Abstract
Aims
To examine the impact of active only (A) vs. combined passive and active (PA) hemostatic products on bleeding-related complications and costs among inpatient surgeries.
Materials and Methods
This retrospective analysis of the US Premier Hospital Database included patients who had an inpatient procedure within a specialty of interest (cardiac, vascular, noncardiac thoracic, solid organ, general, reproductive organ, knee/hip replacement, spinal, or neurosurgery) that utilized a hemostatic product from January 1, 2017 to December 31, 2018. Patients were directly matched 1:1 on surgery code, age categories, and Charlson Comorbidity Index score categories into A or PA cohorts. Unadjusted and adjusted rates of bleeding-related complications, length of stay (LOS) and total hospital costs were compared between cohorts.
Results
A total of 5,934 cardiac, 7,986 vascular, 2,042 noncardiac thoracic, 8,260 solid organ, 9,502 general, 4,616 reproductive organ, 2,758 knee/hip replacement, 42,648 spinal, and 10,716 neuro surgeries were included. Higher unadjusted rates of bleeding-related complications and greater LOS and total hospital costs were observed in the PA cohort vs A cohort across all specialties. The adjusted odds of bleeding complications were significantly higher in solid organ, general, knee/hip replacement, reproductive organ, and spinal surgery (OR range = 1.17–2.48, all p <.01), while incremental costs per hospitalization associated with PA (vs A) controlling for covariates were higher across all specialties (ratio range = 1.04–1.22, all p <.05).
Limitations
This analysis focused on patients who had a single surgery during the hospital encounter; results may not be generalizable to patients undergoing multiple surgeries.
Conclusions
The use of A hemostatic products was associated with significantly lower rates of bleeding-related complications and total hospital costs compared to PA hemostatic products. A treatment approach which considers bleeding-related factors including severity, risk and variability based on surgery type may provide guidance in choosing the optimal hemostatic product to improve surgical outcomes and costs.
Introduction
Effective hemostasis as part of a blood management strategy is a critical requirement for patients undergoing surgery. The delay and/or the inability to achieve hemostasis can lead to excessive bleeding, thereby complicating surgery and increasing the risk of morbidity and mortalityCitation1–3. By effectively controlling peri-operative bleeding, the need for allogenic or autologous blood transfusions as well as the risks associated with transfusions are reducedCitation4–6. Lower risk of blood transfusions equates to significantly shorter hospital length of stay (LOS)Citation7,Citation8. Importantly, preventing excessive intraoperative blood loss has been shown to significantly decrease the risk of major perioperative complicationsCitation4,Citation9,Citation10. This decreased risk of major perioperative complications may translate into reduced surgery time, shorter intensive care unit (ICU) LOS and overall LOS which in turn may result in potential savings in overall hospital costsCitation3,Citation5,Citation6.
Primary methods of surgical hemostasis include sutures, clips, and a wide variety of electrosurgical devices. Adjunctive topical hemostatic products are used in addition to primary methods to achieve hemostasisCitation11–14. Hemostatic products that are widely used today have extensive evidence of effective and safe use across a wide variety of surgeries and grade of bleedingCitation15–17. Hemostatic products have been classified in different ways based on their mechanism of action. One of the most widely used classifications for hemostatic products is based on whether the product provides a physical structure around which platelets can aggregate so a clot can form (passive) or whether the product delivers their mechanism of action on the clotting cascade in a biologically active manner (active). Passive products (e.g. oxidized cellulose, collagen, gelatin, etc.), or mechanical hemostats, provide a physical structure for platelet activation and clot formation. Further, they rely on the patient’s ability to generate clotting factors. Passive products are most effective for minimal bleeding scenarios and are appropriate for patients who have an adequately functioning coagulation cascade. Active products (e.g. topical thrombin, fibrin sealants, advanced patches, etc.) participate at the end of the coagulation cascade and bypass the initial steps of the clotting cascade. Thus, other aspects of the coagulation cascade can be dysfunctional without impacting product efficacy. Patients with coagulopathies from clotting factors, other than hypofibrinogenemia, platelet dysfunction, or those receiving antithrombotic medications may be ideal candidates for active hemostatsCitation15,Citation18,Citation19. Details on the classification of hemostatic products assessed in this study are listed in .
