Abstract
Background
NYVEPRIA, a pegfilgrastim (a long-acting granulocyte colony-stimulating factor [G-CSF]) biosimilar, was recently recommended for marketing authorization in Europe for decreasing the incidence of febrile neutropenia (FN) in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs. The present study aimed to evaluate the financial impact of introducing a new pegfilgrastim biosimilar from a French healthcare system perspective.
Methods
An Excel-based budget impact model was developed to estimate the financial impact by introducing a new pegfilgrastim biosimilar (NYVEPRIA) to France over a 5-year time horizon. Comparators included existing long-acting and short-acting G-CSFs. The burden of FN was obtained from existing literature. Costs (2021 Euros) included drug acquisition and administration, estimated based on drug dosage in both clinical trial and real-world settings. Scenario analyses were conducted to examine the robustness of key model assumptions.
Results
In a total French population of 67.19 million, 79,873 patients were estimated to be treated with G-CSFs annually. The annual number of patients to be treated with NYVEPRIA was estimated to be 1593, 3195, 3674, 3782, and 4052 in years 1 to 5, respectively. Using real-world data, NYVEPRIA resulted in total annual cost savings of €8,620, €868,498, €868,498, €814,102, and €958,952 over years 1 to 5, respectively, leading to a cumulative 5-year cost savings of €3,518,669. Using data from clinical trials, NYVEPRIA resulted in total annual cost savings of €14,366, €1,447,496, €1,447,496, €1,356,836, and €1,598,253 over years 1 to 5, respectively, leading to a cumulative 5-year cost savings of €5,864,448.
Conclusions
The introduction of a new pegfilgrastim biosimilar (NYVEPRIA) is potentially associated with substantial cost savings for the French healthcare system.
Introduction
Chemotherapy-induced febrile neutropenia (FN) is a potentially fatal hematologic toxicity of myelosuppressive cancer chemotherapyCitation1. FN is defined as an oral temperature >38.3 °C or 2 consecutive readings of >38.0 °C for 2 h and an absolute neutrophil count (ANC) of <0.5 × 109/LCitation2. Chemotherapy-induced FN may lead to delays in chemotherapy cycles of treatment and dose reductions and has the potential to adversely affect tumor controlCitation3–5. In France, an analysis of newly diagnosed cancer patients estimated that 7.4% of those receiving chemotherapy were hospitalized for FN and mortality was 7% among hospitalized patientsCitation6. From 2010 to 2011, mean per patient treatment costs in France for chemotherapy-induced FN were €7,821 for hematologic tumors and €4,908 for solid tumors, totaling a cost of €59.6 million for the French Statutory Health InsuranceCitation7.
Granulocyte-colony stimulating factors (G-CSF) including pegfilgrastim (a long-acting G-CSF) can be used to prevent treatment-related FNCitation2,Citation8. Several meta-analyses indicated that primary prophylaxis with G-CSF reduces the risk of FN by at least 50% in patients with solid tumors without significantly affecting tumor response or overall survivalCitation9–11.
Budgetary pressures, including high costs for innovative medicines, have led to growing inequalities in access to cancer care between and within European countriesCitation12. Biosimilars are biological products which are highly similar to their reference product with no clinically meaningful differences in quality, biological activity, safety, efficacy, and immunogenicityCitation13. Oncology biosimilars present an opportunity for decreased health expenditures and increased patient access to biologics. According to a recent health economics analysis, appropriate use of generics and biosimilars in France, the UK, Germany, Italy, Belgium, Denmark, Poland, the Netherlands, and Sweden could bring an estimated €7.1 billion in savingsCitation14.
NYVEPRIAFootnotei (pegfilgrastim biosimilar) was recently approved by the European Medicines Agency (EMA) for the reduction in the duration of neutropenia and the incidence of FN in adult patients treated with cytotoxic chemotherapy for malignancy (with the exception of chronic myeloid leukemia and myelodysplastic syndromes), but has not yet been launched in FranceCitation15. Analytic, non-clinical, and clinical studies support that pegfilgrastim biosimilar has no clinically meaningful differences in terms of safety, efficacy, purity, or potency when compared to pegfilgrastimCitation16.
