Abstract
Aim
Multiple screening strategies are guideline-endorsed for average-risk colorectal cancer (CRC). The impact of real-world adherence rates on the cost-effectiveness of non-invasive stool-based CRC screening strategies remains undefined.
Methods
This cost-effectiveness analysis from the perspective of Medicare as a primary payer used the Colorectal Cancer and Adenoma Incidence and Mortality Microsimulation Model (CRC–AIM) to estimate cost and clinical outcomes for triennial multi-target stool DNA (mt-sDNA), annual fecal immunochemical test (FIT) and annual fecal occult blood test (FOBT) screening strategies in a simulated cohort of US adults aged 65 years, who were assumed to either be previously unscreened or initiating screening upon entry to Medicare. Reported real-world adherence rates for initial stool-based screening and colonoscopy follow up (after a positive stool test result) were defined as 71.1% and 73.0% for mt-sDNA, 42.6% and 47.0% for FIT, and 33.4% and 47.0% for FOBT, respectively. The incremental cost-effectiveness ratio using quality-adjusted life years (QALY) was defined as the primary outcome of interest; other cost and clinical outcomes were also reported in secondary analyses. Multiple sensitivity and scenario analyses were conducted.
Results
When reported real-world adherence rates were included only for initial stool-based screening, mt-sDNA was cost-effective versus FIT ($62,814/QALY) and FOBT ($39,171/QALY); mt-sDNA also yielded improved clinical outcomes. When reported real-world adherence rates were included for both initial stool-based screening and follow-up colonoscopy (when indicated), mt-sDNA was increasingly cost-effective compared to FIT and FOBT ($31,725/QALY and $28,465/QALY, respectively), with further improved clinical outcomes.
Limitations
Results are based on real-world cross-sectional adherence rates and may vary in the context of other types of settings. Only guideline-recommended stool-based strategies were considered in this analysis.
Conclusion
Comparisons of the effectiveness and benefits of specific CRC screening strategies should include both test-specific performance characteristics and real-world adherence to screening tests and, when indicated, follow-up colonoscopy.
Introduction
Colorectal cancer (CRC) is both preventable and curable; effective, population-based screening can reduce the incidence and mortality of CRCCitation1,Citation2. Nonetheless, CRC remains the second-leading cause of cancer-related mortality, accounting for 8.8% of all cancer deaths in the United States (US)Citation3. Recommended screening options for average-risk individuals include invasive tests that directly visualize CRC and precancerous lesions (e.g. colonoscopy, flexible sigmoidoscopy, computed tomography colonography) and non-invasive tests that either detect blood (e.g. fecal immunochemical test [FIT] and fecal occult blood test [FOBT]) or DNA (e.g. multi-target stool DNA [mt-sDNA]) markers associated with CRC and advanced precancerous lesions in the stoolCitation4,Citation5. As positive stool-based screening results must be followed by a colonoscopy to evaluate the presence of neoplasiaCitation4, the benefits of stool-based screening are dependent upon adherence to the completion of both the initial stool-based test and the follow-up colonoscopyCitation6,Citation7.
Previous modeling analyses have consistently demonstrated that guideline-recommended CRC screening strategies are cost-effective compared to no screeningCitation8–10. However, existing data on the relative cost-effectiveness amongst recommended stool-based screening strategies are more heterogeneous, in part due to differences in assumptions related to patient participationCitation11–14. Adherence to screening may have more impact on cost-effectiveness than the screening test strategy usedCitation15; compared with theoretical assumptions of 100% adherence to screening, the relative cost-effectiveness of screening strategies can shift based on imperfect adherence assumptionsCitation6. Previous microsimulation models have explored the impact of imperfect adherence on the relative benefits of guideline-endorsed stool-based screening strategiesCitation16, but have not estimated the impact of real-world reported adherence on cost-effectiveness.
The Colorectal Cancer and Adenoma Incidence and Mortality Microsimulation Model (CRC-AIM) was developed to model the natural sequence of adenoma detection to carcinoma progression in unscreened patients as well as to evaluate the contribution of test-related attributes, such as patient adherence to both initial screening and follow-up colonoscopy when indicatedCitation17. Insights regarding the effectiveness, rather than the efficacy, of CRC stool-based screening strategies, necessitate assumptions using reported real-world dataCitation18,Citation19. The objective of this analysis was to use the CRC-AIM model to estimate the cost-effectiveness of non-invasive stool-based screening strategies using test-specific real-world adherence data.
Methods
Overview of CRC–AIM model structure
CRC microsimulation models include both a natural history component and a screening component. The natural history component incorporates assumptions around the progression of adenomas to CRC in the unscreened population, while the screening component incorporates assumptions relevant to the screening process, such as the type of screening test used, screening frequency, test sensitivity and specificity, complications related to colonoscopies, and adherence to screening. Within this model, adenomas may grow and transition to preclinical cancer, which in turn may progress to symptomatic CRC. The screening strategies modeled then potentially detect an adenoma or preclinical CRC, as a function of their test performance and patient adherence. CRC–AIM has demonstrated substantial cross-model validity when comparing natural history, screening outputs and probability curves to those from other Cancer Intervention and Surveillance Modeling Network (CISNET) modelsCitation16,Citation17.