Table 1. Brand name and generic name of active and passive hemostatic products used during surgery.
Despite the growing evidence of the significant clinical and economic outcome benefits of active productsCitation13,Citation20–22, most surgeons typically employ an approach where a passive product is universally utilized as the first choice. This pattern of passive first use is often repeated multiple times at the same bleeding site regardless of the ineffectiveness of the hemostat. Thus, the objective of this analysis was to identify the most beneficial clinical and economic treatment approach to treat intraoperative bleeding during surgery through the comparison of active product use only vs combination passive then active product use approach.
Methods and materials
Data source
This retrospective observational analysis utilized the Premier US Perspective Hospital Database (PHD), a nationally representative hospital administrative database that has captured >8 million inpatient admissions per year from >600 hospitals (>25% of annual US inpatient admissions) since 2012. All data analyzed were de-identified and in compliance with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA).This analysis using the Premier US PHD did not meet the criteria for human subject research and was not subject to Institutional Review Board approval.
Study population
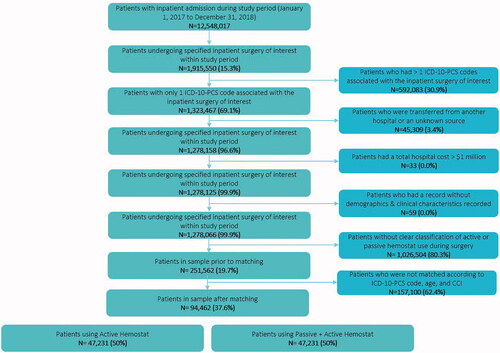
Patients undergoing an inpatient surgery of interest (i.e. cardiac, vascular, noncardiac thoracic, solid organ, general, knee/hip replacement, reproductive organ, spinal and neuro surgery) during January 1, 2017 to December 31, 2018 based on ICD-10-PCS codes (see Supplemental Appendix 1) where a hemostat was used during surgery (see Supplemental Appendix 2) were initially selected for analysis. The index date for each patient was defined as the date of the first claim for the most recent hospitalization (i.e. index hospitalization) for the surgery of interest. Patients were excluded if they had >1 ICD-10-PCS code associated with the index hospitalization, were transferred from another hospital or an unknown source, had a total hospital cost >$1 million, or did not have demographics and clinical characteristics reported. For patients who had multiple procedures during the index hospitalization, it was not possible within the Premier database to determine which hemostatic product was used for each procedure, nor to associate the specific costs or bleeding complication with a specific procedure. Thus, in order to identify the association between hemostatic product type and the aforementioned outcomes for each surgery type, only surgeries with 1 ICD-10 PCS code for the index hospitalization were included in the analysis.
Patients in the active hemostat cohort (A; i.e. where only an active hemostat was utilized during the index hospitalization) and patients in the combined passive & active hemostat cohort (PA; i.e. where both passive and active hemostats were utilized during the index hospitalization) were extracted via a 1:1 direct matching procedure. The passive and active hemostatic products used during surgery are defined in . The final study sample () included patients that were matched on ICD-10-PCS code of the index hospitalization, age categories (0–17, 18–45, 46–64, and 65+) and Charlson Comorbidity Index (CCI) score categories (0–2, 3–4, 5+).
Study variables
Patient and hospital characteristics
Patient demographics (age, gender, race, geographic location of provider, and primary payer), hospital characteristics (hospital bed size, teaching hospital status, hospital location [urban or rural], and hospital length of stay) and pre-/peri-operative use of antiplatelet/anticoagulant products were evaluated during the index hospitalization while clinical characteristics (CCI score, any-cause hospitalization within 6 months prior to index hospitalization, and prior surgery) and comorbidities were evaluated during pre-index period (i.e. period starting from 12 months prior to index hospitalization to index hospitalization unless otherwise noted). CCI scores were calculated for each patient by identifying diagnoses using ICD-10-CM codes that were associated with hospitalizations occurring within the 12-month pre-index period. Higher CCI scores indicated a greater likelihood of mortality or resource use, whereas a CCI score of zero signified no comorbidityCitation23.