In order to understand the financial impact of introducing a new pegfilgrastim biosimilar to the formulary for a reduction in the duration of neutropenia and the incidence of FN in adult patients treated with cytotoxic chemotherapy from a French healthcare system perspective, a budget impact model (BIM) was developed.
Methods
Model overview
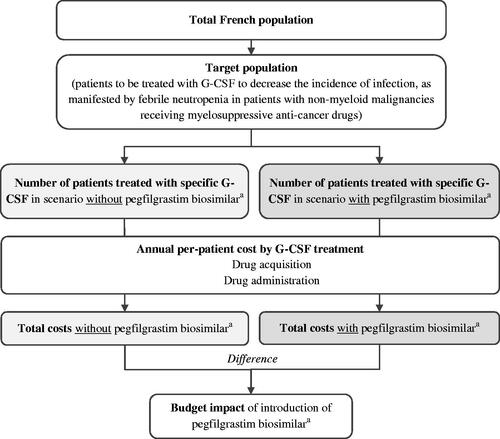
A budget impact model was developed in Microsoft Excel (Microsoft Corp., Redmond, WA) to estimate the financial impact of a new pegfilgrastim biosimilar (NYVEPRIA) from the perspective of the French healthcare system with a total French population of 67.19 million. The budget impact of pegfilgrastim biosimilar was estimated by comparing the total costs between 2 scenarios with and without pegfilgrastim biosimilar annually over a 5-year time horizon (). Costs included drug acquisition and drug administration, reported in 2021 Euros. Two approaches were adopted for estimating drug utilization: a clinical trial basis approach and a real-world data approach. The clinical trial basis approach relied on the drug label and clinical trial data for treatment dosage and duration, whereas the real-world data approach employed the drug utilization pattern observed in clinical practice. Given that the aim of the BIM was to compare annual cash flows, no discounting was appliedCitation17. Reporting of the study was guided by the consolidated health economic evaluation reporting standards (CHEERS) statement (Appendix A)Citation18.
Target population
The target population was patients treated with G-CSFs for non-myeloid malignancies receiving myelosuppressive anti-cancer drugs. It was assumed that that only patient who would have been treated with G-CSFs would switch to pegfilgrastim biosimilar upon market entry of pegfilgrastim biosimilar (NYVEPRIA). Epidemiology and G-CSF utilization inputs were used to estimate the target population ().
Table 1. Epidemiology, treatment utilization of G-CSF, and target population estimation.
This analysis only included incident population but not prevalent population, assuming that previously diagnosed patients would have already completed the course of treatment with G-CSFs in the previous year(s) given that a treatment course of G-CSFs usually takes less than a year (e.g. 4-8 chemotherapy cycles, with 14–21 days per cycleCitation8).
Treatment distribution
Treatment comparators to a new pegfilgrastim biosimilar (NYVEPRIA) included existing G-CSF products available in the French market: long-acting G-CSFs consist of pegfilgrastim reference product, currently available pegfilgrastim biosimilars, and lipegfilgrastim; short-acting G-CSFs consist of filgrastim reference product, currently available filgrastim biosimilars, and lenograstim. Market share in the market scenarios with and without pegfilgrastim biosimilar (NYVEPRIA) was based on historical biosimilars uptake trends observed at the time of the last new pegfilgrastim biosimilar (ZIEXTENZO) entrant to the market. For the market scenario without pegfilgrastim biosimilar (NYVEPRIA), it was assumed that only patients who would be treated with long-acting G-CSF could switch to NYVEPRIA following the introduction of pegfilgrastim biosimilar (NYVEPRIA) ().
Table 2. G-CSF treatment distribution in market with and without pegfilgrastim biosimilar.
