cells and the restoration of thrombotic-fibrinolytic balanceCitation13. The efficacy and safety of defibrotide for the treatment of VOD/SOS post-HCT have been demonstrated in a number of studiesCitation14–17. In a phase 3 study of defibrotide in patients with VOD/SOS with MOD, day 100 survival was 38% in defibrotide-treated patients and 25% in historical controls (23% estimated difference between groups; 95.1% confidence interval [CI] = 5.2–40.8; p = 0.0109, using a propensity-adjusted analysis)Citation14.
Defibrotide is designated as an orphan medicine by the European Medicines Agency (EMA) and was approved under exceptional circumstances due to the rarity of hepatic VOD/SOSCitation13 and, thus, collection and evaluation of additional data on efficacy and safety are ongoingCitation18. In the US, defibrotide received orphan drug status and FDA approval based on the results of two prospective trials and an expanded access studyCitation19.
Although the safety and efficacy of defibrotide in patients with VOD/SOS post-HCT is established, data on cost-effectiveness of defibrotide in Europe is needed in order to inform the health technology assessment process. This study sought to determine the cost-effectiveness of defibrotide for the treatment of patients who develop severe VOD/SOS (defined as VOD/SOS with MOD) post-HCT in the Spanish setting.
Methods
Model structure
A Markov model, with an acute phase (until year 1, with daily cycles) and a long-term phase (from year 1 to the end of time horizon, with annual cycles), was adapted to the Spanish settingCitation20. To ensure that the adaptation of the model fit the current clinical practice in Spain, all the inputs and assumptions were validated by a panel of six experts.
The acute phase consisted of three health states: severe VOD, complete response (CR), and death. The long-term phase had two states, since the severe VOD and CR states were merged into the ‘survival state’ ().
Figure 1. Model diagram. Abbreviations. VOD/SOS, veno-occlusive disease/sinusoidal obstruction syndrome; MOD, multiorgan dysfunction; CR, complete response.
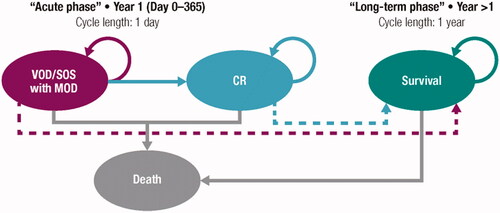
The time horizon of the analysis included the entire life of the patient (lifetime) with a theoretical maximum of 100 years (99.8% of the patients would have died). A discount rate of 3% was applied for both costs and future effects, in accordance with published recommendations on the evaluation of health technologies in SpainCitation21,Citation22.
The model compared estimated costs and health outcomes for defibrotide vs best supportive care (BSC) alone. The results of the cost-effectiveness analysis were expressed using the incremental cost-effectiveness ratio (ICER) and incremental cost-utility ratio (ICUR) of defibrotide vs BSC alone.
Study population and compared alternatives
The study population of the analysis replicated the cohort included in the phase 3 clinical study of defibrotide. The study included 102 patients (44 children and 58 adults; mean age of 26 years) with severe VOD/SOS who received 25 mg/kg of defibrotide (in addition to BSC) compared with 32 historical controls (identified from the clinical records of 6,867 HCT patients who received BSC alone). Severe VOD/SOS was defined as VOD/SOS with MOD (renal and/or pulmonary dysfunction by day 28 post-HCT)Citation14. In the phase 3 study, efficacy analyses were based on propensity score-adjusted estimators; propensity scores provided an adjustment for prognostic factors that may have been unbalanced between treatment groups.
Efficacy parameters
The Markov model used transition probabilities calculated using raw data from the phase 3 clinical study on overall survival (OS) and probability of reaching CR.
The Kaplan-Meier (KM) curves for CR and OS were both extrapolated to day 365 to complete the time horizon for the acute phase. Subsequently, all patients who either reached CR or were still alive at day 365 entered the ‘survival state’ of the long-term phase.