Key study outcomes
Occurrence of any bleeding-related complications and/or blood product transfusions and total hospital costs (i.e. total cost to treat the patient during the hospitalization, including both fixed and variable costs) were evaluated during the index hospitalization (see Supplemental Appendix 3). Total hospital costs were standardized to 2019 US Dollars using the medical care component of the Consumer Price IndexCitation25.
Statistical analysis
Statistical analyses were conducted using R version 3.6.0Citation24. Patient demographics, clinical characteristics, hospital characteristics and study outcomes were summarized descriptively using the mean (standard deviation [SD]) and median (range or minimum and maximum) for continuous variables, and ‘N’ and proportion (%) for categorical variables across study cohorts. Comparisons of patient demographics, clinical characteristics, and hospital characteristics were conducted with a t-test for continuous variables and chi-squared test for categorical variables to test for equivalency between cohorts. Unadjusted generalized estimating equations models were used to determine whether LOS, ICU LOS, and total hospital costs were significantly different between cohorts.
The odds ratio for bleeding-related complications between A (reference) and PA cohorts was calculated for all surgeries as well as by surgery type, adjusting for pre-/peri-operative use of antiplatelet/anticoagulant products, bleeding-related complications, demographics (including age, gender, and race), clinical and hospital characteristics (including geographic region, year of surgery, hospital size, teaching hospital status, admission type, and any-cause hospitalization within 6 months prior to index hospitalization), and presence of comorbidities including diabetes, obesity, COPD, cancer, liver cirrhosis, thrombocytopenia, non-MI coronary disease, deep vein thrombosis, congestive heart failure, hypertension, renal disease, and peripheral vascular disease; all comorbidities modeled as separate covariates) within a generalized estimating equations model with logit link function. Adjusted total hospital costs and the ratio of average total hospital costs between A (reference) and PA cohorts were calculated for all surgeries as well as by surgery type within a generalized estimating equations model with gamma log link function, adjusting for the same variables as the bleeding-related complications regression model. For (adjusted and unadjusted) total hospital costs, patients with $0 total hospital costs were excluded from the analysis. A p <.05 was considered statistically significant.
Results
Of the 12,548,017 patients with inpatient admission during the study period, 251,562 patients met eligibility criteria and were included in the 1:1 direct matching procedure. Of these, 157,100 patients did not have a direct match, thus a final study sample of 94,462 directly matched patients were included in the analysis (). The top 20 most reported procedures (ICD-10-PCS codes) by surgery type are provided in Supplemental Appendix 4. There were no statistically significant differences between A vs PA cohorts in terms of mean age (60.0 vs 60.0, p = .543), proportion of males (52.6% vs 52.4%, p =.658), CCI score categories (0–2: 83.0% vs 83.0%, 3–4: 13.2% vs 13.2%, 5+: 3.8% vs 3.8%, p = 1), and whether they had a prior hospitalization in the pre-index period (12.0% vs 11.9%, p = .968). Statistically significant differences in demographic and clinical characteristics between the study cohorts were found () in the distribution of race, geographic region, primary payer type, diagnosis of cancer, proportion of patients that had a prior surgery, and pre- and/or peri-operative use of antiplatelet and/or anticoagulant products within cardiac, solid organ, reproductive organ, knee/hip replacement, spinal and neuro surgeries; however, a vast majority of these characteristics may not necessarily be clinically relevant. Regarding hospital characteristics, statistically significant differences between the study cohorts were observed in the hospital size, proportion of teaching hospitals, hospital location (i.e. urban vs rural), and hospital length of stay ().
Table 2. Baseline demographics and clinical characteristics by study cohort.
Table 3. Hospital characteristics by study cohort.