Model population
This analysis assumes patients are either previously unscreened or their previous screening status is unknown; thus, it is assumed that upon entry into Medicare, regardless of their previous screening status, all patients will be eligible for regular screening. Screening outcomes are simulated for an average-risk, US cohort of one million 65 year-olds (Medicare eligible years for screening), free of diagnosed CRC at age 40, and focuses on guideline-recommended, stool-based screening strategies (triennial mt-sDNA, annual FIT, annual FOBT). This population aligns with previous cost-effectiveness papers of CRC screening in Medicare beneficiariesCitation12.
Test inputs
This model focuses on guideline-endorsed non-invasive stool-based screening tests. The sensitivity and specificity of the tests are identical to those used in previous CISNET modeling analyses and those used to inform the 2016 USPSTF guideline recommendations (Supplemental Table 1)Citation20. Distinct sensitivities were used for CRC, as well as for small (1–5 mm), medium (6–9 mm), or large (≥10 mm) adenomas, across the various screening modalities. Specificity was the same regardless of adenoma size or symptomatic cancer detection. Patients with a history of adenomas of any size are assumed to undergo surveillance with colonoscopy. The time to the next surveillance colonoscopy is simulated based on findings at the last exam: three years when a large adenoma or ≥3 adenomas (any size) were detected, or 5 years if ≤2 small or medium-adenomas were detected. Adherence to surveillance colonoscopy was assumed to be 100% in all modeled scenarios.
Table 1. Reported test-specific adherence rates.
Costs inputs
Only direct medical costs are included (Supplemental Table 2). The cost of mt-sDNA was obtained from the Medicare Physician Fee ScheduleCitation21. Screening costs for FIT were obtained from a study that evaluated the cost-effectiveness of CRC screening of Medicare beneficiaries using CISNET modelsCitation12. Screening costs for FOBT and surveillance colonoscopies were sourced from an observational cohort study of outpatient colorectal test utilization rates, indications, and payments among 21 million 18–64 year-old adultsCitation22. Costs associated with colonoscopy complications were sourced from a budget impact and cost-consequence studyCitation23. CRC-related direct medical costs were stratified by stage and time since diagnosisCitation24. All costs were inflated to 2020 US dollars (USD) using the medical care component of the consumer price indexCitation25.
Table 2. Discounted clinical outcomes per 1,000 patients.
Utility inputs
Health state utilities may vary as a function of age and population; as such, baseline health state utilities used in the model were age-stratified values based on EuroQoL-5D US populationCitation26. Utility loss for CRC was stratified by care level and cancer stage (stages I–III and stage IV) and was applied per person-year of CRC care (Supplemental Table 3)Citation27.
Table 3. Discounted total costs and QALYs with associated incremental cost-effectiveness ratios.
Reported real-world adherence rates
Reported real-world screening estimates were sourced from various studies. For mt-sDNA, reported rates were taken from a cross-sectional study in a Medicare population which determined adherence over a 12-month period from order dateCitation28. No studies reporting adherence rates to FIT or FOBT in a Medicare population were identified, therefore a retrospective study reporting adherence rates within 1-year of test order was used to determine adherence for FIT and FOBTCitation29; these adherence rates are consistent with that of a meta-analysisCitation30. Rates of follow-up colonoscopy following a positive stool-based screening test were taken from a study among patients with a positive mt-sDNA or FIT testCitation18. As test-specific adherence rates for follow-up colonoscopy were not identified for FOBT, the rate was assumed to be the same as FIT.
Modeled adherence scenarios
Three scenarios were considered to evaluate the impact of reported real-world adherence on screening (): (i) Scenario 1, where 100% adherence to both stool-based screening and follow-up colonoscopy completion was assumed, similar to previous CISNET models; (ii) Scenario 2, where reported real-world adherence rates were used for initial stool-based screening, and, when indicated, 100% adherence was assumed for follow-up colonoscopy; and (iii) Scenario 3, where reported real-world adherence rates were used for both initial stool-based screening and follow-up colonoscopy when indicated. Patients who did not undergo their required follow-up colonoscopy were assumed to be non-adherent until they became symptomatic.
Cost-effectiveness analysis
Outcomes
The primary outcome was the incremental cost-effectiveness ratio (ICER) of mt-sDNA versus FIT and FOBT. In addition, clinical outcomes, presented as totals over the model lifetime per 1,000 patients, describing the benefits and harms of CRC screening were reported within each Scenario, including incidence and mortality reduction. Total lifetime CRC screening costs (which include screening, complications and CRC treatment), as well as quality-adjusted life years (QALYs) gained, were calculated.
