Abstract
Objectives
This study documents real-world patterns and additional costs of above-label (≥10% above the recommended maintenance dose) use of biologics in patients with plaque psoriasis.
Materials and methods
This was a descriptive, retrospective cohort analysis using the IBM MarketScan Commercial and Medicare Supplemental Databases. Adult patients diagnosed with plaque psoriasis initiating treatment with etanercept, adalimumab, ixekizumab, or secukinumab between 1 January 2015 and 30 November 2019 (index) were eligible. Only biologics approved prior to 2017 were included to ensure data for all agents was available throughout the study period. Patients had no other indications for biologics of interest. Outcomes were measured in the 12-month follow-up period after start of maintenance dosing.
Results
Of 6453 patients included, 708 (11.0%) received etanercept, 4654 (72.1%) received adalimumab, 228 (3.5%) received ixekizumab, and 863 (13.4%) received secukinumab. Above-label dosing was recorded in 326 (46.0%) patients receiving etanercept, 513 (11.0%) receiving adalimumab, 40 (17.5%) receiving ixekizumab, and 79 (9.2%) receiving secukinumab. Mean time to above-label use for all treatments was 50.7–99.5 days; the median was 21–37 days. Mean duration of above-label use for all treatments was 130–196 days; the median was 35–175 days. Mean total additional annual psoriasis-related medical/pharmacy costs associated with above-label use were $312/$16,475 for etanercept, $278/$9,773 for adalimumab, $124/$5,202 for ixekizumab, and $277/$9,288 for secukinumab. Above-label use was generally not associated with safety concerns; however, gastrointestinal and combined “other” nonrespiratory infections were significantly more frequent in patients receiving adalimumab above-label.
Conclusions
Above-label use of biologics for psoriasis treatment was most frequent for patients receiving etanercept, followed by ixekizumab, adalimumab, and secukinumab. Above-label vs on-label use resulted in additional costs but few significant safety concerns.
Introduction
Psoriasis is a chronic inflammatory condition of the skin estimated to affect approximately 2–3% of the US populationCitation1. Previously, topical or traditional systemic medications have been utilized but have inconsistent successCitation2. Patients with moderate-to-severe psoriasis may not respond favorably to topical medications used alone or combined with phototherapy; therefore, other therapeutics, like biological agents, may be consideredCitation2. Biologics available for psoriasis include tumor necrosis factor alpha inhibitors, anti-interleukin (IL) 12/23 inhibitors, IL-17 inhibitors, and IL-23 inhibitorsCitation2. While these medications have exhibited favorable efficacy and safety profiles, there is a subset of patients who either fail to respond or lose response over timeCitation3. Additionally, psoriasis—as a chronic inflammatory condition with several comorbidities—constitutes a substantial economic burden to society. In the US, direct costs associated with psoriasis in 2006 were estimated to be $5 billion, and most of these costs were attributed to patients with moderate-to-severe psoriasisCitation4. Direct and indirect costs of psoriasis—including loss of work productivity associated with psoriasis-related disease activity and absenteeism, and intangible costs due to decreased quality of life—impose an estimated total annual cost of approximately $112 billionCitation5. Patients diagnosed with moderate-to-severe psoriasis are also more likely to incur inpatient admissions, emergency room visits, and outpatient visits compared with control patients not diagnosed with psoriasisCitation4. Therefore, cost-effective treatments are needed to mitigate the economic burden of psoriasis.
Biologics in particular can provide effective treatment for psoriasis but are perceived as expensiveCitation3,Citation6. Due to the favorable safety profiles of standard dosing regimens, many clinicians will consider utilizing nonstandard or off-label biologic dosing regimens, such as dose escalation or dose intensification, in the subset of patients with a nonsatisfactory response to the approved regimenCitation6. Dose escalation or intensification includes abbreviating the scheduled dosing interval and/or increasing the amount of medication per single dose. In patients treated with etanercept, adalimumab, and ustekinumab, dose escalation in nonresponders can result in greater efficacy compared with standard dosingCitation6,Citation7. Some patients may benefit from dose escalation above the labeled dose; however, this can significantly increase the cost of therapy.