Unadjusted bleeding-related complications
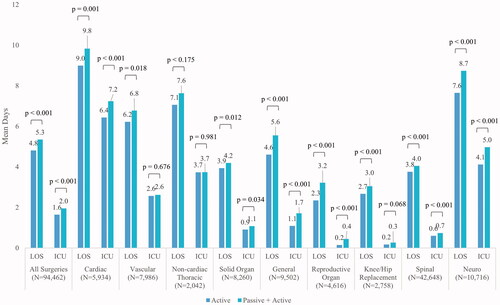
Significantly fewer patients in the A cohort had any bleeding-related complications compared to patients in the PA cohort () across all surgeries (3,705 [7.8%] vs. 4,521 [9.6%], respectively; p < .001) as well as within solid organ (310 [7.5%] vs. 384 [9.3%], respectively; p = .004), general (346 [7.3%] vs. 634 [13.3%], respectively; p <.001), reproductive organ (116 [5.0%] vs. 202 [8.8%], respectively; p < .001), knee/hip replacement (83 [6.0%] vs. 207 [15.0%], respectively; p <.001), and spinal surgery (801 [3.8%] vs. 954 [4.5%], respectively; p <.001). Patients undergoing spinal surgery exhibited the lowest rates of any bleeding-related complications (3.8% and 4.5% for A and PA, respectively), while patients undergoing cardiac surgery exhibited the highest rates of any bleeding-related complications (24.3% and 24.8% for A and PA, respectively).
Table 4. Percentage of patients with bleeding-related complications* by surgery type.
Unadjusted hospital LOS and ICU LOS
During the index hospitalization, the A cohort had shorter unadjusted mean [SD] hospital LOS (4.8 [6.8] vs 5.3 [8.1] days; p < .001) and ICU LOS (1.6 [4.6] vs 2.0 [5.5] days; p < .001) compared to the PA cohort across all surgeries as well as within each surgery type (). Patients undergoing reproductive organ surgery exhibited the shortest mean [SD] hospital LOS (2.3 [2.5] and 3.2 [5.0] days for A and PA cohorts, respectively; p <.001), while patients undergoing knee/hip replacement surgery exhibited the shortest mean [SD] ICU LOS (0.2 [1.1] and 0.3 [1.6] days for A and PA cohorts, respectively; p =.068), compared to other surgery types. In contrast, patients undergoing cardiac surgery exhibited the longest mean [SD] hospital LOS (9.0 [8.8] and 9.8 [10.4] days for A and PA cohorts, respectively; p < .001) as well as mean [SD] ICU LOS (6.4 [7.5] and 7.2 [9.3] days for A and PA cohorts, respectively; p < .001), compared to other surgery types.
Odds of bleeding-related complications and total hospital costs
Regarding the odds of bleeding-related complications, patients in the PA cohort had significantly greater odds (OR = 1.22; 95% CI: 1.16 − 1.28, p <.001) of bleeding-related complications compared to patients in the A cohort over all surgeries. Specifically, the odds of bleeding-related complications were significantly greater for the PA cohort within spinal surgery (OR = 1.17; 95% CI: 1.05 − 1.29, p =.003), solid organ (OR = 1.37; 95% CI: 1.16 − 1.63, p < .001), knee/hip replacement (OR = 1.85; 95% CI: 1.42 − 2.40, p <.001), general (OR = 2.00; 95% CI: 1.72 − 2.32, p < .001), and reproductive organ (OR = 2.48; 95% CI: 1.81 − 3.40, p < .001) (). No significant differences in the odds of a bleeding-related complication were observed between the PA and A cohorts for cardiac, vascular, noncardiac thoracic, and neuro surgery.
Table 5. Impact of active vs combined passive and active hemostatic product on odds of bleeding-related complicationsa.
Unadjusted mean (SD) total hospital costs across all surgeries were $25,606 ($26,777) and $28,293 ($32,697) for A and PA cohorts, respectively (p <.001). The adjusted mean (95% CI) total hospital costs across all surgeries were $38,295 ($37,876–$38,719) and $41,335 ($40,883–$41,792) for A and PA cohorts, respectively (p <.001). The unadjusted and adjusted total hospital costs within each surgery type was lower for the A cohort compared to the PA cohort ( and ).
Figure 3. Unadjusted mean total hospital costs by surgery type and study cohort. Patients with $0 total hospital cost were excluded from this figure; N excluded from all surgeries = 176; cardiac surgery = 9; vascular surgery = 20; Noncardiac thoracic surgery = 2; solid organ surgery = 9; general surgery = 6; reproductive organ surgery = 1; knee hip replacement surgery = 16; spinal surgery = 91; neuro surgery = 22.