The model used a lifetime time horizon, from the perspective of Medicare as a primary payer. All costs and outcomes were discounted at 3.0%Citation31. Though there is no official willingness-to-pay threshold (WTP) in the US, ICERs less than $100,000 per QALY are considered to provide good valueCitation32. As such, we assumed a WTP threshold of $100,000 per QALY.
Base case scenarios
The base case scenarios (Scenario 1, Scenario 2, Scenario 3) assumed fixed cross-sectional adherence rates over time, where the same yearly adherence value is assumed for all patients, resulting in all patients eventually getting screened over time.
Capped adherence over time
In order to explore the impact of fixed yearly adherence, a “capped adherence” sensitivity analysis was explored based on National Health Interview Survey (NHIS) data, which found that 62.4% of patients are adherent to CRC screening over a 10-year period. In this sensitivity analysis, a calibration factor was determined to reflect that adherence to screening within a 10-year period may not reach 100%Citation6. Using the base case of 38% adherence for colonoscopies in a given yearCitation33, screening is then offered every year to non-compliant individuals declining at a constant rate (r) each year to ensure that these individuals remain non-compliant: 38% × (1 − r)t, if colonoscopy is delayed by t years, where 0 ≤ t ≤ 10. This results in a capped adherence rate where simulated individuals who are up-to-date with CRC screening match the reported NHIS rate (62.4%)Citation34. The calibration factor was then applied to all stool-based screening strategies to simulate a capped adherence approach.
Sensitivity analyses
To account for the uncertainty around model parameters, and to test the robustness of the model, multiple sensitivity analyses were conducted. One-way sensitivity analyses were conducted for Scenario 3 by varying adherence inputs ±20% and costs and utility inputs ±10% from their base case value. Two-way sensitivity analyses for Scenario 3 were conducted by varying adherence to both stool-based screening and follow-up colonoscopy (when indicated) for mt-sDNA vs. FIT and mt-sDNA vs. FOBT. A probabilistic sensitivity analysis, where all parameters are varied simultaneously, was conducted. Threshold analyses were conducted to present the ICER for mt-sDNA vs. FIT and mt-sDNA vs. FOBT in two different scenarios. In the first scenario, the screening test adherence for both strategies was varied from 0 − 100%, while adherence to follow-up colonoscopy was held constant at 100%. In the second scenario, the adherence to the screening test was held constant at 71.1% for mt-sDNACitation28, 42.6% for FIT and 33.4% for FOBTCitation29, while the adherence to follow-up colonoscopy was varied from 0–100%.
Results
Scenario 1
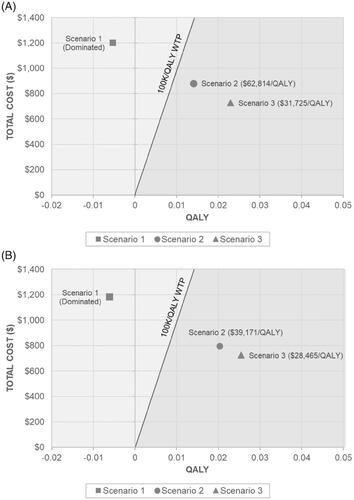
When 100% adherence is assumed for all stool-based screening strategies and follow-up colonoscopies (when indicated), the total number of screening tests per 1,000 patients was highest for FIT (6,704) and the total number of colonoscopies per 1,000 patients were highest for FOBT (1,248) (). FOBT also resulted in the highest CRC incidence reduction (46.3%) and greatest mortality reduction (55.5%) amongst the screening strategies. The total discounted lifetime costs were highest with mt-sDNA, while QALYs were highest with FOBT (). The primary outcome of the ICER comparing mt-sDNA vs. FIT and FOBT is presented in , respectively. Assuming 100% adherence to all stool-based screening and follow-up colonoscopies, mt-sDNA was dominated (i.e. costs more and is less effective) by both FIT and FOBT.
Scenario 2
When reported real-world adherence inputs were used for initial stool-based screening, and 100% adherence was assumed for follow-up colonoscopies, the total number of screening tests per 1,000 patients remained highest for FIT (3,296) compared to other screening strategies (). While the total number of colonoscopies per 1,000 patients was highest for mt-sDNA (850), mt-sDNA also reported the greatest reduction in CRC incidence and mortality (37.4% and 45.9%, respectively) across all screening strategies. Total costs and QALYs in this scenario were also highest for mt-sDNA ($6,325 and 9.3853 QALYs, respectively), though compared to Scenario 1, were lower in magnitude (). When considering reported real-world adherence rates for stool-based screening, mt-sDNA becomes cost-effective versus both FIT ($62,814/QALY) and FOBT ($39,171/QALY) at a WTP threshold of $100,000/QALY ().