There are limited data on real-world dose-escalation patterns and associated costs and safety concerns among patients with plaque psoriasis treated with biologics. Previous studies involving off-label dosing regimens of biologic agents for the treatment of plaque psoriasis enrolled a small number of patients, and therefore adverse event (AE) rates were difficult to interpretCitation6,Citation7. More studies are needed in order to further characterize the costs associated through both dose-escalation regimens and duration of dose escalationCitation8. The objectives of this study are to document real-world dose-escalation patterns in patients with psoriasis, examine additional costs associated with above-label use of biologics, and assess the safety of above-label use of these biologics.
Methods
Study design
This was a descriptive retrospective cohort analysis using the IBM Watson Health MarketScan Commercial and Medicare Supplemental Databases. Adult patients diagnosed with plaque psoriasis and newly initiating biologic treatment with etanercept, adalimumab, ixekizumab, and secukinumab between 1 January 2015 and 30 November 2019 were eligible. Only biologics approved prior to 2017 were included to ensure data for all agents was available throughout the study period. Patients receiving ustekinumab were excluded due to lack of data on weight; it could not be determined whether ustekinumab 90 mg was prescribed above label or as on-label weight-based dosing. All subgroups were defined, and study outcomes were measured based on inpatient medical, outpatient medical, and outpatient pharmaceutical claims data using enrollment records; service dates; International Classification of Diseases, 9th or 10th Revision Clinical Modification codes; Current Procedural Technology 4th edition codes; Healthcare Common Procedure Coding System codes; and National Drug Codes, as needed.
Data source
The MarketScan Commercial Database contains the inpatient and outpatient medical and outpatient prescription-drug experience of millions of individuals and their dependents, covered under a variety of fee-for-service and capitated health plans. This includes exclusive provider organizations, preferred provider organizations, point of service plans, indemnity plans, and health maintenance organizations. The MarketScan Medicare Supplemental Database includes the healthcare experience of individuals with Medicare supplemental insurance paid for by employers; both the Medicare-covered portion of the payment (represented as Coordination of Benefits Amount) and the employer-paid portion are included in this database.
Study period
The study period was from 1 January 2015 to 30 November 2019, with the index date being the date of the first claim for the index treatment. The baseline period was 12 months prior to the start of index treatment, and the induction period was 2–12 weeks based on the dosing schedule for each agent. The follow-up period was 12 months following the start of maintenance dosing.
Patients
Adult patients diagnosed with plaque psoriasis from 1 January 2011, to the index date with ≥12 months of continuous enrollment with medical/pharmacy benefits before the index date and after the start of maintenance dosing, and ≥1 prescription filled for the index biologic during the 12-month follow-up period, were eligible for inclusion. Key exclusion criteria were use of the index medication during the baseline period; switching biologics during the follow-up period (to ensure a 12-month data window); or a diagnosis of psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, juvenile idiopathic arthritis, HIV infection, or cancer during the baseline period. Full selection criteria are shown in .
Table 1. Sample selection.
Outcomes
The outcomes measured were the percentage of patients with above-label use (defined as ≥10% above the recommended maintenance dose of each biologic), time to dose escalation (days), number of days of above-label use, and additional costs associated with above-label use. AEs were identified during the baseline period and from the index date through the 12-month period following the start of maintenance using ICD-9-CM/ICD-10-CM diagnosis codes in any position on medical claims. Additional costs included total all-cause healthcare costs, total psoriasis-related costs, and psoriasis-related biologic costs, which were calculated during the 12-month baseline period and the 12-month follow-up period, separately. Costs were calculated using paid amounts of adjudicated claims, including insurer and health plan payments, as well as patient cost-sharing in the form of co-payment, deductible, and coinsurance. Reported costs were mean total cost per patient during the entire 12-month follow-up period, and pharmacy costs were annualized or extrapolated for the whole year. All costs are reported in 2019 dollars, adjusted using the medical component of the Consumer Price Index. All outcomes were measured in the 12-month follow-up period after the start of the maintenance period.