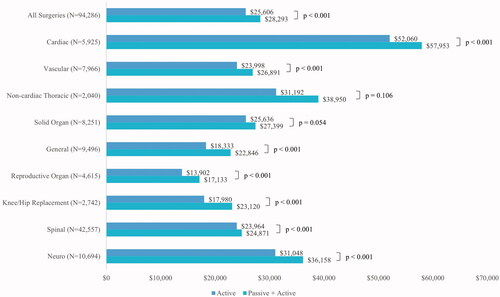
Figure 4. Adjusted mean total hospital costs by surgery type and study cohort. Patients with $0 total hospital cost were excluded from this figure; N excluded from all surgeries = 176; cardiac surgery = 9; vascular surgery = 20; Noncardiac thoracic surgery = 2; solid organ surgery = 9; general surgery = 6; reproductive organ surgery = 1; knee hip replacement surgery = 16; spinal surgery = 91; neuro surgery = 22. Total hospital costs between active vs combined passive & active hemostatic cohort are statistically significantly different in all surgeries as well as within each surgery type (all p < .05).
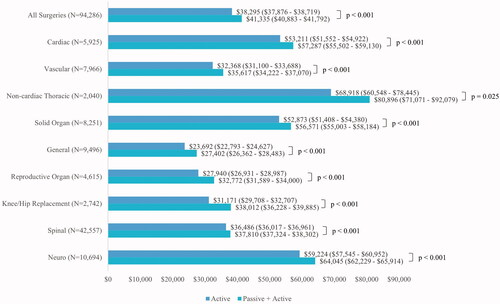
Based on the ratio of the adjusted mean total hospital costs (), patients in the PA cohort had significantly higher (OR = 1.07; 95% CI: 1.06 − 1.08, p < .001) total hospital costs compared to patients in the A cohort over all surgeries. The ratio of the adjusted mean total hospital costs was lowest amongst patients undergoing spinal surgery (OR = 1.04; 95% CI: 1.02 − 1.05, p < .001), while the ratio was as high as 1.22 (95% CI: 1.16 − 1.28, p <.001) among patients undergoing reproductive organ surgery. Within each surgical specialty, the adjusted mean total hospital costs were significantly higher for patients in the PA vs A cohorts (all p <.05).
Table 6. Impact of active vs combined passive and active hemostatic product on total hospital costsa.
Discussion
Using patient-level data from a large, nationally representative US hospital database, the present analysis aimed to address a current disconnect or lack of awareness by comparing the real-world clinical and economic outcomes in patients treated with active only vs combined passive then active hemostatic products across a wide range of surgical specialties. The results of this study found significantly improved clinical and economic outcomes among the active product cohort compared to the combined passive & active product cohort. Patients treated for bleeding with the combination of passive & active products had longer unadjusted mean hospital LOS (5.3 vs 4.8 days) and ICU LOS (2.0 vs 1.6 days), greater adjusted odds of bleeding-related complications across all surgeries (OR = 1.22, p <.001) as well as within solid organ, general, knee/hip replacement, reproductive organ, and spinal surgery (all p < .05), and greater adjusted total hospital costs, across all surgeries ($41,335 vs $38,295, p < .001) as well as within each surgery type (all p <.001) when compared to patients treated with an active agent hemostatic approach.
This study is strengthened by its use of a large national hospital database that covers a large sample size of 94,462 inpatient surgeries treated with a hemostatic product, a large number of surgeons from teaching and non-teaching hospitals from a broad geographic and demographic setting, and the utilization of a direct matching approach (i.e. 1:1 direct matching on ICD-10-PCS code of the index hospitalization, age categories, and CCI score categories) to minimize potential selection bias.
The results from this study are consistent with a retrospective database study conducted by Ramirez et al. that showed spinal surgeries utilizing a commercial active flowable topical hemostat were associated with better clinical (i.e. lower blood transfusion and blood loss complications) and economic outcomes (i.e. lower hospital LOS, reduced surgical time, and less hemostat volume) compared to surgeries utilizing a non-commercial combination of passive (e.g. gelatin and thrombin) product and active flowable topical hemostatCitation25. Specifically, patients treated with a commercial active flowable topical hemostat alone had a shorter hospital length of stay (mean difference = −0.446 days, p < .0001) and lower rates of intraoperative transfusions (1.4% vs 2.5%, p < .0001), peri-operative transfusions (1.6% vs 2.8%, p < .0001), post-operative transfusions (1.6% vs 3.0%, p <.0001), pure-blood/pBRC transfusions (2.3% vs 4.3%, p <.0001), and blood loss complications (0.5% vs 0.8%, p = .0022) compared to patients treated with a tableside combination of sheet gelatin and thrombin in addition to a commercial active flowable topical hemostat during spinal surgery. The results from our study expands upon the analysis by Ramirez et al. by examining trends across all active hemostatic products and across a range of surgery types, in addition to spinal surgeries.