Acute phase
For the acute phase, an area under the curve approach was used to model the transition between health states based on parametric curves and assuming that patients who reached CR did not have a subsequent recurrence of severe VOD/SOS.
Survival data from the phase 3 study were available up to days 150 and 94 for patients treated with defibrotide and BSC, respectively. A semiparametric approach was found to be the most appropriate method to respond to the plateau observed in the KM curves from day 100 to 150 and from day 69 to 94 in the defibrotide and control arms, respectively. Therefore, the available KM data were incorporated into the noted ‘plateau’ of the curves of each treatment arm, and the remaining time until day 365 was extrapolated using an exponential function and the hazard ratio (HR) for the ‘plateau’ of each survival curve (HR = −0.0012 for defibrotide and historical controls; Supplementary Figure S1).
In the phase 3 study, data on CR were available up to days 137 and 97 for patients treated with defibrotide and BSC, respectively. Similar to the survival data, a ‘plateau’ in the time to CR was observed from day 101 to 137 in the defibrotide arm and from day 50 to 97 for the control arm. In order to calculate the time-dependent cycle probabilities of achieving CR, the KM curve of ‘1 – CR’ from the phase 3 study was scanned and inverted (Supplementary Figure S2).
The KM curves for ‘1 ‒ CR’ only included patients who reached CR and were alive at the end of the study and did not account for the OS rate. Therefore, the ‘1 ‒ CR’ curve was adjusted to incorporate OS data and ensure that the proportion of responding patients did not exceed the proportion of surviving patients. As an example of the adjustment, the proportion of patients with a CR by day 100 was 23.54% and the proportion of survivors by day 100 was 37.89%, so the ratio used to adjust the CR curve (for the example at day 100) was: 23.54% × 37.89% = 9.92%. Since data were only available for day 100, this ratio was used throughout the acute phase, which spanned from day 1 to day 365. These adjustments ensured that the proportion of patients with a CR at day 100 was consistent with the results of the phase 3 study.
Long-term phase
A literature review was performed to develop an estimate of the life expectancy of patients who recovered from VOD/SOS post-HCT. The literature review identified a study published in 2011 by Remberger et al., which evaluated survival in 953 patients who underwent allogeneic HCT during four different time periods (1992–1995, 1996–2000, 2001–2005, and 2006–2009)Citation23. In order to capture any recent improvements post-HCT while also capturing data from the longest period of observation, the survival curves of the first (1992–1995) and the last period (2006–2009) of the study were combined into a single curve and used to model the first 9 years of the long-term phase (year 2 to year 10).
For the following years (year 11 to end of life), Spain’s general population survival data (Instituto Nacional de Estadística 2019) were used. However, patients who have undergone HCT tend to have a shorter life expectancy compared to the general population. To account for this, the panel of experts agreed to adjust the general population survival data using the relative survival of 0.995 reported by Nivison-Smith et al.Citation24.
Safety parameters
It was assumed that treatment-related adverse events (AEs) did not develop after a patient was discharged from the hospital. Thus, treatment-related AEs are reflected in the hospital stay and were not considered to contribute to an extension of the hospital stay or additional costs. Therefore, the panel of experts agreed not to include AEs in the model.
Cost inputs
The analysis was carried out from the perspective of the Spanish Health System, so only direct health costs were considered (these included pharmacologic costs and costs of extended hospital stay due to severe VOD/SOS). All costs were expressed in Euros for the year 2019.
Pharmacologic costs of defibrotide were calculated based on an ex-factory price of €426, considering a mean weight of 53.7 kg based on data from the phase 3 study and, for the base case, assumed optimization of defibrotide vials. Based on prevailing clinical practice in Spain, the panel of experts determined the mean duration of defibrotide treatment was 17.5 (range = 14–21) days. Administration costs were not considered separately but were included in the cost of hospital stay since defibrotide is administered during the management of severe VOD/SOS.