Analysis
IBM Watson Health (Cambridge, MA, USA) created an analytic file to conduct descriptive analyses separately for patients diagnosed with plaque psoriasis who newly initiated therapy with secukinumab, ixekizumab, adalimumab, and etanercept. The analytic file included inpatient, outpatient, and prescription drug claim records as well as accompanying coding for the outcomes measured among the eligible patients identified for inclusion in the analysis. Patient demographics and clinical characteristics were measured on the index date, as well as during the baseline period before initiation of biologics, and stratified by on-label vs above-label use. Continuous measures are presented as mean, standard deviation, and median. Categorical measures are presented as counts and percentages. Time to dose escalation was examined using Kaplan-Meier curves. Chi-squared tests were used to compare whether the proportion of patients experiencing AEs was different between on-label and above-label use. A priori p value <.05 was considered as statistically significant.
Results
Patients
Of 56,522 patients in the database who had a claim for etanercept, adalimumab, ixekizumab, or secukinumab during the study period, 6,453 patients were included in the study after application of study inclusion and exclusion criteria (). Of the 6,453 total patients included, 708 (11.0%) received etanercept, 4,654 (72.1%) received adalimumab, 228 (3.5%) received ixekizumab, and 863 (13.4%) received secukinumab. Patient age and sex were similarly distributed across the treatment categories (). While diverse geographic regions were included, the South and urban areas were overrepresented in this study. A majority of patients included had commercial insurance with preferred provider organization plans ().
Table 2. Patient demographics.
Above-label use patterns
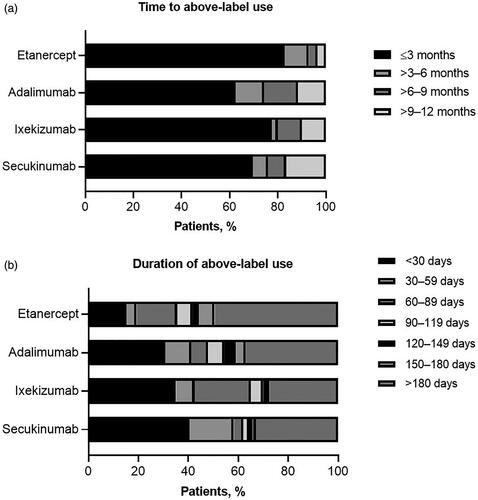
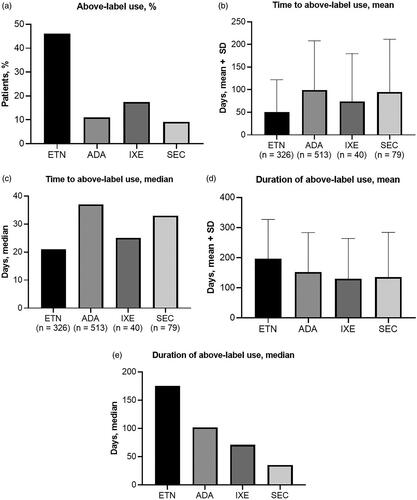
Above-label dosing was recorded in 326 (46.0%) patients receiving etanercept, 513 (11.0%) receiving adalimumab, 40 (17.5%) receiving ixekizumab, and 79 (9.2%) receiving secukinumab (). Time to above-label use was similar between different biological agents. Mean time to above-label use for all treatments ranged from 50.7 days for etanercept to 99.5 days for adalimumab (). The median time to above-label use for all treatments ranged from 21 days for etanercept to 37 days for adalimumab (). Mean duration of above-label use for all treatments ranged from 130 days for ixekizumab to 196 days for etanercept (), with median duration ranging from 35 days for secukinumab to 175 days for etanercept (). Categorical distribution of time to above-label use and duration of above-label use for each agent are shown in . The majority of patients initiated above-label use within 3 months of starting maintenance dosing, including 82.8% of patients receiving etanercept, 62.4% of patients receiving adalimumab, 77.5% of patients receiving ixekizumab, and 69.6% of patients receiving secukinumab (). The distribution of duration of above-label use was bimodal, with the largest subcategories of patients generally being those with ≥30 days or ≥180 days of above-label use ().
Figure 1. Above-label use patterns. (a) proportion of patients with above-label use, (b) mean time to above-label use, (c) median time to above-label use, (d) mean duration of above-label use, and (e) median duration of above-label use for patients receiving etanercept, adalimumab, ixekizumab, and secukinumab. Error bars represent standard deviation. Abbreviations. ADA, adalimumab, ETN, etanercept, IXE, ixekizumab; SD, standard deviation; SEC, secukinumab.