Other prior studies comparing the impact of active only vs passive only hemostatic approach also demonstrated similar results; active product use during surgery was associated with lower rates of blood product utilization and blood transfusionsCitation4–6, lower rates of revisions for bleedingsCitation22, and lower rates of major perioperative complicationsCitation4,Citation9,Citation10. This current analysis builds upon prior studies to demonstrate that active hemostatic products have improved outcomes even when compared to passive and active products used in combination. Collectively, the improved outcomes demonstrated from the use of active hemostatic products are most likely due to the differences in product efficacy based on the patient’s coagulation status and effectiveness of active hemostats over a broader range of bleeding as compared to passive hemostats given the differences in the mechanism of action. Active hemostatic products act biologically at the end of the coagulation cascade thus other aspects of the coagulation cascade can be dysfunctional without significantly impairing the hemostatic efficacy. Passive hemostatic products are most effective for minimal bleeding scenarios and are appropriate for use in patients who have an adequately functioning coagulation system.
As continuous effort and emphasis is being placed on providing effective care while reducing waste in healthcare, one key area that can further provide clinical and economic benefits is effective management of intraoperative bleeding. The risk involving unresolved bleeding and extended blood loss during surgery has increased dramatically in recent decades due to the rise in the utilization of antiplatelet and anticoagulant productsCitation25. As this analysis and other recent studies have shown, achieving effective hemostasis during surgeries is a key component that leads to reduced healthcare resource utilization and decreased risk of morbidity and mortality for patients. As noted earlier, most surgeons employ an approach where a passive product is universally utilized as a first choice and used repeatedly despite the lack of effectiveness of the hemostat. There are many reasons why surgeons employ this approach of initial and repeated use of passive products including familiarity with the passive products, surgeon training and education as well as the routine opening of these products as part of the standard surgical case set up.
As no evidence-based practice guidelines are currently available to facilitate clinically informed decisions on approaches to hemostasis, certain tools or processes may be utilized to help guide surgeons on selecting the optimal hemostatic agent based on critical factors such as bleeding severity, bleeding risk and surgery type. The VIBe scaleCitation26, among others, is a validated measure to evaluate the level of bleeding severity in open surgical procedures across surgical specialtiesCitation26. Graded on a 5-point scale from 0 (no bleeding) to 4 (life threatening), this measure provides a standardized assessment of bleeding severity, which may provide the surgeon with critical information to help inform hemostatic product choice. During higher grades of bleeding, surgeons should consider an active hemostatic agent as the first approach.
Additionally, the level of bleeding risk of the patient should also be considered in choosing the optimal approach to achieving intraoperative hemostasis. Certain procedure types such as cardiacCitation27 and liver surgeryCitation13 may be associated with a higher bleeding intensity or VIBe bleeding grade. Therefore, a proposed strategy would be to introduce an active hemostat approach proactively before bleeding begins, thus an active first approach is immediately available. Conversely, in surgeries that may vary widely in bleeding frequency and intensity, and the patient has lower bleeding risk, the VIBe bleeding scale may be a useful tool to help determine the optimal hemostatic product for efficient bleed management.