To estimate costs associated with extended hospital stay due to the development of severe VOD/SOS, the average length of stay (LoS) of patients undergoing HCT was established. LoS was obtained from the Spanish National Hospital Discharge Database (SNHDD) for groups related to the diagnoses 803 (allogeneic HCT) and 804 (autologous HCT) and weighted by VOD/SOS incidence since VOD/SOS is more frequently associated with allogeneic HCT than autologous HCTCitation25. An average LoS of 38.83 days was established for patients undergoing HCT. Secondly, extended hospital stay costs due to severe VOD/SOS were calculated based on the time to CR reported in the phase 3 study; mean time to CR was 46.3 and 62.0 days in the defibrotide and historical control groups, respectivelyCitation7,Citation14. Extended hospital stay due to severe VOD/SOS was defined as the difference between the mean LoS due to HCT and the time to CR for the corresponding treatment group (). shows the calculated values for extended hospital stay due to severe VOD/SOS (7.5 and 23.2 days in the defibrotide and BSC arms, respectively).
Figure 2. Use of resources, unit costs, and cost per cycle for the health states of the model. Abbreviations. HCT, hematopoietic cell transplantation; VOD/SOS, veno-occlusive disease/sinusoidal obstruction syndrome; CR, complete response; BSC, best supportive care.
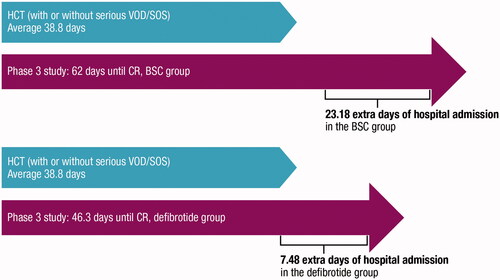
Table 1. Summary of the costs associated with extended hospital stay due to severe VOD/SOS.
Finally, the cost associated with extended hospital stay due to severe VOD/SOS was estimated based on the time spent in each hospital unit. The panel of experts considered the proportion of time spent in the intensive care unit (ICU) and transplant unit (recovery ward) to be different for patients treated with BSC vs defibrotide. The panel estimated that patients treated with BSC spent 60% (sensitivity analysis range = 40–80%) of their time in the ICU and 40% (sensitivity analysis range = 20–60%) of their time in the transplant unit, while defibrotide-treated patients were estimated to spend 30% (sensitivity analysis range = 20–40%) and 70% (sensitivity analysis range = 60–80%) of their time in the ICU and transplant units, respectively.
Based on these proportions and the unit costs per day obtained from the SNHDDCitation25, the costs per extended hospital stay due to severe VOD/SOS were calculated ().
Utilities
For the cost-effectiveness analysis, the literature was searched for utility values; these values represent the impact on quality-of-life associated with a particular health state on a scale of 0 (death) to 1 (perfect health). However, no utilities data specific to patients with VOD/SOS post-HCT were identified. Therefore, utility values from studies with patient populations that approximate the VOD/SOS post-HCT population were used.
In a study of patients with hepatic insufficiency prior to liver transplantation with Model for End-Stage Liver Disease >30, a population with comorbidities and a survival expectancy similar to patients with severe VOD/SOS, a utility of 0.208 was reportedCitation26. Hence, this utility value was used to approximate the severe VOD/SOS health state. For patients with severe VOD/SOS who reached CR, it was assumed that there was no continuing impact on quality-of-life after resolution of VOD/SOS; thus, the utility would be similar to that of the general population (adjusted for age). In the absence of Spanish data, the age-dependent utilities reported in Szende et al.Citation27 were used. summarizes the utility values included in the model.
Table 2. Utility values.
Sensitivity analysis
Both deterministic and probabilistic sensitivity analyses were performed in order to assess the uncertainty of the variables used in the model and determine the robustness of the results.
In the scenario analyses, alternatives to the base-case analysis were proposed. Modified variables included time horizon (70, 50, 30, or 10 years vs a lifetime horizon), pharmacologic costs (considering vial wastage vs vial sharing), extrapolation of KM data for OS (exponential [HR from the last observation; HR = −0.0075 for defibrotide and HR = −0.0147 for controls] vs the semiparametric approach), long-term survival adjustment (no adjustment or adjustment per Goldman et al. [HR = 2.5; 95% CI = 1.3–3.7]Citation28 vs Nivison-Smith et al.Citation24) and utilities (constant over time vs decreasing with age).