Treatment dosage
Clinical trial data approach
The number of G-CSF doses per patient was estimated based on the treatment dosage from drug labels and clinical trial data (). Given that chemotherapy is usually administered for 4-8 cyclesCitation8, the base case analysis assumed 6 cycles of treatment for all G-CSFs (including both filgrastim and pegfilgrastim), in line with previous cost analyses for G-CSFsCitation19,Citation20. For long-acting G-CSFs, 1 dose (i.e. 1 pre-filled syringe) per chemotherapy cycle was applied per drug labelCitation15,Citation21–24. For short-acting G-CSFs that are administered for no more than 14 days per drug labelCitation25–27, a duration of 11 days per chemotherapy cycle, which was commonly observed in clinical trials, was applied in the base case analysisCitation19,Citation20. In addition, for those short-acting G-CSFs that are dosed based on weight or body surface area (e.g. 0.5 MU/kg for filgrastim products with 30 MU per syringe and 19.2 MU/m2 for lenograstim with 34 MU per syringe), it was conservatively assumed that 1 syringe would be used per dose.
Table 3. Drug dosage.
Real-world data approach
The average number of syringes used per patient was directly obtained from observed drug utilization in the real-world clinical practice, which was calculated from the average number of chemotherapy cycles per patient and number of doses per chemotherapy cycle ()Citation28.
Drug acquisition and administration cost
For both clinical trial basis and real-world data approaches, the drug acquisition costs per syringe were based on the current manufacturing prices obtained from L’Assurance Maladie ()Citation29. It was projected that the manufacturing prices of pegfilgrastim reference product and existing long-acting pegfilgrastim biosimilars will further decrease in the year following the new pegfilgrastim biosimilar (NYVEPRIA) entry and then remain stable throughout the modeled time horizon, whereas the manufacturing prices of short-acting G-CSFs will remain constant over time.
Table 4. Drug cost inputs.
It was expected that 20% of patients would be self-administered while others would be administered by nurses at patients’ homes, according to previous cost analyses for G-CSFs conducted in the United States and BelgiumCitation19,Citation20. For self-administered drugs, no associated administration cost was included in the analysis. For administration by a nurse, a cost per administration of €7.32 was applied, regardless of G-CSF products, inclusive of €3.15 for nursing acting, €1.35 for an additional charge for performing the actCitation30, €2.50 for a lump-sum travel allowance, and €0.32 for extra allowance (estimated as a product of €0.35/kilometer and average journey of 0.9 kilometers for a nurse in France)Citation31,Citation32.
Analysis
The total annual budget impact was calculated as the difference in total annual costs between the scenarios with and without pegfilgrastim biosimilar (NYVEPRIA). For both market scenarios with and without pegfilgrastim biosimilar (NYVEPRIA), the total annual costs were estimated as the sum of the product of the number of G-CSF-treated patients in each year and the annual per-patient costs of specific G-CSFs. In addition, an additional number of patients treatable with the new pegfilgrastim biosimilar (NYVEPRIA) for 1 treatment course was estimated by dividing the total cost savings in a specific year by the annual per-patient of pegfilgrastim biosimilar (NYVEPRIA).
To assess the robustness of the model assumptions, a range of scenario analyses were conducted where 1 parameter or analysis setting was varied at a time while the other parameters or analysis settings were held unchanged. Key parameters related to the clinical trial basis approach assessed in the scenario analyses included drug acquisition cost of both pegfilgrastim biosimilar (NYVEPRIA) and other long-acting G-CSFs, self-administration rate, number of cycles per chemotherapy treatment course for all G-CSFs, and number of days of administrations of short-acting G-CSFs per chemotherapy cycle.
Results
Base case analysis
For a total French population of 67.19 million, 79,873 patients were estimated to be treated with G-CSFs annually. After accounting for the projected uptake of a new pegfilgrastim biosimilar (NYVEPRIA) over years 1 to 5, the annual number of patients to be treated with this new pegfilgrastim biosimilar (NYVEPRIA) was estimated to be 1593, 3195, 3674, 3782, and 4052 in year 1 to 5, respectively.