Costs associated with above-label vs on-label use
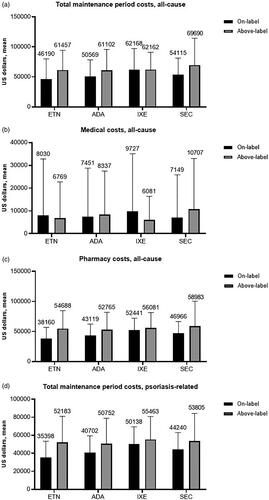
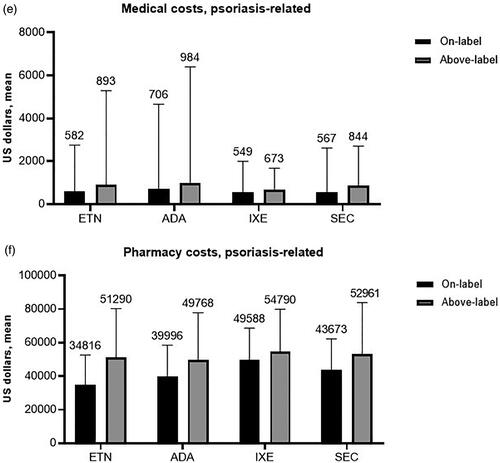
Baseline period all-cause costs were mainly associated with outpatient medical (nonpharmacy, e.g. office visits) costs and were generally similar among treatment categories; the mean per patient for the 12-month baseline period ranged from $1,470 for patients who received adalimumab as the index treatment, to $2,140 for those receiving ixekizumab (). Psoriasis-related baseline costs were a small share of total costs ($319–$513) and were also mainly associated with outpatient medical claims (). All-cause and psoriasis-related follow-up costs are shown in . Total maintenance period costs (all cause) were similar among biologics, with above-label use resulting in an increase when compared with on-label use except for ixekizumab (by a negligible $6 margin) (). Generally, the majority of all-cause costs were pharmacy rather than medical (). Mean total additional annual psoriasis-related medical costs associated with above-label use ranged from $124 to $312 (). Above-label use was associated with higher psoriasis-related total maintenance period costs compared with on-label use (). The majority of these costs were also pharmacy-related, with mean per-patient total additional annual psoriasis-related pharmacy costs ranging from $5,202 to $16,475 (ixekizumab, etanercept) (). Above-label use generally resulted in increased additional costs driven mainly by additional pharmacy costs.
Figure 3. Pre-index period pharmacy, outpatient, and inpatient cost assessments. (a) Pre-period all-cause costs and (b) pre-period psoriasis-related costs. Values above each subset column represent the mean cumulative cost. Abbreviations. ADA, adalimumab, ETN, etanercept, IXE, ixekizumab; PsO, plaque psoriasis; SEC, secukinumab.
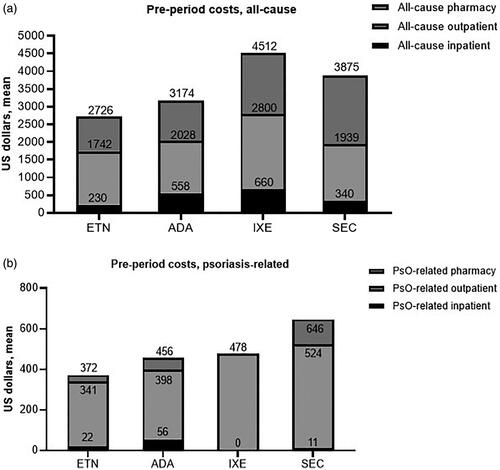
Figure 4. All-cause and psoriasis-related costs within the maintenance period. (a) All-cause maintenance period total costs, (b) all-cause maintenance period medical costs, (c) all-cause maintenance period pharmacy costs, (d) psoriasis-related maintenance period total costs, (e) psoriasis-related maintenance period medical costs, and (f) psoriasis-related maintenance period pharmacy costs. Values above the columns represent the mean, and the error bars represent standard deviation. Abbreviations. ADA, adalimumab, ETN, etanercept, IXE, ixekizumab; SEC, secukinumab.