Interestingly, this study reports statistically significant differences in total hospital costs within each of the surgery types examined, while the odds of bleeding-related complications were significantly different in patients undergoing certain surgeries (solid organ, general, reproductive organ, knee/hip replacement and spinal), but not others (cardiac, vascular, non-cardiac thoracic, and neuro). This particular observation may be due to the variability in bleeding (both frequency and intensity) being lower in certain surgeries (e.g. bleeding in all cardiac surgeries usually occur in high frequency and intensity) while higher levels of variability in bleeding is most likely observed in other types of surgeries (e.g. general surgeries range from low-frequency/intensity bleeding procedures such as appendectomy to high-frequency/intensity bleeding procedures such as liver resection). The data suggests that while patients undergoing cardiac, vascular, noncardiac thoracic, and neuro surgeries were likely to have similar rates of bleeding-related complications, patients immediately treated with active products as a first-line adjunctive hemostat (i.e. active product only) may have experienced more effective or sustained hemostasis that led to shorter hospital LOS as well as lower risk of complications thereafter, thus lower total hospital costs. Additional randomized controlled trials and real-world studies are needed to elucidate the exact mechanism regarding the effect of hemostatic products in those surgeries where variability in bleeding is likely to be lower (e.g. cardiac and vascular surgery) as well as to better understand the patterns of use related to combined passive then active product utilization.
Limitations to this study include its retrospective observational evaluation of claims data, which is less robust compared to prospective randomized trials. Although a direct matching approach was employed to minimize potential selection bias, other potential nonmeasured confounding factors may exist and should be acknowledged. Specifically, in-hospital or 30-day mortality was not incorporated in this analysis. A large number of surgeries were excluded due to not having a clear classification of active or passive hemostat use during surgery, which may have introduced potential selection bias. However, the large sample size of this study (94,462 surgeries) provides greater confidence in the reliability of the results and minimizes the likelihood of bias. Within the combined passive then active product cohort, the order and amount of the hemostatic product or products actually received cannot be definitively determined within this database. In the surgical setting, it is possible for the surgical staff to open both types of hemostatic products regardless of actual usage. During the 1:1 direct matching procedure, more than 50% of the potential surgeries were excluded, including those with multiple procedures during the index hospitalization, which may limit the generalizability of the findings. Finally, other clinical factors that were not controlled for include the degree of bleeding severity, the expertise/skill level of surgeon, and site variation in bleeding and transfusion requirements for commonly performed procedures, though these effects should be normalized by the large sample size.
The findings of this retrospective analysis of real-world data could be further validated through observational studies and clinical trials. Additionally, as the profile of the surgical patient has changed over the years (e.g. increased age and comorbidities) as well as the increased utilization of antiplatelet/anticoagulant products, the relationship between these important clinical factors and the use of active hemostatic products on surgical outcomes should be explored to further inform and guide surgeons on the optimal approach to hemostasis.
Conclusion
This observational, direct-matched analysis of patients undergoing surgery found that the use of active hemostatic products alone was associated with significantly lower rates of bleeding-related complications and total hospital costs compared to combined use of passive & active hemostatic products. A treatment approach which considers critical bleeding-related factors such as severity, risk and variability based on surgery type combined with the use of a standardized bleeding severity assessment tool may provide guidance to surgeons in choosing the optimal hemostatic product to improve surgical outcomes and costs.
Transparency
Declaration of funding
This analysis was sponsored by Baxter.
Declaration of financial/other interests
David Iannitti has served as a consultant to Baxter. Diane Ito, Chong Kim and Josh Epstein are current employees at Stratevi; Stratevi is a consulting firm that has received funding from Baxter to conduct the analysis of this study.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
Diane Ito, Chong Kim and Josh Epstein were involved in the conception and design of this study, analysis and interpretation of results, and drafting/revising the paper for intellectual content. David Iannitti was involved in the interpretation of the results and drafting/revising the paper for intellectual content.
Previous presentations
This study has not been previously presented.
Supplemental Material
Download MS Word (73.1 KB)Acknowledgements
None reported
Data availability statement
The data that support the findings of this study are available from Premier. Restrictions apply to the availability of these data, which were used under license for this study.
References
- Boucher BA, Traub O. Achieving hemostasis in the surgical field. Pharmacotherapy. 2009;29(7, part 2):2S–7S.
- Karkouti K, Wijeysundera DN, Yau TM, et al. The independent association of massive blood loss with mortality in cardiac surgery. Transfusion. 2004;44(10):1453–1462.
- Renkens KL, Jr Payner TD, Leipzig TJ, et al. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine (Phila Pa 1976)). 2001;26(15):1645–1650.
- Block JE. Severe blood loss during spinal reconstructive procedures: the potential usefulness of topical hemostatic agents. Med Hypotheses. 2005;65(3):617–621.