Using the drug dosage observed from real-world practice, the introduction of a new pegfilgrastim biosimilar (NYVEPRIA) resulted in a total annual cost savings of €8,620 in year 1, €868,498 in year 2, €868,498 in year 3, €814,102 in year 4, and €958,952 in year 5, leading to a cumulative 5-year cost savings of €3,518,716.
Using data from clinical trials, the introduction of a new pegfilgrastim biosimilar (NYVEPRIA) resulted in total annual cost savings of €14,366 in year 1, €1,447,496 in year 2, €1,447,496 in year 3, €1,356,836 in year 4 and €1,598,253 in year 5, resulting in a cumulative 5-year cost savings of €5,864,448 ().
Table 5. Base case analysis results.
Scenario analyses
For the real-world data approach, decreases in drug acquisition costs by 10% and 20% for the new pegfilgrastim biosimilar (NYVEPRIA) and other long-acting G-CSFs that are not pegfilgrastim reference products increased the 5-year cost saving to €4,647,797 and €5,776,925, respectively. Increasing the market share of the new pegfilgrastim biosimilar by 50% and 100% increased the 5-year cost savings (from €3,518,716 in the base case) to €4,316,987 and €5,111,746, respectively. Assuming a 100% market shift from the pegfilgrastim reference product to the new pegfilgrastim biosimilar led to a 5-year cost saving of €9,951,256. In contrast, switching completely from other long-acting G-CSFs that are not pegfilgrastim reference products led to zero cost saving, due to the same drug acquisition cost of the new pegfilgrastim biosimilar and other long-acting G-CSFs already on the market ().
Table 6. Scenario analyses results.
For the clinical trial basis approach, in a scenario of 4 cycles per chemotherapy treatment course for all G-CSFs, the total 5-year cost-saving decreased slightly to €3,909,632. When the number of administrations per chemotherapy cycle for short-acting G-CSFs reduced to 8 or 6, the total 5-year cost-saving increased slightly to €5,864,448. Decreases in drug acquisition costs by 10% and 20% for the new pegfilgrastim biosimilar (NYVEPRIA) and other long-acting G-CSFs that are not pegfilgrastim reference products increased the 5-year cost saving to €7,746,328 and €9,628,208, respectively. Increasing the market share of the new pegfilgrastim biosimilar by 50% and 100% was associated with cumulative cost savings of €7,194,979 and €8,519,577, respectively, over 5 years. Assuming a 100% market shift from the pegfilgrastim reference product to the new pegfilgrastim biosimilar led to a 5-year cost saving of €16,585,426, whereas switching completely from other long-acting G-CSFs that are not pegfilgrastim reference products led to zero cost-saving ().
Decreasing the self-administration rate from 20% to 10% for all G-CSFs did not result in a change in the total 5-year cost savings, using either real-world data or clinical trial basis approaches ().
Discussion
Our budget impact analysis suggested that the introduction of a new pegfilgrastim biosimilar (NYVEPRIA) in France would lead to substantial cost savings to the French healthcare system. With advances in effective cancer treatment and an aging French population, more oncology patients are expected to benefit from lower-cost biosimilars. However, our results should be interpreted with caution, given the key drivers of the observed savings were the lower drug acquisition costs associated with pegfilgrastim biosimilars as well as an assumed gradual market shift from the pegfilgrastim reference product to pegfilgrastim biosimilars, both of which have uncertainties and could be influenced by many factors, e.g. market competitions.