Scenario 3
When reported real-world adherence rates were used for initial stool-based screening strategies and, when indicated, for follow-up colonoscopies, the total number of screening tests per 1,000 patients were highest for FIT (3,300) and exceeded the total number of screening tests for any strategy in Scenario 2 (). While the total number of colonoscopies decreased across all screening strategies compared to Scenario 2, mt-sDNA still reported the highest total number of colonoscopies per 1,000 patients (632). As in Scenario 2, reductions in CRC incidence and mortality were highest for mt-sDNA (27.0% and 33.5%, respectively), though the magnitude of reduction was decreased compared to Scenario 2. Total costs and QALYs remained highest for mt-sDNA under this scenario ($6,525 and 9,3694 QALYs, respectively) (). When considering reported real-world adherence rates for all initial stool-based screening and follow-up colonoscopies, the cost-effectiveness of mt-sDNA vs. FIT and FOBT is improved ($31,725/QALY and $28,465/QALY, respectively) as compared to Scenario 2 ().
Capped adherence over time
Capping adherence resulted in decreased incremental costs and increased incremental QALYs for mt-sDNA vs. FIT or FOBT (). Capped adherence also resulted in lower ICERs (increased cost-effectiveness) for mt-sDNA versus FIT for Scenario 2 ($18,433/QALY) and Scenario 3 ($19,812/QALY). The same trend was observed for mt-sDNA vs. FOBT: $12,348/QALY (Scenario 2) and $16,691/QALY (Scenario 3).
Table 4. ICER for capped adherence sensitivity analyses.
Sensitivity analyses
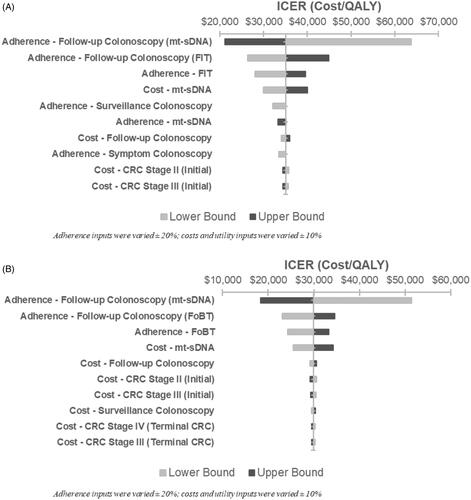
In one-way sensitivity analyses of mt-sDNA vs. FIT, the adherence to follow-up colonoscopy for mt-sDNA had the largest impact on the ICER, followed by the adherence to follow-up colonoscopy for FIT (). For mt-sDNA vs. FOBT, adherence to follow-up colonoscopy for mt-sDNA had the largest impact on the ICER, followed by adherence to follow-up colonoscopy for FOBT (). Across all analyses, the ICER remained under the $100,000/QALY WTP threshold.
Figure 2. One-way sensitivity analysis of incremental cost-effectiveness of mt-sDNA vs. (A) FIT or (B) FOBT (scenario 3).
Two-way sensitivity analyses are highlighted in Supplemental Table 4. ICER results for mt-sDNA vs. FIT were most sensitive to changes in adherence to follow-up colonoscopy following a positive mt-sDNA screening test. ICER results for mt-sDNA vs. FOBT were also most sensitive to changes in adherence to follow-up colonoscopy following a positive mt-sDNA test. Across all analyses, the ICER remained below the WTP threshold of $100,000/QALY.
Probabilistic sensitivity analysis
In the probabilistic sensitivity analysis, mt-sDNA was cost-effective over FIT or FOBT in 100% of the simulations at a WTP threshold of $100,000/QALY (Supplemental Figure 1).
Threshold analyses
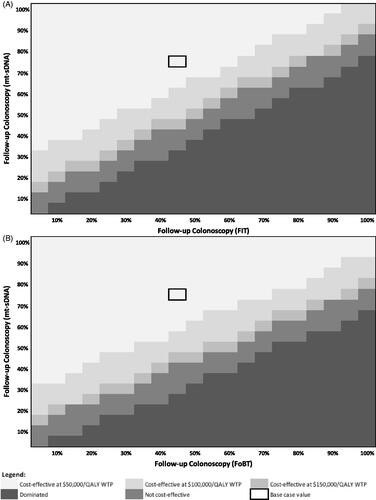
To evaluate the cost-effectiveness of mt-sDNA vs. FIT and mt-sDNA vs. FOBT at various screening test adherence rates (assuming 100% follow-up colonoscopy adherence), a threshold analysis was performed, as shown in . At a screening test adherence rate of 71.1% for mt-sDNA, annual screening test adherence would need to exceed 55% for FIT () and 50% for FOBT (), for mt-sDNA to no longer be cost-effective at a willingness to pay (WTP) of $100,000/QALY.