Adverse events by treatment and dose escalation
There were few safety concerns significantly associated with above-label vs on-label biologic use (). Gastrointestinal-related infections and “other” infections (urinary tract infection, tinea infections, herpes simplex infection, oral herpes, osteomyelitis, oral candidiasis, conjunctivitis, and cellulitis) occurred significantly more frequently in patients receiving adalimumab above label vs on label. Patients who received above-label dosing of adalimumab were significantly less likely to have had no “other” infections pre-index but had significantly higher frequency of new “other” infections during the follow-up compared with patients receiving on-label dosing. Respiratory infections (including upper respiratory tract infections, lower respiratory tract infections, sinusitis, nasopharyngitis, influenza, pneumonia, rhinitis, pharyngitis, and bronchitis) occurred numerically more frequently with above-label dosing of ixekizumab (62.5%) compared with on-label dosing (47.3%); however, this could be due to the small pool of patients receiving ixekizumab.
Table 3. Incident adverse events by treatment and dose escalation.
Discussion
This study documented real-world dose-escalation patterns and associated additional costs and safety concerns in patients with psoriasis treated with biologics. Above-label use was most frequent among patients receiving etanercept (46%) and ixekizumab (17.5%). Mean time to above-label use was 51–100 days for all biologics. Adalimumab-treated patients had the longest mean time to above-label use (100 days), while etanercept-treated patients had the shortest (51 days). Duration of above-label use was longest for patients receiving etanercept (mean, 196 days) and shortest for those receiving ixekizumab (mean, 130 days). Baseline period costs were similar among index treatments, and the majority of those costs were medical rather than pharmacy-related; however, the majority of costs in the follow-up period were pharmacy-related. Total additional annual pharmacy and psoriasis-related costs associated with above-label use ranged from $5,202 to $16,475, with ixekizumab having the lowest cost and etanercept having the highest. The large discrepancy between psoriasis-related pharmacy costs associated with above-label use is possibly due to the smaller pool of patients receiving above-label ixekizumab (n = 228) compared with those receiving above-label etanercept (n = 708).
Patients diagnosed with psoriasis who have certain comorbidities—including psoriatic arthritis, metabolic syndrome, cardiovascular diseases, and other autoimmune diseases—utilize more healthcare resources, ultimately resulting in higher indirect and direct costs for those patients and adding to the already high economic burden of psoriasisCitation4. While new biologic agents are available for use, a subset of patients diagnosed with psoriasis do not respond to on-label dosing and may benefit from above-label dosingCitation6. In order to treat psoriasis both effectively and cost-effectively, more comprehensive studies need to be completed to assess the efficacy, safety, and affordability of above-label use of all available biologic agentsCitation6,Citation7.
This study has several limitations. There was insufficient data available for ustekinumab and guselkumab to include them in this study; agents approved after the study period were also not included. Future studies should be conducted to assess above-label use for these agents. This study did not assess effectiveness of above-label biologic use as we were not able to determine the reasoning behind dose escalation or if dose escalation itself was effective. Data were available only for patients with commercial health coverage or private Medicare supplemental coverage. Psoriasis diagnoses, comorbidities, or study outcomes may have been misclassified, as patients were identified through administrative claims data and not through medical records. Additionally, claims data can only identify if a patient has filled a prescription and cannot identify causality of drug use and AEs. Doses that were provided through promotional programs (or as samples) and claims that were denied by the payer were also not captured.
Above-label dosing of biologics for psoriasis treatment was associated with substantial additional pharmacy costs but few significant safety concerns. More comprehensive studies are needed to encompass all available biologic agents, include larger numbers of patients to reduce “background noise” in cost data, and capture effectiveness to allow cost-benefit analysis of above-label biologic use. Future analyses of interest include cost-effectiveness of dose escalation outcomes in the clinical context of patient characteristics and medical history, propensity matching to adjust for possible selection biases, treatment adherence in the on-label vs above-label groups, and comparisons after adjustment switches vs dose augmentation or escalation.
Transparency
Declaration of funding
The study was funded by Sun Pharmaceutical Industries, Inc.