The difference in estimated cost savings between the clinical trial basis approach and real-word data approach was primarily driven by the underdosing of G-CSFs in clinical practice. In the present study, an average of 3–5 days treatment duration per chemotherapy cycle for patients with shorting-acting G-CSFs was observed in practice, which was much shorter than an average treatment duration of 11 days adopted from clinical trialsCitation19,Citation20,Citation33. Notably, our data of short-acting G-CSFs usage obtained from real-world practice were consistent with two multicenter observational studies conducted in FranceCitation34,Citation35. Laribi et al.Citation34 have found that among 1,119 elderly cancer patients, the median treatment duration of filgrastim was 5 days per chemotherapy cycle, regardless of primary prophylaxis or secondary prophylaxis. Similarly, the ZOHe study which assessed the use of biosimilar filgrastim in routine practice in patients ≥18 years who were at risk of FN-inducing chemotherapy has shown that the median duration of planned filgrastim treatment was 5 days for all tumor subgroups and no patients were treated for more than 14 daysCitation35. In contrast, for long-acting G-CSFs, despite a lack of published real-world study for long-acting G-CSFs specific to France, it was reported in a meta-analysis that in the real-world settings across multiple countries (e.g. UK, Greece, Canada), the overall risk of dose reductions was statistically significantly lower (risk ratio = 0.69) for long-acting G-CSFs compared to short-acting G-CSFsCitation36. Given the savings estimated using clinical trial data were likely to be overestimated, we expected that the savings calculated using real-world data to be more accurate from a French healthcare system perspective.
It is worth mentioning that some observational studies have pointed out that pegfilgrastim is probably more effective than filgrastim in reducing FN and FN-related complications among patients receiving myelosuppressive chemotherapyCitation37–41. A meta-analysis that evaluated 36 publications have found that in randomized clinical trials (RCTs), there was no statistically significant difference in the incidence of FN and FN-related complications between short- and long-acting G-CSFs, whereas in non-RCTs, the overall risk was lower within long-acting G-CSF than with short-acting G-CSF for incidence of FNCitation36. Mitchell et al.Citation42 conducted a review of comparative effectiveness of long- vs. short-acting G-CSFs in real-world clinical settings and also found that risks of FN and FN-related complications were generally lower for prophylaxis with pegfilgrastim versus prophylaxis with short-acting G-CSFs, which might be attributed to under-dosing of short-acting G-CSFs per chemo-cycle in routine clinical practiceCitation36,Citation42,Citation43. In the present analysis, only patients who would be treated with existing long-acting G-CSFs would switch to the new pegfilgrastim biosimilar (NYVEPRIA). Further studies might be needed to make switching from short-acting G-CSFs to long-acting G-CSFs into consideration.
Scenario analyses using real-world data have indicated that the savings were sensitive to changes in drug acquisition costs. Assuming a 10% and 20% decrease in drug acquisition costs for pegfilgrastim biosimilars, the total 5-year cost savings would double and triple compared to the base case analysis, respectively. In the present study, we did not take the potential market competition into account in the next few years following pegfilgrastim biosimilar (NYVEPRIA) entry. However, the Institute for Health Informatics (IMS) which assessed G-CSF biosimilar uptake and competition in Europe found that, from 2006 to 2013, concurrent to the introduction of biosimilar filgrastim, the cost of G-CSF has been reduced by 22% in FranceCitation44. IMS also suggested that introduction of more biosimilar G-CSFs may result in competitive price pressure, further reducing prices of G-CSF productsCitation44. It is possible that the introduction of a new pegfilgrastim biosimilar (NYVEPRIA) could result in further price reduction for both long-acting and short-acting G-CSFs, and thus provide more savings to the French healthcare system.
In summary, pegfilgrastim biosimilars potentially have a significant favorable impact on the affordability of oncology therapies for both French patients and French healthcare systemCitation14. An economic analysis of resource allocations in cancer care in France, the UK, Germany, Italy, Belgium, Denmark, Poland, and Sweden has reported that France spends more than the European Union average in all cancer treatments except for lung cancerCitation14. The authors conclude that countries such as France could bring an estimated €7.1 billion in savings by the additional use of generics and biosimilarsCitation14.