In addition, a one-way sensitivity analysis was performed, in which some variables were individually modified to minimum or maximum values (). Minimum and maximum values were obtained based on standard deviation (SD) or standard error (SE) and, in cases where SD/SE was not available, a variation of ±20% (in unit costs) was applied or extreme ranges were assumed, as in the cost per day of extended LoS due to severe VOD/SOS (100% ICU cost and 100% transplant unit cost).
Table 3. Univariate sensitivity analysis parameters.
In the probabilistic sensitivity analysis, 1,000 simulations were carried out using the Monte Carlo method and were found to be consistent with the recommendations of the literatureCitation29. Costs were assumed to follow a generalized gamma distribution and, for the parameters related to patients or treatment, a normal distribution was selected. For utilities and for variables with probabilities, a beta distribution was used since these were limited to a range of 0–1.
Results
Base case
The results of the cost-effectiveness analysis for the base case are shown in . The deterministic results of the base case show that the use of defibrotide provided more life-years (LYs) and quality-adjusted LYs (QALYs) than BSC alone (+1.348 LYs and +1.214 QALYs for a lifetime horizon) at a higher cost (+€33,708 for a lifetime horizon). The ICER and ICUR were both <€30,000 per LY and per QALY, the efficiency threshold commonly accepted in SpainCitation30,Citation31.
Table 4. Results of the base case.
Sensitivity analyses
Deterministic sensitivity analysis
shows the results of the scenario analysis, including the ICER and ICUR resulting from the comparison of defibrotide vs BSC alone. The cost-effectiveness threshold of €30,000/QALY was not exceeded in any of these scenarios. The results of the one-way sensitivity analysis are represented by a tornado diagram, showing the impact of the minimum and maximum values of each variable on the ICUR of the base case (). Variation on the extended hospital stay showed the greatest impact on the results of the base case.
Figure 3. Results of the univariate analysis: tornado diagram. Abbreviations. VOD/SOS, veno-occlusive disease/sinusoidal obstruction syndrome; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year.
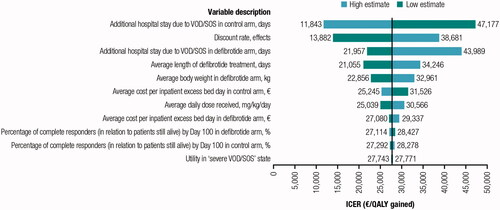
Table 5. Results of deterministic analysis.
Probabilistic sensitivity analysis
The mean ICER (expressed in QALYs) obtained from 1,000 simulations was 1.215 (95% CI = 1.180–1.252), and the mean incremental cost was €33,821 (95% CI = €16,845–€49,553). The resultant mean ICUR was €27,829/QALY, which was in line with the base-case results.
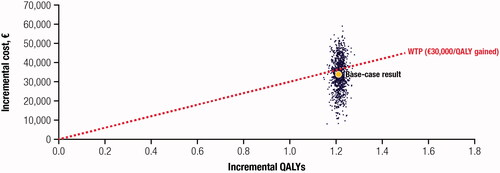
shows the incremental results of defibrotide vs BSC alone using a cost-effectiveness plane; 61.9% of the simulations fell under the willingness-to-pay threshold of €30,000/QALYCitation32. All simulations were located in the first quadrant, representing the higher cost and greater efficiency for defibrotide.
Discussion
Given the poor outcome associated with severe VOD/SOS, the survival benefit provided by defibrotide treatment for VOD/SOS patients fills a significant need for the treatment of this rare but frequently fatal complication in patients who receive HCTCitation14. For this reason, it is important to analyze the cost-effectiveness of defibrotide for the treatment of severe VOD/SOS.