Figure 3. Heatmap of mt-sDNA vs. FIT (A) and mt-sDNA vs. FOBT (B) when varying screening test adherence (follow-up colonoscopy adherence fixed at 100%).
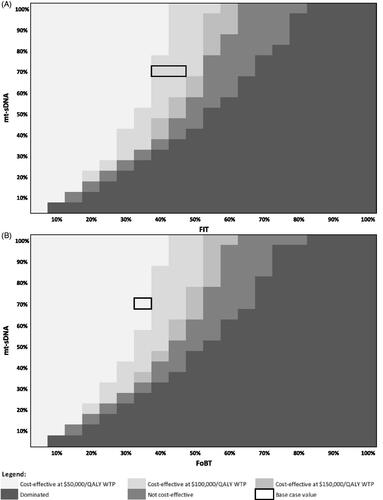
Similarly, the cost-effectiveness of mt-sDNA vs. FIT and FOBT at various rates of follow-up colonoscopy (assuming base case real-world screening test adherence) was evaluated as a threshold analysis as shown in . When adherence to follow-up colonoscopy for mt-sDNA is 73.0%, the adherence to follow-up colonoscopy would need to exceed 80% following a positive FIT test () and 95% following a positive FOBT test () for mt-sDNA to no longer be cost-effective at a WTP of $100,000/QALY.
Discussion
Using the CRC–AIM microsimulation model, we evaluated the impact of three different adherence scenarios on the cost-effectiveness of guideline-endorsed, non-invasive stool-based CRC screening strategies. As observed in prior studies, all stool-based screening modalities were cost-effective (less costly and higher QALYs) compared to no screeningCitation8–10. We also found that when adherence was assumed to be 100% for all stool-based screening tests and follow-up colonoscopies (when indicated), mt-sDNA cost more and was less effective than FIT or FOBT, which is consistent with other simulations. However, when reported real-world adherence rates were considered, mt-sDNA was the more cost-effective option compared to both FIT and FOBT at a WTP threshold of $100,000/QALY, and also resulted in greater reductions in CRC incidence and mortality. Additionally, when considering a model of capped adherence over time, the ICER for mt-sDNA is further reduced, thus improving the estimated cost-effectiveness of mt-sDNA compared to FIT and FOBT. Our threshold analysis also provided a benchmark for the necessary level of adherence to render FIT cost-effective: adherence to mt-sDNA or follow-up colonoscopy following a positive mt-sDNA test would need to drop below 45% for FIT to be cost-effective versus mt-sDNA at $100,000/QALY WTP.
Our study contributes to the growing body of evidence that adherence has a substantial impact on the cost-effectiveness of CRC screening modalities. Naber et al. also found that under adherence assumptions of 100%, mt-sDNA was dominated by FIT and FOBTCitation12. However, when adherence to mt-sDNA screening increased by 31% to 53% relative to other screening tests, mt-sDNA became cost-effective at a base case reimbursement of $512. This supports our findings which used a more conservative relative increase in adherence between mt-sDNA and FIT and FOBT of 28.5% and 37.7%, respectively. Ladabaum et al. identified that, compared to 100% adherence rates, mt-sDNA would only need 68% of individuals to participate consistently and the remaining 32% to participate intermittently in screening for this modality to be preferred over FIT at a WTP threshold of $100,000 per QALY gainedCitation13. It should be noted that the assumed adherence rate of 68% is below the reported adherence rate used for mt-sDNA in this study (71%), which was based on a large cross-sectional study of Medicare patientsCitation29.
Our study also supports the existing literature that adherence rates have a significant impact on CRC screening outcomesCitation7. Under assumptions of imperfect adherence (i.e. adherence less than 100%), Piscitello et al. found that mt-sDNA predicted more life-years gained (LYG), and higher incidence and mortality reduction, consistent with our studyCitation16. Our results are also in line with D’Andrea et al. who found that the number of CRC cases and deaths varied based on assumptions of either 100% adherence or reported adherence for specific testsCitation6. Despite the much lower estimate assumed for adherence to mt-sDNA in their analysis (42.6%), under the reported adherence scenarios, mt-sDNA averted more cases and deaths than FIT and FOBTCitation6. Peterse et al. also explored adherence scenarios similar to our studyCitation10. Under 100% adherence rates, LYG and reductions in the incidence of CRC were highest for FOBT, then FIT, then mt-sDNACitation10. However, when test-specific adherence rates for stool-based screening tests were considered, mt-sDNA resulted in more LYG compared to FIT and FOBT. It is important to note that none of these previously reported analyses accounted for real-world adherence to follow-up colonoscopies, which, as evidenced in this study, has an impact on the estimated cost and clinical outcomes in CRC screening.