Declaration of financial/other relationships
JB has received research funds for the Psoriasis Treatment Center from AbbVie; Amgen; Arcutis Biotherapeutics; Boehringer Ingelheim; Bristol-Myers Squibb; Celgene Corporation; Corrona LLC; Dermavant Sciences, LTD; Dermira; UCB; Eli Lilly and Company; Glenmark Pharmaceuticals Ltd.; Janssen Biotech; Kadmon Corporation; LEO Pharma; Lycera Corp; Menlo Pharmaceuticals; Novartis; Pfizer; Regeneron Pharmaceuticals; Sun Pharmaceutical Industries, Inc.; Taro Pharmaceutical Industries Ltd.; and Ortho Dermatologics; is or has been a consultant for AbbVie; Amgen; Celgene Corporation; Eli Lilly and Company; Janssen Biotech; Novartis; Sun Pharmaceutical Industries, Inc.; and Valeant Pharmaceuticals; and is or has been a speaker for AbbVie, Celgene Corporation, Eli Lilly, Janssen Biotech, and Novartis.
BG is or has been an investigator, speaker, and advisor for AbbVie; Celgene; Dermavant; Dermira; Eli Lilly; and Sun Pharmaceutical Industries, Inc.; is or has been an advisor and speaker for Novartis, Ortho Dermatologics, and Sanofi Genzyme; is or has been an investigator for Brickell Biotech, ChemoCentryx, Corrona Registry PSO, Corrona Registry AD, and Prose Registry; is or has been a speaker for Janssen and Regeneron; and is or has been an advisor for Pfizer.
JJW is or has been an investigator, consultant, or speaker for AbbVie; Almirall; Amgen; Arcutis; Aristea Therapeutics; Boehringer Ingelheim; Bristol-Myers Squibb; Dermavant; Dr. Reddy's Laboratories; Eli Lilly; Galderma; Janssen; LEO Pharma; Mindera; Novartis; Regeneron; Sanofi Genzyme; Solius; Sun Pharmaceutical Industries, Inc.; UCB; Valeant Pharmaceuticals North America LLC; and Zerigo Health.
IC was an employee of IBM Watson Health during the time of the study.
XS and MB are employees of IBM Watson Health.
AM is a former employee of Sun Pharmaceutical Industries, Inc.; and has individual shares in Johnson and Johnson, and as a part of retirement account/mutual funds.
SR is an employee of Sun Pharmaceutical Industries, Inc.
GH is or has been an investigator for Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Eli Lilly, Novartis, Janssen, MC2, PellePharm, Pfizer, and UCB; and a consultant, advisor, or speaker for AbbVie; Boehringer Ingelheim; Dermavant; Eli Lilly; Janssen; LEO Pharma; Ortho Dermatologics; Pfizer; Regeneron; Sanofi Genzyme; Sun Pharmaceutical Industries, Inc.; and UCB.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
AM, SR, XS, and IC contributed to the study concept and design.
XS, IC, and MB performed database review and data analysis.
JB, BG, JJW, and GH provided guidance on manuscript content and data interpretation.
All authors had access to the study data, reviewed the manuscript critically for intellectual content, and the final version for submission.
Acknowledgements
Medical writing and editorial support was provided by Hilary Durbano, PhD, of AlphaBioCom, LLC, and funded by Sun Pharmaceutical Industries, Inc.
References
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9(2):136–139.
- Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82(1):161–201.
- Shahwan KT, Kimball AB. Managing the dose escalation of biologics in an era of cost containment: the need for a rational strategy. Int J Womens Dermatol. 2016;2(4):151–153.
- Feldman SR, Zhao Y, Shi L, et al. Economic and comorbidity burden among patients with moderate-to-severe psoriasis. J Manag Care Spec Pharm. 2015;21(10):874–888.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151(6):651–658.
- Brezinski EA, Armstrong AW. Off-label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PLoS One. 2012;7(4):e33486.
- Cassano N, Loconsole F, Miracapillo A, et al. Treatment of psoriasis with different dosage regimens of etanercept: preliminary results from the Tαranta Plastic Study Group. Int J Immunopathol Pharmacol. 2010;23(3):797–802.
- Bagel J. Parting Words from Jerry Bagel, MD. The psoriasis treatment landscape has changed dramatically during this editor’s tenure. 2019. [cited 2020]. https://practicaldermatology.com/articles/2019-aug/parting-words-from-jerry-bagel-md.