The results of this analysis demonstrate that defibrotide treatment in patients who develop severe VOD/SOS after HCT is a cost-effective option vs BSC alone. The higher cost associated with defibrotide (+€33,708) occurred exclusively at the beginning of the acute phase and, as demonstrated by the results of the model, the long-term benefits (+1.348 LYs and +1.214 QALYs) offset the incremental costs. The cost-effectiveness ratios obtained were below the cost-effectiveness threshold of €30,000/QALY commonly used in SpainCitation32. It should also be noted that the cost of defibrotide therapy, relative to the overall costs of transplantation and of managing HCT patients, especially those with post-HCT complicationsCitation33, is low. Indeed, it has been estimated that post-HCT VOD/SOS can increase hospital and ICU costs by up to 3-foldCitation34.
The economic evaluations of defibrotide published in Spain are sparse. In Spain, in a retrospective cohort study of adults diagnosed with VOD/SOS after HCT, the cost-effectiveness of defibrotide was calculated using the median treatment-related cost and the median OS, without any long-term modeling techniques. The median-based cost-effectiveness ratio was €800/life-day gainedCitation35. Based on a retrospective hospital database analysis, Veenstra et al.Citation36 estimated the budget impact and cost-effectiveness of introducing defibrotide to a transplant center in the US. The reported ICER was $47,736/QALY gained, with an 88% probability of defibrotide being cost-effective at a $100,000/QALY threshold. In summary, this analysis, which uses a Markov model, based, in part, on prospective data and which considered long-term effects to determine the cost-effectiveness of defibrotide for the treatment of severe VOD/SOS post-HCT in Spain, appears to be unique.
In this study, the main efficacy variables were obtained from the phase 3 studyCitation14. Consistent with previous clinical studies of defibrotide, the efficacy endpoints evaluated in the phase 3 study demonstrated that defibrotide use in patients with severe VOD/SOS and MOD post-HCT was associated with a clinically significant improvement in day 100 survival and CR ratesCitation14,Citation37.
It should be noted that there were some limitations inherent to the design of the phase 3 study. While patients in the defibrotide treatment arm were enrolled prospectively, the historical controls were selected by an independent, blinded medical review committee through a retrospective review of patient recordsCitation14. Although use of a historical control group provides a feasible mechanism for comparison when a randomized controlled group is not possible for ethical issues, the different methodologies for identifying patients is a potential source of variation between the treatment groups, and the number of patients in the historical control group was small. Since data from the phase 3 study were used to build the model presented here, these limitations should be considered when evaluating this cost-effectiveness model. However, it should be noted that, in the case of the phase 3 data, the records for the historical control group were screened from the same medical centers that enrolled the defibrotide-treated group, with minimal temporal disparity between the historical and treatment populations, in order to limit potential variation.
It should be emphasized that the cost of defibrotide could be affected by a number of variables, including mean treatment length, average body weight, and vial wastage. Furthermore, based on guidance from a panel of experts, this study assumed a treatment duration that was shorter (17.5 [range = 14–21] days) than the median of 21.5 (range = 1–58) days observed in the phase 3 study or the 21-day duration of therapy recommended in the summary of product characteristicsCitation13,Citation14.
Finally, the sensitivity analysis showed that the results of the base case were robust because the cost-effectiveness threshold of €30,000/QALY was not exceeded in any of the given scenarios, showing that defibrotide is a cost-effective alternative in patients experiencing severe VOD/SOS when compared with BSC aloneCitation30–32.
Conclusions
In brief, this economic analysis demonstrated that defibrotide therapy provides a substantial QALYs gained for patients with severe VOD/SOS (defined as VOD/SOS with MOD), making defibrotide a cost-effective treatment in this setting despite its higher cost in the acute phase.
Transparency
Declaration of funding
This study was supported by Jazz Pharmaceuticals.
Declaration of financial/other interests
DCR and AV are employees of Hygeia Consulting SL; Hygeia received funding from Jazz Pharmaceuticals to conduct this analysis. ACR has served in an advisory role for Jazz Pharmaceuticals. MGV has consulted for Jazz Pharmaceuticals. GGG has received grants from Jazz Pharmaceuticals, Pfizer, and Gilead. MCH is an employee of and holds stock ownership and/or stock options in Jazz Pharmaceuticals. TAU, EGT, and ARG have no conflicts to disclose.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Previous presentations
These data were previously presented, in part, at the 46th Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), held virtually from 29 August to 1 September 2020.