Multiple factors influence adherence to both initial CRC screening and follow-up colonoscopy. Reasons for low adherence to screening may include patient- (fear and bowel preparation), provider- (lack of knowledge) and system-level factorsCitation35,Citation36. A meta-analysis identified patient navigation as a key component to increasing CRC screening completion ratesCitation37 which is also a critical factor in increasing adherence rates over timeCitation38. Patient navigation is fully integrated within the cost of mt-sDNA, providing multilingual assistance, up-front education, periodic reminders and ongoing call center support to all patients. Weiser et al. found that the majority (61.5%) of patients who adhered to mt-sDNA screening did so within the first 30 days when patient navigation is most activeCitation28. This is consistent with a previous study that found that when mt-sDNA was offered to Medicare patients previously non-adherent with CRC screening, 88% completed mt-sDNA tests within 12 months and, of those with a positive mt-sDNA test, 96% went on to complete a follow-up colonoscopyCitation39. Though mt-sDNA is currently the only stool-based strategy with fully integrated patient navigation, other studies, including a systematic review, have noted that the use of patient navigation and patient reminders are associated with increased adherence to CRC screening across various modalities, including FITCitation37,Citation40,Citation41. Other differences in the practical aspects of stool-based screening strategies may also contribute to test-specific variations in adherence to follow-up colonoscopies. In addition to system-level navigation, higher adherence to a follow-up colonoscopy after a positive mt-sDNA, relative to FIT or FOBT, may be due to the patient and/or provider perceptions regarding the assay approach, or the lower screening interval of 3 years for mt-sDNA versus 1 year for FIT or FOBTCitation42.
There are limited data on the adherence to stool-based testing and follow-up colonoscopies. For our analyses, reported adherence data for mt-sDNA was taken from a recent study of Medicare patients; Medicare data was not available for FIT and FOBT, therefore rates were obtained from a Veterans Affairs health system population. While there are differences between the Medicare and Veterans Affairs health system, a systematic review found that the Veterans Affairs performed similarly or better across a wide variety of quality dimensions, including cancer screening to non-Veterans Affairs health settings, including MedicareCitation43. Despite this difference in settings, the adherence rates for FIT and FOBT used in the base case scenario are conservative when compared with other reported adherence rates in other non-Medicare populations, ranging from 14%–29%Citation38,Citation41,Citation44. Few data also exist on reported real-world adherence data for follow-up colonoscopiesCitation41,Citation45. Our multiple sensitivity analyses through two-way scenario analyses and threshold analyses include these adherence rates for both initial stool-based screening and follow-up colonoscopy.
The strengths of this analysis include the use of real-world adherence data for both stool-based screening and follow-up colonoscopies, with minimal changes in other model inputs (e.g. costs and utilities), as compared to other recent cost-effectiveness analyses using similar microsimulation models of CRC screening and natural history. This study used the CRC–AIM microsimulation model, which has been previously validated and is based on an established CRC–SPIN modelCitation16,Citation17. While we modified the inputs within the CRC–AIM model, Scenario 1 confirms that under assumptions of 100% adherence to both stool-based screening and follow-up colonoscopy, our results are consistent with other simulation analyses, providing validity to our modelCitation12.
Our study should be interpreted in the context of its limitations. Our analysis is based on cross-sectional adherence to stool-based screening modalities, that is, we assumed that individuals remain adherent to their initial screening modality over time. Jensen et al., however, found that adherence to FIT increased over time: adherence rates in round 1 were found to be 48.2% (similar to our baseline adherence rate), and for those who completed their initial screening and remained eligible, adherence to annual screening reached 86.1% in round 4Citation46. While longitudinal screening rates are not explicitly considered in our model, calculations indicate that after 4 years, ∼89% of patients in our model were adherent to FIT, similar to Jensen et al. Longitudinal screening rates over time for individuals who are not adherent at baseline, however, remains incompletely defined. We are unaware of models that have incorporated longitudinal screening rates over time and this should be considered for future study. The adherence rates in this model are based on observed real-world cross-sectional adherence and may not be generalizable to all settings. This analysis also did not consider additional program-related costs, such as patient reminder or navigation programs that have been shown to increase adherence to stool-based screening testsCitation37. The impact of additional program costs for FIT or FOBT in order to increase adherence was outside the scope of this paper. Finally, while recommended screening strategies include both invasive and non-invasive tests, only non-invasive stool-based tests were considered in this cost-effectiveness analysis as they may be considered comparable in the lack of need for bowel preparation, do not require time off work and have lower complication rates.
Increased adherence to guideline-endorsed screening strategies would enhance the programmatic effectiveness of average-risk CRC screening, leading to reductions in CRC incidence and mortality. Comparisons of the effectiveness and benefits of specific CRC screening strategies should include both test performance characteristics along with real-world adherence rates to both the test and, when indicated, follow-up colonoscopy, to better inform clinical practice decision-making.
Transparency
Declaration of funding
Financial support for this study was provided by a contract with Exact Sciences Corporation. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report.