Limitations
Our study has limitations. First, our analysis did not take patients who might switch from short-acting G-CSFs to pegfilgrastim biosimilar (NYVEPRIA) into account. Future studies might be needed to take this scenario into consideration. Second, the market share projection was based on historical biosimilars uptake trends observed at the time of the last new pegfilgrastim biosimilar (ZIEXTENZO) entrant to the market which might not represent the complex market dynamics over the years due to uncertainties of competitions and reimbursement policies for biosimilars. Third, the estimated proportion of patients who are treated with G-CSFs in France was based on the patients who had emergency department visits after myelotoxic treatment for cancer, which might not be generalizable to the target population in our model, and therefore the targeted patient population was likely underestimated. Lastly, the impact of evolving treatment paradigm in oncology care on the usage of G-CSFs was not considered in the present analysis. Advances in less intense cancer regimens, such as antibody–drug conjugates (ADCs) and immunotherapies, and chemotherapy de-escalation strategies, all of which are likely associated with relatively low risk of FN and related complications, and therefore the use of G-CSFs may be reduced.
Conclusion
Our results have demonstrated substantial savings by introducing a new pegfilgrastim biosimilar (NYVEPRIA) to the French healthcare system. The model has incorporated robust methodologies estimating drug utilization in both clinical trial/label-based scenarios and real-world clinical practices.
Transparency
Declaration of funding
The study was sponsored by Pfizer Inc.
Declaration of financial/other relationships
Rongzhe Liu, Omer Zaidi and Jennifer Stephens are employees of Pharmerit – an OPEN Health Company, which received research funding from Pfizer Inc. Jennifer Stephens owns OPEN Health stock. Jingyan Yang and Anna Granghaud are employees of Pfizer Inc. and own Pfizer stocks.
The peer reviewers on this manuscript have received an honorarium from JME for their review work. In addition, a reviewer on this manuscript has disclosed that the agency they work for has had contracts related to biosimilar GCSFs (both standard and pegylated) from Sandoz/Novartis, Coherus Biosciences, and Mylan/Viatris. The reviewers have no other relevant financial relationships or otherwise to disclose.
Author contributions
Jingyan Yang: Conceptualization, methodology, development of the original draft. Rongzhe Liu: Conceptualization, methodology, modeling, development of the original draft. Omer Zaidi: Conceptualization, input collection, modeling, development of the original draft. Jennifer Stephens: Conceptualization, supervision, manuscript review and revision. Anna Granghaud: Input collection, manuscript review and revision. All authors approved of the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Acknowledgements
Medical writing support was provided by Gregory Poorman, an employee of Pharmerit – an OPEN Health Company, Bethesda, MD, USA., and was funded by Pfizer Inc.
Notes
i NYVEPRIA is a registered trademark of Pfizer Inc., New York, NY, USA.
References
- Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295–3304.
- Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann Oncol. 2016;27(suppl 5):v111–v118.
- Chang J. Chemotherapy dose reduction and delay in clinical practice. Evaluating the risk to patient outcome in adjuvant chemotherapy for breast cancer. Eur J Cancer. 2000;36(Suppl 1):11–14.
- Khan S, Dhadda A, Fyfe D, et al. Impact of neutropenia on delivering planned chemotherapy for solid tumours. Eur J Cancer Care. 2008;17(1):19–25.
- Pettengell R, Schwenkglenks M, Leonard R, et al. Neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC-EU prospective observational European neutropenia study. Support Care Cancer. 2008;16(11):1299–1309.
- Freyer G, Scotte F, Borget I, et al. Clinical burden caused by hospitalization for febrile neutropenia in France in 2010–2011: an analysis of the PMSI database. Bull Cancer. 2016;103(6):552–560.
- Vainchtock A, Cohen S, Zaleski I. PCN84. Analysis of public and private hospital databases (PMSI) 2010/2011 to estimate the frequency and associated costs for febrile neutropenia in France. Value Health. 2013;16(7):A406.
- Aapro MS, Bohlius J, Cameron DA, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47(1):8–32.
- Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–3167.
- Cooper KL, Madan J, Whyte S, et al. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404.