Supplemental Material
Download MS Word (137.7 KB)Acknowledgements
Medical writing and editorial assistance were provided by Erica Chevalier-Larsen, PhD, CMPP, of SciFluent Communications, Inc., and were financially supported by Jazz Pharmaceuticals.
Data availability statement
Data that support the findings of this study are openly available in Ministerio de Sanidad, Consumo y Bienestar Social - Portal Estadístico del SNS - Registro de Altas de los Hospitales Generales del Sistema Nacional de Salud at https://www.mscbs.gob.es/estadEstudios/estadisticas/cmbd.htm and from the literature cited in References. Additional data that support the findings of this study are available from the corresponding author, DCR, upon reasonable request.
References
- World Health Organization. Haematopoietic stem cell transplantation HSCtx. 2020. Available from: https://www.who.int/transplantation/hsctx/en/.
- Cheuk DK. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: prophylaxis and treatment controversies. World J Transplant. 2012;2(2):27–34.
- Carreras E. Veno-occlusive disease of the liver after hemopoietic cell transplantation. Eur J Haematol. 2000;64(5):281–291.
- Svahn BM, Remberger M, Alvin O, et al. Increased costs after allogeneic haematopoietic SCT are associated with major complications and re-transplantation. Bone Marrow Transplant. 2012;47(5):706–715.
- Carreras E. Early Complications after HSCT. In: The EBMT handbook. 6th ed. European School of Haematology. Cham (Switzerland): Springer Nature Switzerland AG; 2012. p. 177–195.
- Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16(2):157–168.
- NHS England Clinical Commissioning. Clinical commissioning policy: use of defibrotide in severe veno-occlusive disease following stem cell transplant; 2015. Available from: https://www.england.nhs.uk/wp-content/uploads/2020/11/Defibrotide-in-severe-veno-occlusive-disease-following-stem-cell-transplant.pdf
- Corbacioglu S, Carreras E, Ansari M, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018;53(2):138–145.
- Carreras E, Diaz-Ricart M, et al. Early complications of endothelial origin. In: Carreras E, Dufour C, Mohty M, editors. The EBMT handbook: hematopoietic stem cell transplantation and cellular therapies. Cham (Switzerland): Springer Nature Switzerland AG; 2019. p. 315–322.
- Dalle JH, Giralt SA. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: risk factors and stratification, prophylaxis, and treatment. Biol Blood Marrow Transplant. 2016;22(3):400–409.
- Wallhult E, Kenyon M, Liptrott S, et al. Management of veno-occlusive disease: the multidisciplinary approach to care. Eur J Haematol. 2017;98(4):322–329.
- Carreras E. [Prevention and treatment of hepatic veno-occlusive disease]. Gastroenterol Hepatol. 2011;34(9):635–640.
- Defitelio. Defitelio. Summary of product characteristics. 2019 [cited 2019 Apr 2]. Available from: https://pp.jazzpharma.com/pi/defitelio.gb.SPC.pdf
- Richardson PG, Riches ML, Kernan NA, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127(13):1656–1665.
- Richardson PG, Soiffer RJ, Antin JH, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16(7):1005–1017.
- Kernan NA, Grupp S, Smith AR, et al. Final results from a defibrotide treatment-IND study for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Br J Haematol. 2018;181(6):816–827.
- Corbacioglu S, Carreras E, Mohty M, et al. Defibrotide for the treatment of hepatic veno-occlusive disease: final results from the international compassionate-use program. Biol Blood Marrow Transplant. 2016;22(10):1874–1882.
- European Medicines Agency. Defitelio EPAR public assessment report; 2013. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/defitelio
- FDA. FDA approves first treatment for rare disease in patients who receive stem cell transplant from blood or bone marrow; 2016 [cited 2020 May 6]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-rare-disease-patients-who-receive-stem-cell-transplant-blood-or-%E2%80%A61/3.