Declaration of financial/other interests
D.A. Fisher is a consultant for Exact Sciences and Guardant Health. J.J. Karlitz is an advisor for Exact Sciences, a consultant for Myriad Genetics, and has an equity position in Gastro Girl. P.J. Limburg serves as Chief Medical Officer for Screening at Exact Sciences through a contracted services agreement with Mayo Clinic. Dr. Limburg and Mayo Clinic have contractual rights to receive royalties through this agreement. D. Lieberman serves as a consultant for Ironwood and serves on scientific advisory boards for Check-Cap and Freenome. A.M. Fendrick has been a consultant for AbbVie, Amgen, Centivo, Community Oncology Association, Covered California, EmblemHealth, Exact Sciences, Freedman Health, GRAIL, Harvard University, Health & Wellness Innovations, Health at Scale Technologies, MedZed, Penguin Pay, Risalto, Sempre Health, the State of Minnesota, U.S. Department of defense, Virginia Center for Health Innovation, Wellth, and Zansors; has received research support from the Agency for Healthcare Research and Quality, Gary and Mary West Health Policy Center, Arnold Ventures, National Pharmaceutical Council, Patient-Centered Outcomes Research Institute, Pharmaceutical Research and Manufacturers of America, the Robert Wood Johnson Foundation, the State of Michigan, and the Centers for Medicare and Medicaid Services.
JME peer reviewers on this manuscript have received an honorarium from JME for their review work, but have no other relevant financial relationships to disclose.
Author contributions
All authors were involved in the conception and design of the study. All authors were involved in either the generation, collection, assembly, analysis or interpretation of the data. All authors provided input on the drafting of the manuscript and all authors approved the final version of the manuscript.
Supplemental Material
Download MS Word (96.8 KB)Acknowledgements
Lianne Barnieh who is a consultant with Maple Health Group provided medical writing support.
References
- Mandel JS, Church TR, Ederer F, et al. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91(5):434–437.
- Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369(12):1106–1114.
- Surveillance epidemiology and end results. Cancer stat facts: Colorectal cancer 2020. [cited 2020 Aug 31]. Available from: https://seer.cancer.gov/statfacts/html/colorect.html
- Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315(23):2564–2575.
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68(4):250–281.
- D'Andrea E, Ahnen DJ, Sussman DA, et al. Quantifying the impact of adherence to screening strategies on colorectal cancer incidence and mortality. Cancer Med. 2020;9(2):824–836.
- Limburg P, Saoud L, Borah BJ, et al. Higher impact on clinical outcomes from delays in colorectal cancer screening with the fecal immunochemical test vs multitarget stool DNA: CRC-AIM microsimulation model results. Gastroenterology. 2020;158(6):S-1176.
- Ran T, Cheng CY, Misselwitz B, et al. Cost-effectiveness of colorectal cancer screening strategies-a systematic review. Clin Gastroenterol Hepatol. 2019;17(10):1969–1981.
- Zhong GC, Sun WP, Wan L, et al. Efficacy and cost-effectiveness of fecal immunochemical test versus colonoscopy in colorectal cancer screening: a systematic review and meta-analysis. Gastrointest Endosc. 2020;91(3):684–6975.
- Peterse EFP, Meester RGS, de Jonge L, et al. Comparing the cost-effectiveness of innovative colorectal cancer screening tests. J Natl Cancer Inst. 2021;113(2):154–161.
- National Cancer Institute. Cancer intervention and surveillance modeling network. CISNET model registry 2020. [cited 2020 Mar 29]. Available from: https://resources.cisnet.cancer.gov/registry/site-summary/colorectal/
- Naber SK, Knudsen AB, Zauber AG, et al. Cost-effectiveness of a multitarget stool DNA test for colorectal cancer screening of Medicare beneficiaries. PLOS One. 2019;14(9):e0220234.
- Ladabaum U, Mannalithara A. Comparative effectiveness and cost effectiveness of a multitarget stool DNA test to screen for colorectal neoplasia. Gastroenterology. 2016;151(3):427–439.
- Ladabaum U, Mannalithara A, Brill JV, et al. Contrasting effectiveness and cost-effectiveness of colorectal cancer screening under commercial insurance vs. Medicare. Am J Gastroenterol. 2018;113(12):1836–1847.
- Vijan S, Hwang EW, Hofer TP, et al. Which colon cancer screening test? A comparison of costs, effectiveness, and compliance. Am J Med. 2001;111(8):593–601.
- Piscitello A, Saoud L, Fendrick AM, et al. Estimating the impact of differential adherence on the comparative effectiveness of stool-based colorectal cancer screening using the CRC-AIM microsimulation model. PLOS One. 2020;15(12):e0244431.