- Clark OA, Lyman GH, Castro AA, et al. Colony-stimulating factors for chemotherapy-induced febrile neutropenia: a meta-analysis of randomized controlled trials. J Clin Oncol. 2005;23(18):4198–4214.
- Wait S, Han D, Muthu V, et al. Towards sustainable cancer care: Reducing inefficiencies, improving outcomes—a policy report from the All.Can initiative. J Cancer Policy. 2017;13:47–64.
- UK National Health Service. What is a biosimilar medicine. NHS England and NHS improvement; 2019. Available from: https://www.england.nhs.uk/wp-content/uploads/2019/05/what-is-a-biosimilar-medicine-guide-v2.pdf
- Cole A, Lundqvist A, Lorgelly P, et al. Improving efficiency and resource allocation in future cancer care. London (UK): Office of Health Economics; 2016. Available from: https://www.ohe.org/publications/improving-efficiency-and-resource-allocation-future-cancer-care
- European Medicines Agency. Nyvepria - summary of product characteristics (placeholder) 2020 [cited 2020 October 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/nyvepria-epar-product-information_en.pdf
- Moosavi S, Borema T, Ewesuedo R, et al. PF-06881894, a proposed biosimilar to pegfilgrastim, versus US-licensed and EU-approved pegfilgrastim reference products (Neulasta®): pharmacodynamics, pharmacokinetics, immunogenicity, and safety of single or multiple subcutaneous doses in healthy volunteers . Adv Ther. 2020;37(7):3370–3391.
- Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 budget impact analysis good practice II task Force. Value Health. 2014;17(1):5–14.
- Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Pharmacoeconomics. 2013;31(5):361–367.
- Fust K, Li X, Maschio M, et al. Cost-effectiveness analysis of prophylaxis treatment strategies to reduce the incidence of febrile neutropenia in patients with early-stage breast cancer or non-Hodgkin lymphoma. Pharmacoeconomics. 2017;35(4):425–438.
- Trautman H, Szabo E, James E, et al. Patient-Administered Biologic and Biosimilar Filgrastim May Offer More Affordable Options for Patients with Nonmyeloid Malignancies Receiving Chemotherapy in the United States: A Budget Impact Analysis from the Payer Perspective. J Manag Care Spec Pharm. 2019;25(1):94–101.
- European Medicines Agency. Ziextenzo - summary of product characteristics 2019 [cited 2020 October 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/ziextenzo-epar-product-information_en.pdf
- European Medicines Agency. Lonquex - summary of product characteristics 2013 [cited 2020 October 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/lonquex-epar-product-information_en.pdf
- European Medicines Agency. Fulphila - summary of product characteristics 2018 [cited 2020 October 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/fulphila-epar-product-information_en.pdf
- European Medicines Agency. Pelgraz - summary of product characteristics 2018 [cited 2020 October 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/pelgraz-epar-product-information_en.pdf
- Ministère des Solidarités et de la Santé. NEUPOGEN 30 MU (0,3 mg/mL), solution injectable - résumé des caractéristiques du produit 2019 [cited 2020 October 20]. Available from: http://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid=69225686&typedoc=R
- European Medicines Agency. Nivestim - summary of product characteristics 2010 [cited 2020 October 18]. Available from: https://www.ema.europa.eu/en/documents/product-information/nivestim-epar-product-information_en.pdf
- Ministère des Solidarités et de la Santé. GRANOCYTE 34 millions UI/ml, poudre et solvant pour solution injectable/perfusion en seringue préremplie - résumé des caractéristiques du produit 2018 [cited 2020 October 20]. Available from: http://base-donnees-publique.medicaments.gouv.fr/affichageDoc.php?specid=65066667&typedoc=R
- Open Innovation Program; OIP Healthcare. Synapps drug market. Extract Oct 2020.