- Belsey J, Kemadjou EN, Isaila M, et al. Defibrotide cost-effectiveness in Canada for the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) with multi-organ dysfunction (MOD) after HSCT. Biol Blood Marrow Transplant. 2019;25:S100–S289.
- Lopez Bastida J, Oliva J, Antonanzas F, et al. [A proposed guideline for economic evaluation of health technologies]. Gac Sanit. 2010;24(2):154–170.
- Puig-Junoy J, Oliva Moreno J, Trapero Bertran M, et al. Guia y recomendaciones para la realizacion y presentacion de evaluaciones economicas y analisis de impacto presupuestario de medicamentos en el ambito del Catsalut; 2014. Available from: https://catsalut.gencat.cat/web/.content/minisite/catsalut/proveidors_professionals/medicaments_farmacia/farmaeconomica/caeip/gaeip_publica_castellano_octubre2014_catsalut.pdf
- Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant. 2011;17(11):1688–1697.
- Nivison-Smith I, Simpson JM, Dodds AJ, et al. Relative survival of long-term hematopoietic cell transplant recipients approaches general population rates. Biol Blood Marrow Transplant. 2009;15(10):1323–1330.
- Ministerio de Sanidad, Consumo y Bienestar Social. Portal Estadístico del SNS – Registro de Altas de los Hospitales Generales del Sistema Nacional de Salud. CMBD. Norma Estatal [2020 Nov 2]. Available from: https://www.mscbs.gob.es/estadEstudios/estadisticas/cmbd.htm.
- Aberg F, Maklin S, Rasanen P, et al. Cost of a quality-adjusted life year in liver transplantation: the influence of the indication and the model for end-stage liver disease score. Liver Transpl. 2011;17(11):1333–1343.
- Szende A, Janssen B, Cabases J, editors. Self-reported population health: an international perspective based on EQ-5D. 2014. Available from: https://www.springer.com/gp/book/9789400775954
- Goldman JM, Majhail NS, Klein JP, et al. Relapse and late mortality in 5-year survivors of myeloablative allogeneic hematopoietic cell transplantation for chronic myeloid leukemia in first chronic phase. J Clin Oncol. 2010;28(11):1888–1895.
- Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17(5):479–500.
- Sacristan JA, Oliva J, Del Llano J, et al. Que es una tecnologia sanitaria eficiente en Espana. Gac Sanit. 2002;16(4):334–343.
- Vallejo-Torres L, Garcia-Lorenzo B, Serrano-Aguilar P. Estimating a cost-effectiveness threshold for the Spanish NHS. Health Econ. 2018;27(4):746–761.
- Sacristan JA, Rovira J, Ortun V, et al. Utilization of economic assessments of health interventions. Med Clin (Barc). 2004;122(20):789–795.
- Perales MA, Bonafede M, Cai Q, et al. Real-world economic burden associated with transplantation-related complications. Biol Blood Marrow Transplant. 2017;23(10):1788–1794.
- Cao Z, Villa KF, Lipkin CB, et al. Burden of illness associated with sinusoidal obstruction syndrome/veno-occlusive disease in patients with hematopoietic stem cell transplantation. J Med Econ. 2017;20(8):871–883.
- Lucas-Alcahuz B, Calpe-Armero P, Ferriols-Lisart R, et al. Cost-effectiveness analysis of treatment with defibrotide for patients with severe sinusoidal obstruction syndrome. Eur J Clin Pharm Atención Farm. 2019;21(1):6–12.
- Veenstra DL, Guzauskas GF, Villa KF, et al. The budget impact and cost-effectiveness of defibrotide for treatment of veno-occlusive disease with multi-organ dysfunction in patients post-hematopoietic stem cell transplant. J Med Econ. 2017;20(5):453–463.
- Richardson P, Aggarwal S, Topaloglu O, et al. Systematic review of defibrotide studies in the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS). Bone Marrow Transplant. 2019;54(12):1951–1962.