- Description and validation of the novel colorectal cancer and adenoma incidence & mortality (CRC-AIM) microsimulation model. [Internet]. bioRxiv: 2020 [cited 2021 Apr 16]. Available from: https://www.biorxiv.org/content/https://doi.org/10.1101/2020.03.02.966838v1
- Cooper GS, Grimes A, Werner J, et al. editors. Barriers to colonoscopic follow-up after positive fecal immunochemical testing or multitarget stool DNA testing, Digestive Disease Week; 2020; Chicago (IL).
- Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172(7):575–582.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US preventive services task force. JAMA. 2016;315(23):2595–2609.
- Centers for Medicare & medicaid services. Physician fee schedule 2020. [cited 2020 Aug 20]. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx
- Ladabaum U, Levin Z, Mannalithara A, et al. Colorectal testing utilization and payments in a large cohort of commercially insured US adults. Am J Gastroenterol. 2014;109(10):1513–1525.
- Hathway JM, Miller-Wilson LA, Jensen IS, et al. Projecting total costs and health consequences of increasing mt-sDNA utilization for colorectal cancer screening from the payer and integrated delivery network perspectives. J Med Econ. 2020;23(6):581–592.
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128.
- St. Louis Fed. Consumer price index for all urban consumers: medical care in U.S. city average (CPIMEDSL) 2020. [2020 Sep 18]. Available from: https://fred.stlouisfed.org/series/CPIMEDSL
- Szende A, Janssen B, Cabases J (editors). Self-reported population health: an international perspective based on EQ-5D. Dordrecht (NL): Springer; 2014.
- Goede SL, Rabeneck L, Van Ballegooijen M, et al. Harms, benefits and costs of fecal immunochemical testing versus guaiac fecal occult blood testing for colorectal cancer screening. PLOS One. 2017;12(3):e0172864.
- Weiser E, Parks PD, Swartz RK, et al. Cross-sectional adherence with the multi-target stool DNA test for colorectal cancer screening: real-world data from a large cohort of older adults. J Med Screen. 2021;28(1):18–24.
- Akram A, Juang D, Bustamante R, et al. Replacing the guaiac fecal occult blood test with the fecal immunochemical test increases proportion of individuals screened in a large healthcare setting. Clin Gastroenterol Hepatol. 2017;15(8):1265–1270 e1.
- Hassan C, Giorgi Rossi P, Camilloni L, et al. Meta-analysis: adherence to colorectal cancer screening and the detection rate for advanced neoplasia, according to the type of screening test. Aliment Pharmacol Ther. 2012;36(10):929–940.
- Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–1258.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness-the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–797.
- Singal AG, Gupta S, Skinner CS, et al. Effect of colonoscopy outreach vs fecal immunochemical test outreach on colorectal cancer screening completion: a randomized clinical trial. JAMA. 2017;318(9):806–815.
- Joseph DA, King JB, Richards TB, et al. Use of colorectal cancer screening tests by state. Prev Chronic Dis. 2018;15:E80.
- Jones RM, Devers KJ, Kuzel AJ, et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010;38(5):508–516.
- May FP, Yano EM, Provenzale D, et al. Barriers to follow-up colonoscopies for patients with positive results from fecal immunochemical tests during colorectal cancer screening. Clin Gastroenterol Hepatol. 2019;17(3):469–476.
- Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(12):1645–1658.
- Liang PS, Wheat CL, Abhat A, et al. Adherence to competing strategies for colorectal cancer screening over 3 years. Am J Gastroenterol. 2016;111(1):105–114.
- Prince M, Lester L, Chiniwala R, et al. Multitarget stool DNA tests increases colorectal cancer screening among previously noncompliant Medicare patients. World J Gastroenterol. 2017;23(3):464–471.
- Selby K, Jensen CD, Levin TR, et al. Program components and results from an organized colorectal cancer screening program using annual fecal immunochemical testing. Clin Gastroenterol Hepatol. 2020;S1542-3565(20)31372-0.
- Issaka RB, Akinsoto NO, Strait E, et al. Effectiveness of a mailed fecal immunochemical test outreach: a Medicare advantage pilot study. Therap Adv Gastroenterol. 2020;13:175628482094538.
- Kisiel JB, Limburg PJ. Colorectal Cancer Screening With the Multitarget Stool DNA Test. Am J Gastroenterol. 2020;115(11):1737–1740.
- O'Hanlon C, Huang C, Sloss E, et al. Comparing VA and non-VA quality of care: a systematic review. J Gen Intern Med. 2017;32(1):105–121.
- Coronado GD, Green BB, West II, et al. Direct-to-member mailed colorectal cancer screening outreach for Medicaid and Medicare enrollees: implementation and effectiveness outcomes from the BeneFIT study. Cancer. 2020;126(3):540–548.
- Finney Rutten LJ, Jacobson DJ, Jenkins GD, et al. Colorectal cancer screening completion: an examination of differences by screening modality. Prev Med Rep. 2020;20:101202.
- Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164(7):456–463.