- L'Assurance Maladie. Base des Medicaments et Informations Tarifaires 2021 [cited 2021 January 26]. Available from: http://www.codage.ext.cnamts.fr/codif/bdm_it/index_presentation.php?p_site=AMELI
- L'Assurance Maladie. Nomenclatures: NGAP and LPP 2020 [cited 2020 November 19]. Available from: https://www.ameli.fr/infirmier/exercice-liberal/facturation-remuneration/nomenclatures-ngap-lpp/nomenclatures-ngap-lpp
- Coldefy M, Com-Ruelle L, Lucas-Gabrielli V, et al. Les distances d’accès aux soins en France métropolitaine au 1er janvier 2007 2011 [cited 2020 October 13]. Available from: https://www.irdes.fr/Publications/Rapports2011/rap1838.pdf
- IRDES. RAPPORTS n° 550 et 551 (biblios n° 1838 et 1839) 2020 [cited 2020 November 19]. Available from: https://www.irdes.fr/EspaceRecherche/BiblioResumeEtSommaire/2011/Rapport1838.htm
- Tilleul PR, Rodgers-Gray BS, Edwards JO. Introduction of biosimilar pegfilgrastim in France: economic analysis of switching from originator. J Oncol Pharm Pract. 2020. DOI:https://doi.org/10.1177/1078155220962208
- Laribi K, Badinand D, Janoray P, et al. Filgrastim prophylaxis in elderly cancer patients in the real-life setting: a French multicenter observational study, the TULIP study. Support Care Cancer. 2019;27(11):4283–4292.
- Roché H, Eymard J-C, Radji A, et al. Biosimilar filgrastim treatment patterns and prevention of febrile neutropenia: a prospective multicentre study in France in patients with solid tumours (the ZOHé study). BMC Cancer. 2018;18(1):1127.
- Cornes P, Gascon P, Chan S, et al. Systematic review and meta-analysis of short- versus long-acting granulocyte colony-stimulating factors for reduction of chemotherapy-induced febrile neutropenia. Adv Ther. 2018;35(11):1816–1829.
- Morrison VA, Wong M, Hershman D, et al. Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3–4 week chemotherapy regimens in community oncology practices. JMCP. 2007;13(4):337–348.
- Tan H, Tomic K, Hurley D, et al. Comparative effectiveness of colony-stimulating factors for febrile neutropenia: a retrospective study. Curr Med Res Opin. 2011;27(1):79–86.
- Henk H, Becker L, Tan H, et al. Comparative effectiveness of pegfilgrastim, filgrastim, and sargramostim prophylaxis for neutropenia-related hospitalization: two US retrospective claims analyses. J Med Econ. 2013;16(1):160–168.
- Almenar D, Mayans J, Juan O, et al. Pegfilgrastim and daily granulocyte colony‐stimulating factor: patterns of use and neutropenia‐related outcomes in cancer patients in Spain–results of the LEARN Study. Eur J Cancer Care). 2009;18(3):280–286.
- Naeim A, Henk HJ, Becker L, et al. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer. 2013;13(1):1–10.
- Mitchell S, Li X, Woods M, et al. Comparative effectiveness of granulocyte colony-stimulating factors to prevent febrile neutropenia and related complications in cancer patients in clinical practice: a systematic review. J Oncol Pharm Pract. 2016;22(5):702–716.
- Phillips J, Ritter S, Starner C, et al. Filgrastim (Neupogen) and pegfilgrastim (Neulasta): cost analysis and utilization management opportunity assessment. J Manag Care Pharm. 2012;18:176–177.
- IMS Institute for Healthcare Informatics. Assessing Biosimilar Uptake and Competition in European Markets. 2014.
- French Population Census [cited Dec 19, 2019]. Available from: https://www.insee.fr/en/metadonnees/source/serie/s1321
- European Commission. ECIS - European Cancer Information System 2020 [cited 2020 October 18]. Available from: https://ecis.jrc.ec.europa.eu/
- Worldometer. France Population 2020 [cited 2020 October 13]. Available from: https://www.worldometers.info/world-population/france-population/
- André S, Taboulet P, Elie C, et al. Febrile neutropenia in French emergency departments: results of a prospective multicentre survey. Crit Care. 2010;14(2):R68.