- Bochicchio G, Dunne J, Bochicchio K, et al. The combination of platelet-enriched autologous plasma with bovine collagen and thrombin decreases the need for multiple blood transfusions in trauma patients with retroperitoneal bleeding. J Trauma Acute Care Surg. 2004;56(1):76–79.
- Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: a review. J Artif Organs. 2005;8(3):137–142.
- Galas FR, Almeida JP, Fukushima JT, et al. Blood transfusion in cardiac surgery is a risk factor for increased hospital length of stay in adult patients. J Cardiothorac Surg. 2013;8(1):54.
- Slonim AD, Joseph JG, Turenne WM, et al. Blood transfusions in children: a multi-institutional analysis of practices and complications. Transfusion. 2008;48(1):73–80.
- McDonnell MF, Glassman SD, John R, Dimar I, et al. Perioperative complications of anterior procedures on the spine. JBJS. 1996;78(6):839–847.
- Carreon LY, Puno RM, Dimar JR, et al. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. JBJS. 2003;85(11):2089–2092.
- Lewis KM, Atlee HD, Mannone AJ, et al. Comparison of two gelatin and thrombin combination hemostats in a porcine liver abrasion model. J Investig Surg. 2013;26(3):141–148.
- Mozet C, Prettin C, Dietze M, et al. Use of Floseal and effects on wound healing and pain in adults undergoing tonsillectomy: randomised comparison versus electrocautery. Eur Arch Otorhinolaryngol. 2012;269(10):2247–2254.
- Nasso G, Piancone F, Bonifazi R, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg. 2009;88(5):1520–1526.
- Raga F, Sanz-Cortes M, Bonilla F, et al. Reducing blood loss at myomectomy with use of a gelatin-thrombin matrix hemostatic sealant. Fertil Steril. 2009;92(1):356–360.
- Samudrala S. Topical hemostatic agents in surgery: a surgeon's perspective. Aorn J. 2008; 88(3):S2–S11.
- Lundblad RL, Bradshaw RA, Gabriel D, et al. A review of the therapeutic uses of thrombin. Thromb Haemost. 2004;91(05):851–860.
- Shander A, Kaplan LJ, Harris MT, et al. Topical hemostatic therapy in surgery: bridging the knowledge and practice gap. J Am Coll Surg. 2014;219(3):570–579. e4.
- Danker W, III DeAnglis A, Ferko N, et al. Comparison of fibrin sealants in peripheral vascular surgery: A systematic review and network meta-analysis. Ann Med Surg. 2021;61:161–168.
- Moss R. Management of Surgical Hemostasis: An Independent Study Guide 2013. Available from: https://www.aorn.org/-/media/aorn/guidelines/tool-kits/medication-safety/management-of-surgical-hemostasis-independent-study-guide.pdf?la=en&hash=9FED3DF8BFDEF8B1C8D1899F8FC7BE79.
- Oz MC, Cosgrove DM, III Badduke BR, et al. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. Ann Thorac Surg. 2000;69(5):1376–1382.
- Maisano F, Kjaergård HK, Bauernschmitt R, et al. TachoSil surgical patch versus conventional haemostatic fleece material for control of bleeding in cardiovascular surgery: a randomised controlled trial. Eur J Cardio-Thorac Surg. 2009;36(4):708–714.
- Tackett SM, Calcaterra D, Magee G, et al. Real-world outcomes of hemostatic matrices in cardiac surgery. J Cardiothorac Vasc Anesth. 2014;28(6):1558–1565.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Clin Epidemiol. 1987;40(5):373–383.
- Team RC. R: a language and environment for statistical computing. Vienna: The R Foundation, 2017. 2020.
- Ramirez MG, Deutsch H, Khanna N, et al. FLOSEAL only versus in combination in spine surgery: A comparative, retrospective hospital database evaluation of clinical and healthcare resource outcomes. Hosp Prac. 2018;46(4):189–196.
- Lewis KM, Li Q, Jones DS, et al. Development and validation of an intraoperative bleeding severity scale for use in clinical studies of hemostatic agents. Surgery. 2017;161(3):771–781.
- Tackett SM, Sugarman R, Kreuwel HT, et al. Hospital economic impact from hemostatic matrix usage in cardiac surgery. J Med Econ. 2014;17(9):670–676.